Abstract
Background
There is increasing interest in performing left atrial appendage (LAA) occlusion at the time of atrial fibrillation (AF) ablation procedures. However, to date there has been no description of the acute changes to the LAA immediately following pulmonary vein (PV) isolation and additional left atrium (LA) substrate modification. This study assessed changes in the size and tissue characteristics of the LAA ostium in patients undergoing PV isolation.
Methods
This series included 8 patients who underwent cardiovascular magnetic resonance evaluation of the LA with delayed enhancement magnetic resonance imaging and contrast enhanced 3-D magnetic resonance angiography pre-, within 48 h of, and 3 months post ablation. Two independent cardiac radiologists evaluated the ostial LAA diameters and area at each time point in addition to the presence of gadolinium enhancement.
Results
Compared to pre-ablation values, the respective median differences in oblique diameters and LAA area were +1.8 mm, +1.7 mm, and +0.6 cm2 immediately post ablation (all NS) and −2.7 mm, −2.3 mm, and −0.5 cm2 at 3 months (all NS). No delayed enhancement was detected in the LAA post ablation.
Conclusion
No significant change to LAA diameter, area, or tissue characteristics was noted after PV isolation. While these findings suggest the safety and feasibility of concomitant PV isolation and LAA device occlusion, the variability in the degree and direction of change of the LAA measurements highlights the need for further study.
Abbreviations: AF, atrial fibrillation; LAA, left atrial appendage; RF, radiofrequency; LA, left atrium; CMR, cardiovascular magnetic resonance imaging; PV, pulmonary vein; CE-3D MRA, contrast enhanced 3-dimensional magnetic resonance angiography; DE-MRI, delayed enhancement magnetic resonance imaging; TR, repetition time; TE, echo time; NEX, number of excitations; FOV, field of view; IR, inversion recovery
Keywords: Atrial fibrillation, Catheter ablation, Left atrial appendage occlusion, Magnetic resonance imaging
1. Introduction
Atrial fibrillation (AF) is a common condition that is associated with a 5-fold increased risk of stroke [1]. While oral anticoagulants have traditionally been utilized for stroke reduction in AF patients, their use has been suboptimal because of concerns over bleeding [2]. Catheter ablation [3–5] and left atrial appendage (LAA) occlusion [6] may reduce stroke risk without the need for oral anticoagulation. The mechanism of the former is related to sinus rhythm maintenance, whereas the latter acts through exclusion of the LAA, which is the dominant site of thrombus formation in patients with non-valvular AF [7,8]. As the long-term freedom from AF associated with catheter ablation procedures is not ideal [9], an appealing solution for stroke reduction would be the placement of an LAA occlusion device at the time of an AF ablation procedure. Such a combined approach may result in very low rates of stroke, potentially similar to those reported in patients undergoing surgical MAZE procedures with LAA ligation [10,11], as well as minimize the need for a repeat invasive procedure requiring transseptal catheterization.
To date, one small series evaluating the feasibility of LAA occlusion at the time of catheter ablation comprising pulmonary vein (PV) isolation has been published [12,13]. In addition, we know of two clinical trials currently under way that are evaluating the merits of this combined strategy (LAALA-AF Registry [14] and LAA Occlusion after catheter ablation of AF (Clinical Trials.gov identifier number: NCT01695824) [15]). However, despite the enthusiasm among electrophysiologists for simultaneous PV isolation and LAA occlusion, it is unknown whether PV isolation triggers morphologic changes of the LAA that may affect the ability to safely perform concomitant LAA occlusion. That is, radiofrequency (RF) ablation in the region of the left PV may alter the size, shape, and tissue characteristics of the LAA ostium. Knowledge of any such changes would be important as it may affect the approach to and safety of percutaneous LAA occlusion at the time of an ablation procedure, thereby influencing future clinical studies in this field. In this pilot study, we address this knowledge gap by reporting on changes in the size and tissue characteristics of the LAA os in patients undergoing PV isolation and additional left atrium (LA) substrate modification as assessed using gadolinium-enhanced cardiovascular magnetic resonance imaging (CMR).
2. Material and methods
Patients who underwent a first-ever RF catheter ablation procedure for AF and were evaluated by CMR pre-, up to 48 h after, and 3 months post procedure were included in this study. Consent for participation was obtained from each patient. Ethics approval was obtained from the Sunnybrook Health Sciences Center Research Ethics Board (Study Number: 357-2006; Initial Approval Date: September 18th 2006; Study Closure Date: October 2013).
2.1. Ablation procedure
Catheter ablation was performed with either a manual or robotic (Stereotaxis Niobe Remote Magnetic Navigation System, St. Louis, USA) approach using an irrigated RF ablation catheter and a 3D-electroanatomic mapping system. Fluoroscopy and the created 3D-electroanatomic maps were used to guide catheter manipulation and ablation in the region of the PVs. Ablation was typically performed along the venous side of the ridge when ablating the anterior aspect of the left PV (Fig. 1). Irrigated RF ablation was typically performed for 30 s at each site with a maximum power of 40 W and temperature of 48°. The goal of pulmonary venous ablation was the elimination of high frequency local electrograms with subsequent PV isolation. Additional LA substrate modification was performed at the operator׳s discretion. No patients underwent electrical isolation of the LAA, LA substrate modification in the region of the LAA, or ablation within the distal coronary sinus or ligament of Marshall. Recurrence of any atrial arrhythmia during follow-up was determined using intermittent Holter, Loop, and electrocardiography monitoring.
Fig. 1.
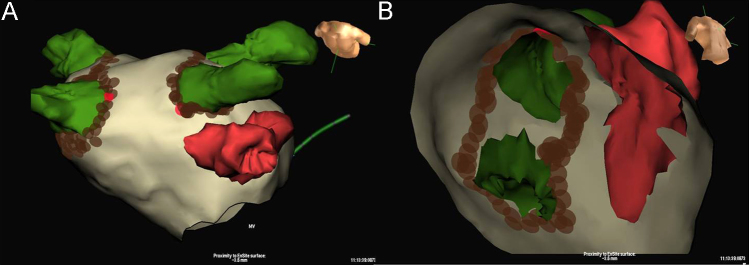
Ablation along the venous side of the left Pulmonary vein – left atrial appendage (PV-LAA) ridge. 3D electroanatomic map reconstruction of the LA and LAA with the NavX (St. Jude Medical, Minneapolis, USA) electroanatomic mapping system. Panel A: Anterior–posterior top-down view showing the relationship between the left PVs (green), the LAA (red), and the sites where ablation lesions are placed (brown dots). Panel B: Sagittal clipping plane showing the relationship between the left PVs (green), the LAA (red), and the sites where ablation lesions are placed (brown dots).
2.2. Magnetic resonance imaging data acquisition
All images were acquired on a 1.5 T Twinspeed Signa magnetic resonance (MR) scanner (GE Healthcare, Inc., Milwaukee, Wisconsin). In all patients, a contrast-enhanced 3D MR angiography (CE-3D MRA) data set was acquired in the coronal orientation following a 13 cc gadolinium chelate injection (Gadobutrol, Bayer Healthcare, Berlin, Germany). To measure the delay time, a test bolus repeating a single axial 2D spoiled gradient echo (2D FSPGR) slice was used after a 1 cc contrast injection. Representative imaging parameters for the 2D FSPGR were as follows: repetition time (TR)/echo time (TE)=13/min, flip angle=90°, sampling matrix=128×128 pixels, number of excitations (NEX)=1, field of view (FOV)=38 mm, and slice thickness=10 mm/0 gap. The parameters for the CE-3D MRA sequence were as follows: TR/TE=3.8/1.3 ms, flip angle=45°, sampling matrix=320×160 pixels, NEX=1, FOV=380 mm, 34 locations/slab, slice thickness=5 mm, and a breath hold time of 20 s. This sequence was acquired twice after a single contrast injection. The inversion recovery (IR)-prepared delayed enhancement MRI (DE-MRI) was obtained in a sagittal oblique plane approximately 20 min after contrast injection. Imaging parameters were as follows: free-breathing navigator gating, TR/TE=5.1/1.6 ms, flip angle=25°, sampling matrix=256×256 pixels, FOV=350 mm, and slice thickness=3.4 mm. An inversion time was selected using a Cine-IR pulse sequence prior to the delayed enhancement scan.
2.3. Image analysis
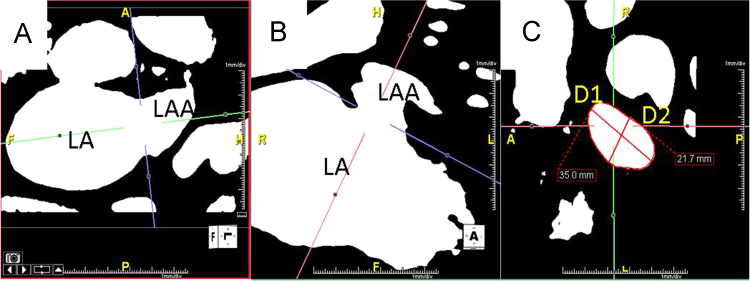
Images were analyzed using commercially available software (Aquarius, Intuition v4.4.8, TeraRecon, Inc., Foster City, California). Two experienced cardiac radiologists independently created multiplanar reformatted images using the double oblique technique (Fig. 2). The “full-width at half maximum” technique of image contrast and brightness adjustment was applied to ensure measurement consistency and reproducibility [16]. The os of the LAA was defined as the site of reflection of this structure with the adjacent LA [17]. Measurements of the LAA ostial diameter were obtained in 2 orthogonal views (D1, D2) (Fig. 2). The area of the LAA at the os was determined by manual planimetry of the LAA os. To minimize bias, each radiologist was blinded to the timing of imaging relative to the ablation procedure.
Fig. 2.
Measurement of the LAA ostial diameters and area. Contrast-enhanced three-dimensional magnetic resonance angiography using the “full-width at half maximum” method of windowing. Panels A and B: Multi-planar reformatted images through the LAA ostium obtained using the “double oblique” technique. Panel C: Two orthogonal dimensions of the LAA ostium (D1, D2) were obtained and the area of the LAA at the ostium was also determined by manual planimetry.
To assess whether differences in measured values were simply due to measurement error, we determined the mean absolute difference between the two radiologists for measurements of the LAA diameter (D1 or D2 at all time points) and LAA area (at all time points).
Each cardiac radiologist independently evaluated the presence of gadolinium enhancement within the LA, and specifically within the ostial region of the LAA. Again, each radiologist was blinded to the timing of imaging relative to the ablation procedure. Finally, measurements of all PVs were obtained to assess for radiographic evidence of PV stenosis (defined as a >50% reduction in the PV diameter [18]).
2.4. Statistical analysis
Continuous variables are reported as median and range. The mean of the pair of values for LAA diameter (D1 and D2) and of the LAA area at each time point were calculated. The mean absolute measurement difference (for both diameters and the area) at all time points between the two cardiac radiologists was determined to provide insight into variability related to the measurement process. A paired analysis of the mean repeated measures was conducted using ANOVA with significance determined using the Wilcoxon test. P-values <0.05 were considered statistically significant. Statistical analysis was performed with R (Version 3.02, Auckland, New Zealand).
3. Results
Eight patients were imaged at all three time points. The median age was 58 years (range: 48–76) and all patients were male. Six of the patients paroxysmal AF, 3 had hypertension, and none had diabetes. The LA diameter on echocardiography was 41 mm (range: 34–53). All patients underwent irrigated RF ablation, and in 4 the procedure was performed with a robotic approach. The median total RF ablation time for each case was 4134 s (range: 3040–4786). No patient developed PV stenosis after the procedure. Long-term freedom from recurrent atrial arrhythmia was noted in 5 of the 8 patients.
A pre-procedure CMR was performed 128 days (range: 1–218) pre-ablation. The immediate post-ablation MRI was performed the following day in 7 patients and 2 days post ablation in the remaining patient. Delayed imaging was performed 67 days (range: 57–103) post ablation. Imaging was performed in sinus rhythm for 7 patients at each study point, and in AF for the eighth patient.
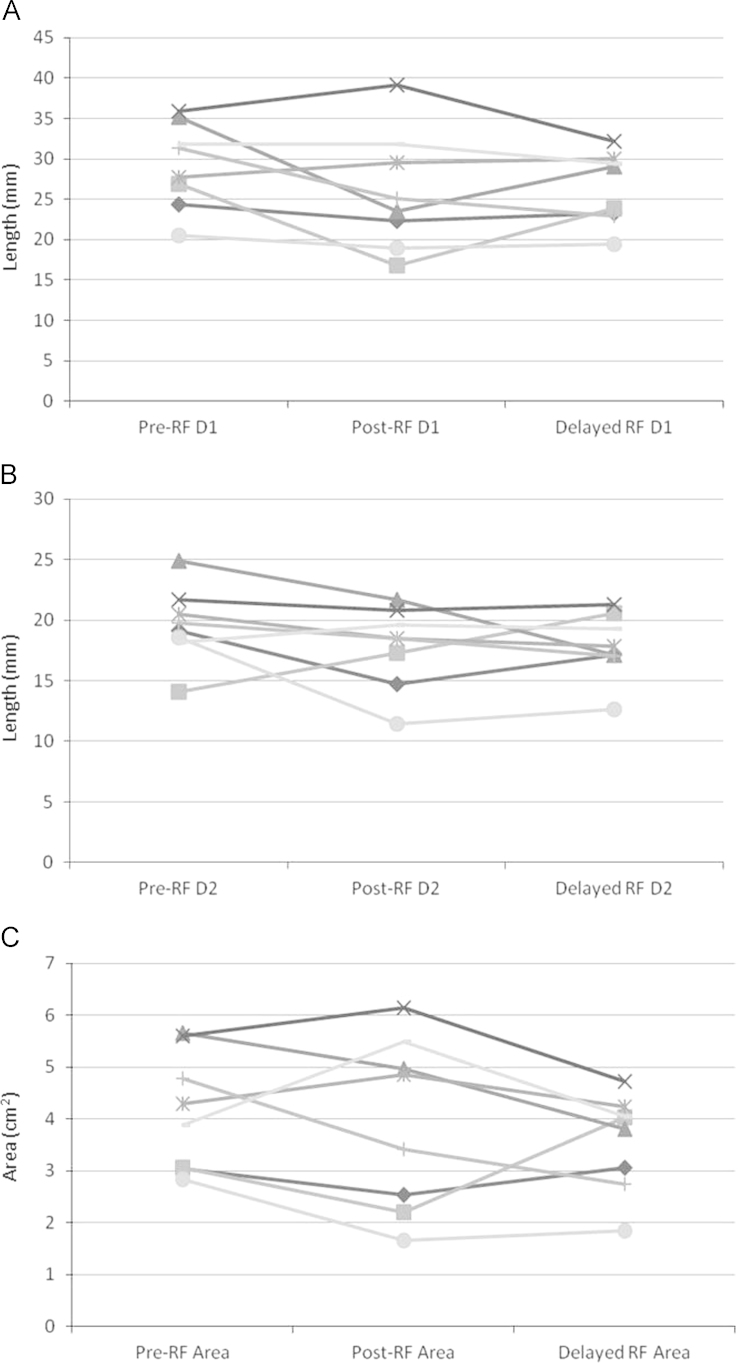
Fig. 3 shows the change in the LAA diameters and area for each of the 8 patients. Compared to pre-ablation values, the respective median differences in the D1 diameter, D2 diameter, and LAA area were +1.8 mm, +1.7 mm, and +0.6 cm2 immediately post ablation (all NS) and −2.7 mm, −2.3 mm, and −0.5 cm2 at 3 months (all NS). Inter-patient variability in the degree and direction of change in the D1 and D2 diameters and the LAA area at the two post-ablation time points was apparent (Fig. 3).
Fig. 3.
LAA ostial dimensions pre-, post-, and 3 months post-ablation. LAA ostial dimensions for each patient pre ablation, immediately following ablation, and 3 months (delayed) post ablation. Panel A: Ostial diameter (D1) measurement. Panel B: Ostial diameter (D2) measurement. Panel C: Ostial area.
The mean absolute differences between the two radiologists for measurements of the LAA diameter (D1 or D2 at all time points) and LAA area (at all time points) were 2.4 mm and 0.7 cm2, respectively.
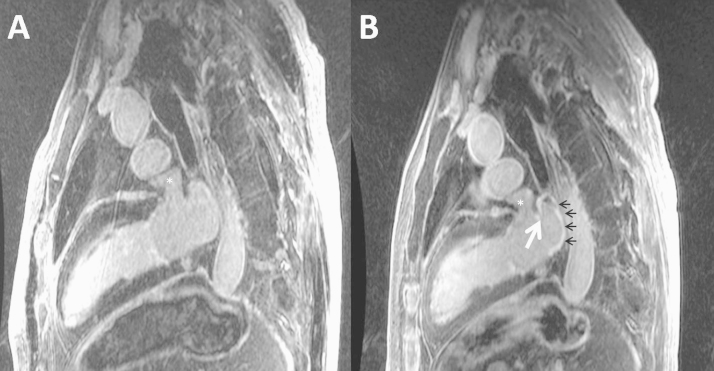
Delayed enhancement was not present in any patient pre ablation but was present within the LA and pulmonary venous antrum in all patients post ablation (Fig. 4). No patient showed evidence of delayed enhancement within the LAA at any time point. Of note, 1 patient had evidence of delayed enhancement along the pulmonary venous ridge immediately post ablation, but not within the LAA itself (Fig. 4, Panel B).
Fig. 4.
Left atrial DE-MRI images pre- and post-ablation. DE-MRI sagittal images of the LA and LAA (*) for patient #6 before (Panel A) and 24 h after (Panel B) ablation. Post ablation (Panel B), note the presence of new DE in the left superior PV, posterior wall (black arrows), and pulmonary venous ridge (white arrow) but not in the LAA (*).
4. Discussion
The results of this pilot imaging study suggest that LAA ostial dimensions and tissue characteristics are unaltered following PV isolation and LA substrate modification. These findings, which may appear obvious to some, are important to document given the increased enthusiasm among electrophysiologists for performing concomitant LAA occlusion and PV isolation.
Current techniques for percutaneous endocardial LAA occlusion rely on attempting occlusion by deploying devices within the ostial region of the LAA. While differences exist between commercially available percutaneous devices, common features include their placement generally at or slightly distal to the os of the LAA and the use of stabilizing barbs that aid in anchoring the device to the adjacent tissue [19]. Therefore, it is important to obtain knowledge of any alteration of the LAA ostial size or tissue characteristics of the LAA wall during an AF ablation procedure prior to proceeding with LAA device occlusion at the time of PV isolation.
The magnitude of the changes observed at all time points throughout our pilot study is probably not clinically important and is potentially related to inherent inaccuracies in measurement. Furthermore, these changes may not affect device implantation given the practice of “over-sizing,” that is, selecting a percutaneous LAA device with a diameter 10–20% larger than the largest measured LAA dimension [12,19]. However, the variability in the magnitude of change observed between patients in our pilot study coupled with the consequences of inappropriate LAA device sizing highlights the need for further study in a larger patient population.
DE-MRI has been shown to identify atrial myocardium changes after ablation procedures [20–22]. Gadolinium diffuses into the extracellular space created by myocardial edema, a reversible form of myocardial injury, as well as into the intracellular space with loss of cell membrane integrity [20,23], which may correspond to varying degrees of coagulation necrosis and intra-lesional hemorrhage [24]. Owing to the inaccuracies associated with catheter localization and stability relating to the presence of cardiac and respiratory motion, RF energy may be unintentionally delivered within the region of the LAA during ablation of the anterior portion of the left PVs. The presence of acute RF tissue injury in this region may be a deterrent to the implantation of an LAA occlusion device, as edematous or necrotic tissue may lead to device instability, resulting in device embolization or LAA perforation secondary to device over-sizing or to the inadvertent positioning of a barb within friable tissue. To the best of our knowledge, our work is the first to evaluate the presence of gadolinium enhancement in the region of the LAA, and suggests that the LAA likely remains free of significant RF injury despite ablation to the adjacent left PV. While this finding may appear intuitive, it is important to confirm this intuition as one would want to be confident that the LAA is free of injury prior to the placement of an LAA occlusion device.
The aim of our work was not to advocate for performing LAA occlusion and AF ablation concomitantly. Indeed, given the potential benefits of a successful catheter ablation procedure for stroke reduction [3–5], some would appropriately advocate performing these procedures in a staged fashion. The aim of this report was simply to provide an objective characterization of the LAA after PV isolation and additional substrate modification. Our findings will provide reassurance to interventional cardiac electrophysiologists contemplating concomitant AF ablation and percutaneous LAA occlusion. This knowledge is important, as combined procedures may have theoretical advantages including a reduction in the number of procedures requiring transseptal catheterization, truncation of the total required duration of oral anticoagulation (as post-procedure anticoagulation would serve a dual purpose of preventing both post-ablation stroke and acute thrombus formation on the LAA occlusion device), and the potential for a long-term reduction in stroke risk without the need for oral anticoagulation. While our current pilot study is important and addresses an important gap in clinical knowledge, it merely provides radiographic data and is no substitute for the additional larger clinical studies needed to elucidate the feasibility and safety of performing these procedures concomitantly prior to the widespread uptake of combined procedures.
Limitations to our work must be noted. First, our sample size was small and comprised patients without severe LA dilation, which may have prevented the detection of statistically significant changes in LAA dimensions. While we suspect that changes in LAA dimension may not be of a sufficient magnitude to affect LAA occlusion device placement, we cannot fully exclude this possibility in all patients and for all ablation strategies and ablation energies. Second, our approach to PV isolation involved ablation along the venous side of the ridge, frequently with the precision of robotic navigation. Deliberate ablation along the pulmonary venous ridge itself, the appendage side of the ridge, or even ablation resulting in LAA electrical isolation may have a more prominent influence on the LAA. Despite this, given the recent uptake of circular ablation techniques that result in more ostial ablation, our findings are applicable [25]. Third, LAA imaging was not performed immediately after ablation. This delay in imaging may have resulted in an underestimation of acute RF injury, which may have resolved during the short waiting period. In addition, our measurements obtained at 24 h post ablation may not reflect acute LAA dimensions due to changes in fluid status. Finally, while it may be argued that the absence of gadolinium uptake in the LAA may reflect technical limitations of DE-MRI and not the absence of actual LAA injury, we suspect that this is not the case as all patients had gadolinium enhancement in other areas of the LA post ablation.
5. Conclusion
The dimensions and tissue characteristics of the LAA remain relatively unchanged after PV isolation and LA substrate modification. Further study is needed to confirm these findings prior to widespread recommendations of concomitant AF ablation and LAA device occlusion procedures.
Conflict of interest
Research funding for this study was provided by General Electric Healthcare and the Ontario Research Fund. A part of Dr. Shmatukha׳s salary was paid by General Electric Healthcare on account of his full-time employment with the company at the earlier stages of the project.
References
- 1.You J.J., Singer D.E., Howard P.A. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531–e575. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baczek V.L., Chen W.T., Kluger J. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunch T.J., May H.T., Bair T.L. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. 2013;10:1272–1277. doi: 10.1016/j.hrthm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Saad E.B., d’Avila A., Costa I.P. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011;4:615–621. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- 5.Oral H., Chugh A., Ozaydin M. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114:759–765. doi: 10.1161/CIRCULATIONAHA.106.641225. [DOI] [PubMed] [Google Scholar]
- 6.Holmes D.R., Reddy V.Y., Turi Z.G. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 7.Stoddard M.F., Dawkins P.R., Price C.R. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic stroke: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 8.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H., Reynolds M.R., Spector P. Treatment of atrial fibrillation with anti-arrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 10.Cox J.L., Ad N., Palaxxo T. Impact of the MAZE procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 11.Ballaux P.K., Geuzebroek G.S., van Hemel N.M. Freedom from atrial arrhythmias after classic MAZE III surgery: a 10 year experience. J Thorac Cardiovasc Surg. 2006;132:1433–1440. doi: 10.1016/j.jtcvs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Swaans M.J., Post M.C., Rensing B.J.W.M. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J Am Heart Assoc. 2012;1:e002212. doi: 10.1161/JAHA.112.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaans M.J., Alipour A., Rensing B.J. Catheter ablation in combination with left atrial appendage closure for atrial fibrillation. J Vis Exp. 2013;26:e3818. doi: 10.3791/3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LAA Ligation as an adjuvant therapy for AF ablation. Available at: 〈http://www.eplabdigest.com/multimedia/LAA-Ligation-Adjuvant-Therapy-AF-Ablation〉 [accessed 12.12.2013].
- 15.Left Atrial Appendage (LAA) occluders after catheter ablation of atrial fibrillation. NCT01695824. Available at: 〈http://clinicaltrials.gov/show/NCT01695824〉 [accessed 12.12.2013].
- 16.Merkx M.A., Bescos J.O., Geerts L. Accuracy and precision of vessel area assessment: manual versus automatic lumen delineation based on full-width at half-maximum. J Magn Reson Imaging. 2012;36:1186–1193. doi: 10.1002/jmri.23752. [DOI] [PubMed] [Google Scholar]
- 17.Heist E.K., Refaat M., Danik S.B. Analysis of the left atrial appendage by magnetic resonance angiography in patients with atrial fibrillation. Heart Rhythm. 2006;3:1313–1318. doi: 10.1016/j.hrthm.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Packer D.L., Kowal R.C., Wheelan K.R. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) Pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 19.Yu C.M., Khattab A.A., Bertog S.C. Mechanical antithrombotic intervention by LAA occlusion in atrial fibrillation. Nat Rev Cardiol. 2013;10:707–722. doi: 10.1038/nrcardio.2013.158. [DOI] [PubMed] [Google Scholar]
- 20.Arujuna A., Karim R., Caulfield D. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2012;5:691–700. doi: 10.1161/CIRCEP.111.966523. [DOI] [PubMed] [Google Scholar]
- 21.Taclas J.E., Nezafat R., Wylie J.V. Relationship between intended sites of RF ablation and post-procedure scar in AF patients, using late gadolinium enhancement cardiovascular magnetic resonance. Heart Rhythm. 2010;7:489–496. doi: 10.1016/j.hrthm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjan R., Kato R., Zviman M.M. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol. 2011;4:279–286. doi: 10.1161/CIRCEP.110.960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim T., Hackl T., Nekolla S.G. Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during 1st week after reperfusion. Radiology. 2010;254:88–97. doi: 10.1148/radiol.09090660. [DOI] [PubMed] [Google Scholar]
- 24.Dickfield T., Kato R., Zviman M. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2006;47:370–378. doi: 10.1016/j.jacc.2005.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy V.Y., Neuzil P., d’Avila A. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm. 2008;5:353–360. doi: 10.1016/j.hrthm.2007.11.006. [DOI] [PubMed] [Google Scholar]