Abstract
Background
Catheter ablation of ventricular tachycardia (VT) is feasible. However, the long-term outcomes for different underlying diseases have not been well defined.
Methods
Eighty-eight consecutive patients who underwent catheter ablation of VT using a three-dimensional mapping system were analyzed. The primary endpoint was any VT or ventricular fibrillation (VF) recurrence. Secondary endpoints were a composite of death or any VT/VF recurrence. Underlying heart diseases were remote myocardial infarction (remote MI) in 51 patients and non-ischemic cardiomyopathy in 37 (arrhythmogenic right ventricular cardiomyopathy [ARVC] in 18 patients, and dilated cardiomyopathy [NIDCM] in 19).
Results
Acute success was achieved in 82 of 88 (93%) patients. During a follow-up period of 39.2±4.6 months, VT recurred in 26 of 87 (30%), and VT/VF recurrence or death occurred in 39 of 87 (45%) patients. ARVC had better outcomes than NIDCM for the primary (p<0.05) and secondary endpoints (p<0.05). Remote MI-VT revealed a midrange outcome.
Conclusions
The long-term outcomes after catheter ablation of VT varied according to the underlying heart disease. ARVC-VT ablation was associated with better long-term prognosis than NIDCM. Remote MI-VT demonstrated a midrange outcome.
Keywords: Catheter ablation, Ventricular tachycardia, Remote myocardial infarction, Dilated cardiomyopathy, Arrhythmogenic right ventricular dysplasia/cardiomyopathy
1. Introduction
Patients with structural heart disease have an increased risk of sudden cardiac death, secondary to ventricular tachyarrhythmias in most cases. Implantable cardioverter–defibrillators (ICDs) are the treatment of choice. However, ventricular tachyarrhythmias cannot be prevented by ICD itself. Moreover, ICD shocks reduce quality of life, and episodes of ventricular tachycardia (VT) predict an increased risk of death and heart failure despite effective treatment with ICD [1–3]. Catheter ablation has been proven to be an effective choice of treatment for VT and may be indicated for some patients as either a primary therapy or an adjunct to an ICD implantation.
Although VT ablation is feasible, multiple morphologies of VT, hemodynamic instability, and non-inducibility limit the success of VT ablation. Recently, ablation of unmappable VTs has become feasible by mapping during sinus rhythm and with energy applications targeting delayed potentials or by creating ablation lesions using a three-dimensional (3D) mapping system [4].
After successful ablation, long-term recurrence of ventricular arrhythmias is not uncommon but the outcomes for different diseases are incompletely defined. This study aimed to clarify the outcomes of VT ablation for patients with different forms of structural heart disease.
2. Materials and methods
2.1. Patients
Between September 2004 and September 2012, endocardial catheter mapping and radiofrequency (RF) current ablation were performed using a 3D mapping system in 88 consecutive patients with clinically documented sustained monomorphic VT. All patients had ischemic (remote myocardial infarction; 51 patients) or non-ischemic heart disease (37 patients). Non-ischemic heart disease was divided into 2 groups: arrhythmogenic right ventricular cardiomyopathy (ARVC, 18 patients) and non-ischemic dilated cardiomyopathy (NIDCM, 19 patients). ARVC was defined based on the Task Force criteria [5]. Non-ischemic DCM was defined as a myocardial disorder in which there is evidence of structurally and functionally abnormal heart muscle, in the absence of significant coronary artery disease.
Written informed consent was obtained from all patients before the procedure.
2.2. Electrophysiological study
A conventional computerized electrophysiological system and CARTO (Biosense Webster, Diamond Bar, CA, USA) or Ensite NavX (St Jude Medical, Minneapolis, MN, USA) electroanatomical mapping system were used. The standard access to the left ventricle was retrograde across the aortic valve. In some patients, an antegrade transseptal access was used because of severe atherosclerosis of the aorta or peripheral arteries.
Programmed ventricular stimulation with up to 2 extra-stimuli at 2 different sites (right ventricular apex and outflow tract) was performed to induce clinical VT and/or any other non-clinical VT. VT was considered clinical if either the 12-lead morphology matched the previously documented VT or if the cycle length was within a range of 30 ms of the VT cycle length documented by the ICD.
2.3. Mapping and ablation
Mapping and ablation were performed using 7F steerable catheters with either a conventional 4-mm tip (NaviStar, Biosense-Webster) or a 3.5-mm irrigated tip electrode (NaviStar ThermoCool, Biosense-Webster) in patients in whom the CARTO systems used. In patients in whom the Ensite NavX system was used, a Celsius Thermocool (Biosense-Webster), Ablaze (8 mm tip, Japan Lifeline, Tokyo, Japan), or Coolflex (St Jude Medical) catheter was inserted.
Briefly, hemodynamically stable VTs were mapped and ablated during VT [6]. VT mapping was combined with conventional entrainment pacing at sites with diastolic potentials according to the methods for entrainment previously described [7–10]. Mapping for hemodynamically unstable VTs was performed during sinus rhythm or right ventricular pacing. The target of the ablation was putative channels and exits within the low-voltage area as identified from a paced QRS morphology similar to the QRS morphology of VT, fractionated potentials, or isolated late potentials during sinus or paced rhythm [11–16]. As for the patients included in the later part of the study period, RF energy was applied targeting all delayed potentials toward dechanneling [17]. Furthermore, when target sites were adjacent to the electrical barriers or a region of electrically unexcitable scar, the ablation lesions were extended to the unexcitable area [18,19]. We placed the mapping catheter at a potential target site during sinus rhythm or pacing. Then, if the VT was inducible and hemodynamically unstable, the RF current was applied as soon as any diastolic activity was recorded. If the endocardial mapping and ablation failed, we switched to epicardial mapping and ablation [20].
Conventional RF applications were delivered using a temperature-controlled mode (maximum 60 °C; maximum 180 s; 30–50 W; Stockert: Biosense-Webster or CABL-IT: Japan Lifeline). For the irrigated tip catheters, RF applications at 30–50 W were applied with a temperature limit of 43 °C.
The endpoint of the RF applications in the VT mapping group was non-inducibility of the clinical VT or all hemodynamically stable VTs, and the elimination of the targeted potentials in the substrate mapping group. The endpoint for linear ablation was the completion of the designed lines. Acute success was defined as the achievement of the ablation endpoint, but allowed for the induction of non-clinical hemodynamically unstable VTs or ventricular fibrillation (VF) [6]. In both groups, chronic success during the follow-up period was defined as the absence of any sustained VT, VF, or ICD therapy.
2.4. Follow-up
Follow-up started after hospital discharge and was performed every 3 months in the ICD outpatient clinic or by the referring physician. The primary endpoint was any VT/VF and the secondary endpoints were any VT/VF or death.
2.5. Statistical analysis
The continuous variables are expressed as the mean±SD, and were compared using the Student׳s t-test. The categorical variables were compared using a chi-square test or Fisher׳s exact test. An overall chi-square test for a 2×n table was performed when comparisons involved >2 groups. A p value <0.05 was considered significant. In the comparisons among the types of cardiomyopathies, survival curves were created using the Kaplan–Meier method, and comparisons between groups were based on the Wilcoxon test.
3. Results
3.1. Patient characteristics
The patient characteristics are shown in Table 1. Between September 2004 and September 2012, 105 ablation procedures were performed in 88 consecutive patients (15 women; age, 64.8±14.5 years). There were significant differences in age between the remote MI and ARVC (p<0.01), and remote MI and NIDCM (p<0.01) groups. Further, the left ventricular ejection fraction (LVEF) was better in the ARVC group than in the remote MI (p<0.01) or NIDCM group (p<0.01). VT storms were more prevalent in the remote MI group (p<0.05). In addition, there was a statistically significant difference in the concomitant diseases, medications, and device implantations.
Table 1.
Patient characteristics.
| Remote MI | ARVC | NI-DCM | P value | |
|---|---|---|---|---|
| Patients (n) | 51 | 18 | 19 | |
| Age (years) | 70.0±11.5⁎† | 55.9±16.2⁎ | 60.2±15.5† | <0.01⁎ |
| <0.01† | ||||
| Sex (male %) | 45 (88) | 12 (67) | 16 (84) | NS |
| LVEF (%) | 33.2±9.5⁎ | 58.1±10.4⁎† | 33.8±10.2† | <0.01⁎ |
| <0.01† | ||||
| VT storm (%) | 12 (24) | 0 (0) | 8 (42) | <0.05 |
| DM (%) | 15 (29) | 1 (6) | 2 (11) | <0.05 |
| HT (%) | 24 (47) | 5 (28) | 4 (21) | N.S. |
| Class 1 AAD (%) | 4 (8) | 6 (33) | 7 (37) | <0.05 |
| ACEI/ARB (%) | 37 (73) | 4 (22) | 16 (84) | <0.01 |
| β-Blocker (%) | 37 (73) | 5 (28) | 16 (84) | <0.01 |
| Amiodarone (%) | 26 (51) | 1 (6) | 13 (68) | NS |
| Sotalol (%) | 3 (6) | 6 (33) | 3 (16) | NS |
| ICD/CRTD (%) | 43 (84) | 8(44) | 19 (100) | <0.01 |
Remote MI=remote myocardial infarction; ARVC=arrhythmogenic right ventricular cardiomyopathy; NIDCM=non-ischemic dilated cardiomyopathy; LVEF=left ventricular ejection fraction; VT=ventricular tachycardia; DM=diabetes mellitus; HT=hypertension; AAD=antiarrhythmic drug; ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; ICD=implantable cardioverter defibrillator; CRT=cardiac resynchronized therapy.
Data are presented as the mean±SD or n (%).
3.2. Remote myocardial infarction
Sixty-one ablation procedures were performed in 51 patients (6 women, age 70.0±11.5 years, Fig. 1). The infarct region was anterior or anteroseptal in 23, inferior in 23, posterior in 4, and anterior and inferior in 1 patient. The mean LVEF was 33.2±9.5% and mean cycle length of the clinical VT 375±80 ms. In 49 of 51 patients (96%), acute success was achieved. During a mean follow-up period of 41.3±28.5 months, 15 patients (29%) died of non-arrhythmic causes and 15 (29%) experienced recurrence. An epicardial approach was performed in 4 patients. Twenty-seven patients (53%) were alive without any recurrence. An ICD or Cardiac resynchronization therapy defibrillator (CRTD) was implanted in 36 and 7 patients, respectively, and no rhythm management device was implanted in 8 patients.
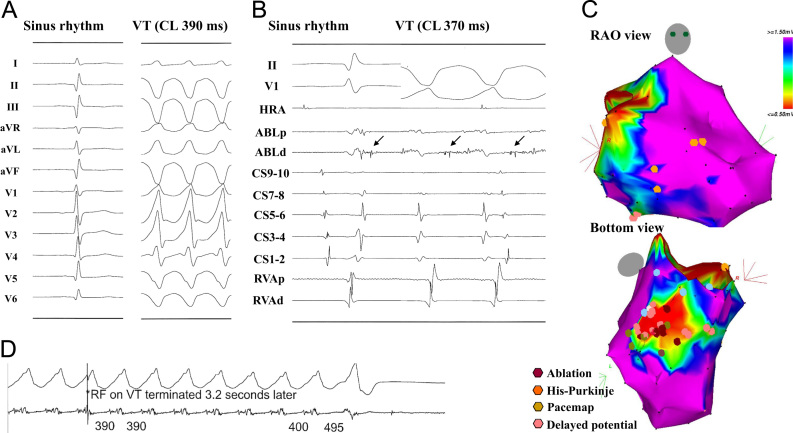
Fig. 1.
Ablation of VT in patient with remote myocardial infarction. (A) Surface ECG during sinus rhythm and VT. (B) Intracardiac electrogram during sinus rhythm and VT at the ablation site. (C) Voltage map in the right anterior oblique (RAO) and bottom views during sinus rhythm. (C) Radiofrequency energy application. The voltage map demonstrates an abnormal low voltage area (<1.5 mV) located in the inferior LV. A delayed potential (B: arrow in the left panel) is recorded inside the low voltage area that changed into a diastolic potential during the VT (B: right panel). An RF energy application terminated the VT immediately (C). VT=ventricular tachycardia; HRA=high right atrium; ABL=ablation; CA=coronary sinus; RVA=right ventricular apex; p=proximal; d=distal.
3.3. Arrhythmogenic right ventricular cardiomyopathy
Twenty ablation procedures were performed in 18 patients (6 female; age, 55.9±16.2 years; Fig. 2) including 2 by an epicardial approach. The mean cycle length of the clinical VT was 334±63 ms. Acute success was achieved in 14 of 18 patients (78%), and a hemodynamically stable VT was still inducible at the end of the procedure in the remaining 4 patients. One patient dropped out. During a mean follow-up period of 44.7±32.8 months, 3 patients experienced VT recurrence, and 4 of 17 patients (24%) died of non-arrhythmic causes (heart failure in 2, pneumonia in 1, and intracranial hemorrhage in 1 patient). Twelve of 17 patients (71%) were alive without any VT recurrence. An ICD was implanted in 8 patients.
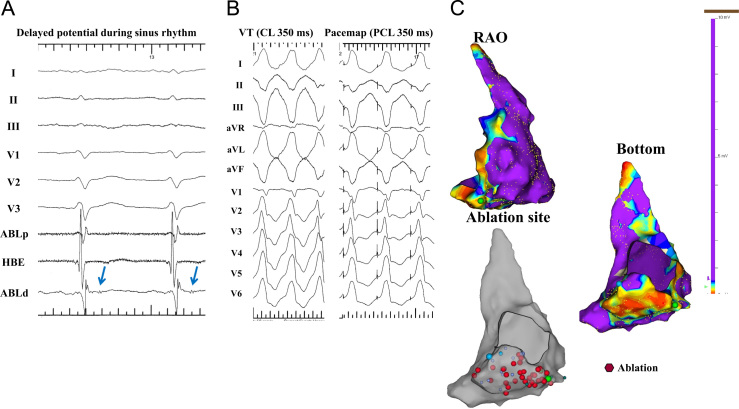
Fig. 2.
Ablation of a VT in a patient with ARVC. (A) Intracardiac ECG. (B) Surface ECG during VT and a pacemap. (C) A voltage map of the RV in the RAO and bottom views during sinus rhythm. An abnormal low voltage area is demonstrated in the inferior wall of the RV (C). Delayed potentials are recorded inside the low voltage area (A: blue arrow). A pacemap at the delayed potential recording site reveals an almost identical QRS morphology as the VT. All delayed potentials disappeared by repeated RF applications inside the abnormal low voltage area (red tag in the gray chamber). HBE=His bundle.
3.4. Non-ischemic dilated cardiomyopathy
Twenty-four ablation procedures were performed in 19 patients (3 women; age, 60.2±15.5 years; Fig. 3). The mean LVEF and mean cycle length of the clinical VT was 33.8±10.2% and 431±88 ms, respectively. Acute success was achieved in all patients. An epicardial approach was performed in 9 patients. During a mean follow-up period of 35.0±27.7 months, 1 patient died of heart failure. VT/VF did not recur in 11 out of 19 patients (58%). An ICD was implanted in 12 patients and the remaining 7 patients received a CRTD.
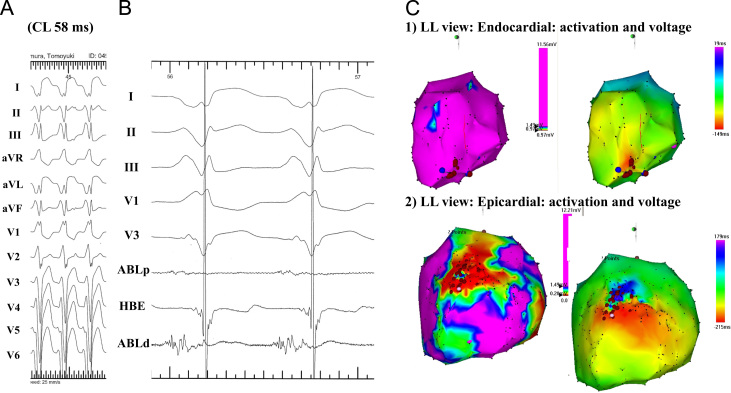
Fig. 3.
Ablation of a VT in a patient with NIDCM. (A) Surface ECG during the VT. (B) Intracardiac ECG at the ablation site during the VT. (C) Voltage and activation maps during the VT of the endocardium (1) and epicardium (2). On the endocardium, there is no abnormal low voltage area and the VT exhibits a focal activation pattern (C-1). However, a low voltage area is seen in the anterior region on the epicardium and the VT shows a macroreentry. An RF application targeting diastolic potentials (B: arrow) inside the low voltage area terminated the VT.
3.5. Differences in the ablation results among the types of cardiomyopathies
The numeric values of the ablation results are shown in Table 2. The mean cycle length of the clinically documented VT was significantly longer in the NIDCM patients than in those with a remote MI (p<0.05) or ARVC (p<0.01). No significant difference was observed in the number of induced VTs, clinical VT induction rate, and irrigation system usage. VT mapping was performed in 35%, 50%, and 68% of remote MI, ARVC and NIDCM patients, respectively (p<0.05), whereas substrate mapping was performed in 80%, 72%, and 68%, respectively (p=nonsignificant). Acute success was less often achieved in the ARVC patients than in the remote MI or NIDCM patients (p<0.05). Non-clinical hemodynamically unstable VT was inducible after RF application in 37%, 44% and 37% of remote MI, ARVC, and NIDCM patients, respectively (p=nonsignificant). An epicardial approach was mostly needed in NIDCM patients (p<0.01).
Table 2.
Procedural details.
| Remote MI | ARVC | NI-DCM | P value | |
|---|---|---|---|---|
| Clinical VTCL (ms) | 375±80† | 334±63⁎ | 431±88⁎† | <0.01⁎ |
| <0.05† | ||||
| Induced VT (n) | 2.2±1.6 | 2.4±1.5 | 2.1±1.1 | NS |
| Clinical VT induction (%) | 40 (78) | 17 (94) | 13 (68) | NS |
| Irrigation system usage (%) | 27 (53) | 7 (39) | 13 (68) | NS |
| Mapping method | ||||
| VT mapping | VT: 18† | VT: 9† | VT: 13† | <0.05† |
| Substrate mapping | SU: 41⁎ | SU: 13⁎ | SU: 13⁎ | NS⁎ |
| Acute success (%) | 49 (96) | 14 (78) | 19 (100) | <0.05 |
| Inducibility of nonclinical, hemodynamically unstable VT (%) | 19 (37) | 8 (44) | 7 (37) | NS |
| Failure | 2 (4) | 4 (22) | 0 (0) | <0.05 |
| Epicardial approach | 4 | 2 | 9 | <0.01 |
CL=cycle length; SU=substrate mapping; VT=VT mapping.
Data are presented as the mean±SD or n (%).
3.6. Follow-up outcomes
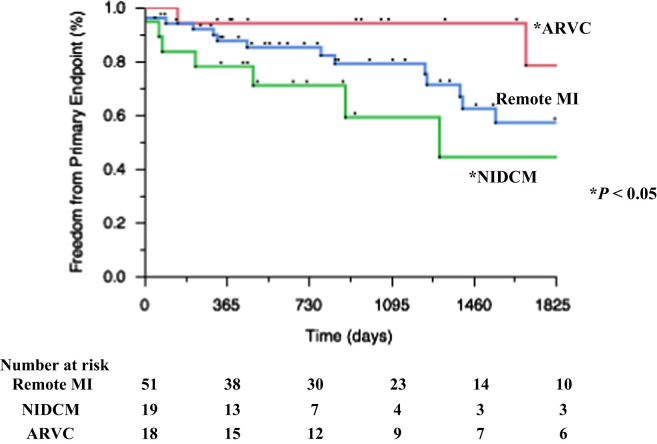
Kaplan–Meier curves of the primary and secondary endpoints are shown in Figs. 4 and 5. During 39.2±4.6 months of follow-up, 26 of 87 (30%) patients reached the primary endpoint. In 39 (45%) patients, the secondary endpoint was reached, including 20 (23%) deaths.
Fig. 4.
Kaplan–Meier curves showing the primary endpoint (VT/VF recurrence).
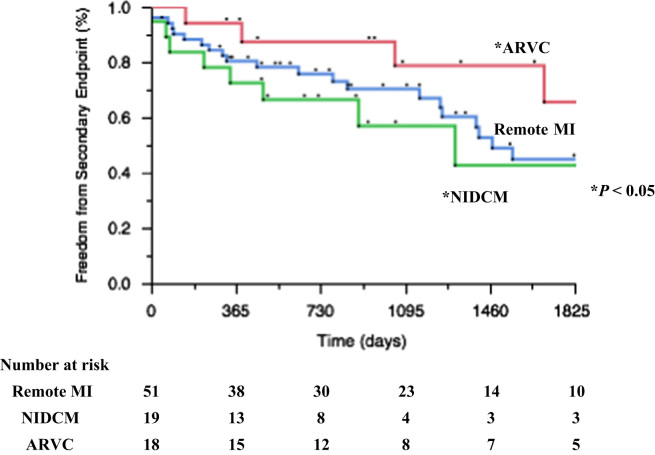
Fig. 5.
Kaplan–Meier curves showing the secondary endpoints (composite of death or any VT/VF recurrence).
4. Discussion
4.1. Main findings
Catheter ablation of VT in ischemic and non-ischemic structural heart disease is feasible. The long-term outcomes differ according to the underlying heart disease. ARVC-VT ablation shows a better long-term prognosis than NIDCM-VT ablation. Remote MI-VT demonstrates a midrange outcome.
4.2. VT mapping and ablation
Slow conduction areas are part of the substrate of the re-entrant circuits in most sustained monomorphic VTs occurring in patients with structural heart disease. These areas are identified by recording using presystolic electrograms during tachycardia. These slow-conduction areas are located in narrow bundles of viable tissue that function as conducting channels bounded by scar tissue [21–23]. However, patients with VT often have multiple reentry circuits with multiple different inducible VTs. In addition, the induced VTs are often hemodynamically unstable, preventing any conventional mapping to localize all the VT circuits.
The electromagnetic anatomical mapping system provides a tool for creating a 3D bipolar electrogram voltage map. Marchlinski and colleagues characterized the electroanatomic substrate of VT [4]. Moreover, they demonstrated that an extensive set of ablation lesions in or around areas of scar could ablate multiple and unstable VTs.
There are two mapping methods: the so-called VT mapping and substrate mapping. In this study, there was no statistically significant difference in the prevalence of substrate mapping between diseases, whereas there were statistically significant differences in the prevalence of VT mapping (p<0.05). This might be attributable to the VT cycle length. The cycle length of NIDCM-VT was significantly longer. This suggests that NIDCM-VT was more hemodyanically stable during mapping.
4.3. Remote myocardial infarction
VT occurs in 1-2% of patients late after an MI, often after an interval of several years. Several papers on the usefulness of catheter ablation for remote MI-VT have been published, but they are mainly from single centers with various degrees of disease severity, stability of the VT for mapping, methods for mapping and ablation, and ablation endpoints.
There have been 3 relevant multicenter studies. In a multicenter trial of 146 patients (82% with coronary artery disease) with a mappable VT who underwent ablation with an internally irrigated catheter, 54% were free of VT during a mean follow-up period of 8 months. Of those with a follow-up period of at least 2 months, 81% experienced a 75% reduction in VT episodes [24].
The Multicenter Thermocool Ventricular Tachycardia Ablation Trial [25] enrolled 231 patients with recurrent VT for ablation with open irrigated RF ablation guided by a 3D mapping system using substrate and/or entrainment mapping approaches. The median LVEF and age was 0.25 and 68 years, respectively, and 94% had an ICD. Ablation eliminated at least 1 VT in 81% of patients and all VTs in 49% of patients. During the following 6 months, VT recurred in 51% of patients. Although recurrences were common, the frequency of VT was markedly reduced in a substantial number of patients. The 1-year mortality was 15% with 38% of deaths due to ventricular arrhythmias and 35% due to heart failure.
In the Euro-VT-Study [26], catheter ablation using external irrigation and an electroanatomical mapping system was performed in 63 patients at 8 centers. The population had a median of 17 VT episodes in the 6 months prior to ablation, age 63 years, and LVEF 0.28; 67% had an ICD. At least 1 VT was ablated in 81% of patients; all inducible VTs were eliminated in 50% of patients. During a follow-up period of 6 months, VT did not recur in 51% of patients. After a mean follow-up period of 12 months, the mortality rate was 8%.
In these 3 multicenter studies, the recurrence rate was 49–54% by 6–8 months of follow-up, which a higher than that during the follow-up period in our study due to the following reasons: (1) difference in the frequency of VTs prior to ablation [25,26]; (2) difference in the LVEF; (3) mapping and ablation performed without a 3D mapping system [24]; and (4) difference in the mapping and ablation strategies. In our patient group, the mean LVEF was 33.2±9.5%, and 15 patients (29%) died during a mean follow-up period of 41.3±28.5 months. It is notable that this value is similar to the SCD-HeFT result (35–43% for 5 years) [2].
4.4. Arrhythmogenic right ventricular cardiomyopathy
Scar-related reentry is the most common cause of ARVC-VT. Although recent technological advances with electroanatomic and voltage mapping systems have significantly improved outcomes [27–28], catheter ablation can reduce the frequency of VT episodes, but long-term follow-up studies have demonstrated a risk of recurrence. However, only an endocardial approach was used in most of the cases previously reported [29–31]. Recently Bai et al. [32] and Berruezo et al. [33] reported the endocardial and epicardial substrate-based mapping and ablation of ARVC-VT. Bai et al. [32] reported absence of VT/VF or ICD therapy in 84.6% in patients who underwent mapping and ablation using endocardial and epicardial approaches during a follow-up period of at least 3 years. Bai et al. [32] and Philip et al. [34] reported the importance of ventricular premature complexes in patients with ARVC-VT. In the present study, the long-term outcomes after ablation were consistent with those in Bai׳s study. An epicardial approach was performed in only 2 patients, and was unsuccessful in 4 of 18 patients. Interestingly, VT recurred in only 1 of those 4 patients, and recent initiation of amiodarone treatment might have contributed to this favorable result.
4.5. Non-ischemic dilated cardiomyopathy
Catheter ablation outcomes in this population are not well defined. In a series of 19 patients [35], endocardial ablation eliminated all inducible VTs in 14 patients. After a follow-up period of 22±12 months, 5 patients were alive without any VT recurrence. In a series of 22 patients [36], epicardial mapping and ablation were performed if endocardial ablation failed. Scar-related reentry circuits were identified in the endocardium in 12 patients and in the epicardium in 7. At least 1 VT was eliminated in 16 of 22 patients and all VTs were eliminated in 12. During a mean follow-up period of 334 days, VT recurred in 46% of patients.
Compared with patients with remote MI-VT, the areas occupied by scars were smaller, transmural scars were fewer, and intramural scars were common in the NIDCM patients. Magnetic resonance imaging with delayed gadolinium-enhancement and voltage mapping demonstrates that scars are often located adjacent to a valve annulus [36,37]. These features likely account for the general perception that patients present with multiple morphologies of VT, and ablation is more difficult than that in the remote MI-VT population.
In our study, an epicardial approach was mostly required in the remote MI-VT patient group. This matches the pathological characteristics of DCM and is consistent with the previous report [36]. Of note, acute success was achieved in all patients, but the recurrence rate was high in this group. There are 3 possible reasons for this unexpected outcome. The first reason is the difficulty of clinical VT induction in this patient group. When clinical VT was non-inducible, entrainment mapping could not be performed and the RF energy was delivered with the guidance of pace mapping and abnormal potentials. The disappearance of the target potential and completeness of the designed lines was defined as acute success but may not have been adequate for long-term success. The second reason is the existence of multiple scars as mentioned previously. Multiple scars are a potential cause of multiple VTs. In this study, there were no statistically significant differences in the number of induced VTs among the 3 groups. According to our protocol, we performed up to 2 extrastimuli for programmed ventricular stimulation. Ventricular stimulation up to 3 extrastimuli might have provided a different result. The third reason is the progression of the underlying disease.
4.6. Long-term outcomes depending on the type of heart disease
Kaplan–Meier curves of the primary endpoint according to the type of heart disease are shown in Fig. 4. Patients with ARVC had significantly better outcomes than those with NIDCM (p<0.05). Moreover the Kaplan–Meier curve for the secondary endpoint is shown in Fig. 5. It shows that patients with ARVC had significantly better outcomes, and that patients with NIDCM had the worst outcomes.
Two papers on the long-term follow-up outcomes after VT ablation in patients with non-ischemic cardiomyopathy have been published. Sacher et al. [38] reported that ischemic cardiomyopathy had a 2-fold increased risk of mortality compared with non-ischemic heart disease, despite a lower VT recurrence. This result is different from ours. In their report, the non-IHD group included not only idiopathic DCM (54%), but also valvular heart disease (19%), congenital heart disease (7%), ARVC (15%), and sarcoidosis (4%) patients. This difference in the underlying diseases might lead to different ablation outcomes. Tokuda et al. [39] recently reported the long-term follow-up outcomes of VT ablation in patients with non-IHD. In that study, the ARVC-VT ablation outcomes were significantly better than the NIDCM-VT ablation outcomes, which is consistent with our study results. Catheter ablation is effective in patients with ischemic and non-ischemic heart disease. Our study suggests that ARVC-VT patients have better outcomes, remote MI-VT patients have midrange outcomes, and NIDCM-VT patients have the worst long-term outcomes. However, the efficacy of VT ablation according to the type of underlying disease is uncertain. Further studies with a larger sample size and longer follow-up periods are required to clarify this issue.
4.7. Study limitations
This study has several limitations. (1) This study was a single center retrospective observational study. There might have been a selection bias. Our institute is a referral center for VT ablation, and therefore our population selection may have been skewed. (2) The sample sizes in the subgroups were small, precluding statistical comparisons. (3) The ablation and mapping technology used in the individual patients was influenced by availability of the equipment. (4) The ablation and mapping strategy varied during the study period.
5. Conclusion
Our study demonstrates that in patients with spontaneous sustained VT due to ischemic and non-ischemic heart disease, catheter ablation is useful, and that the long-term outcomes vary according to the underlying heart disease.
Conflict of interest
None.
References
- 1.Moss A.J., Zareba W., Hall W.J. For the multicenter automatic defibrillator implantation trail II investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-3. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Poole J.E., Johnson G.W., Hellkamp A.S. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchlinski F.E., Callans D.J., Gottlieb C.D., Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 5.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. proposed modification of the Task Force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkmer M., Ouyang F., Deger F. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation. Europace. 2006;8:968–976. doi: 10.1093/europace/eul109. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson W.G., Khan H., Sager P. Identification of reentry circuit during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–1670. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 8.De Chillou C., Lacroix D., Klug D. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105:726–731. doi: 10.1161/hc0602.103675. [DOI] [PubMed] [Google Scholar]
- 9.Bogun F., Bahu M., Knight B.P. Comparison of effective and ineffective target sites that demonstrate concealed entrainment in patients with coronary artery disease undergoing radiofrequency ablation of ventricular tachycardia. Circulation. 1997;95:183–190. doi: 10.1161/01.cir.95.1.183. [DOI] [PubMed] [Google Scholar]
- 10.Kautzner J., Cihak R., Peichl P. Catheter ablation of ventricular tachycardia following myocardial infarction using three-dimensional electroanatomical mapping. Pacing Clin Electrophysiol. 2003;26(Pt. II):342–347. doi: 10.1046/j.1460-9592.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 11.Arenal A., Glez-Torrecilla E., Ortiz M. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 12.Brunckhorst C.B., Delacretaz E.D., Soejima K. Identification of the ventricular tachycardia isthmus after infarction by pace mapping. Circulation. 2004;110:652–659. doi: 10.1161/01.CIR.0000138107.11518.AF. [DOI] [PubMed] [Google Scholar]
- 13.Bogun F., Good F., Reich S. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–2019. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K., Sekiguchi Y., Tanoue K. Feasibility of targeting catheter ablation to the markedly low-voltage area surrounding infarct scars in patients with post-infarction ventricular tachycardia. Circ J. 2008;72:1112–1119. doi: 10.1253/circj.72.1112. [DOI] [PubMed] [Google Scholar]
- 15.Mountantonakis S.E., Park R.E., Frankel D.S. Relationship between voltage map “channels” and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2013;61:2088–2095. doi: 10.1016/j.jacc.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Tung R., Mathuria N.S., Nagel R. Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol. 2013;6:1131–1138. doi: 10.1161/CIRCEP.113.000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergara P., Trevisi N., Ricco A. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23:621–627. doi: 10.1111/j.1540-8167.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 18.Soejima K., Suzuki M., Maisel W.H. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction. Short ablation lined guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104:664–669. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 19.Soejima K., Stevenson W.G., Maisel W.H. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus. Feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106:1678–1683. doi: 10.1161/01.cir.0000030187.39852.a7. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt B., Chun K.R., Baensch D. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Heart Rhythm. 2010;7:1746–1752. doi: 10.1016/j.hrthm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 21.de Bakker J.M., van Capelle F.J., Janse M.J. Reentry as a cause of VT in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 22.de Bakker J.M., Coronel R., Tasseron S. Ventricular tachycardia in the infracted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15:1594–1607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]
- 23.de Bakker J.M., van Capelle F.J., Janse M.J. Slow conduction in the infarcted human heart: zigzag course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 24.Calkins H., Epstein A., Packer D. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi-Center Investigators Group. J Am Coll Cardiol. 2000;35:1905–1914. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson W.G., Wilber D.J., Natale A. Irrigated radiofrequency Catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the Multicenter Thermocool Ventricular Tachycardia Ablation Trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 26.Tanner H., Hindricks G., Volkmer M. Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: results of the prospective multicenter Euro-VT-study. J Cardiovasc Electrophysiol. 2010;21:47–53. doi: 10.1111/j.1540-8167.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 27.Philips B., Madhavan S., James C. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505. doi: 10.1161/CIRCEP.111.968677. [DOI] [PubMed] [Google Scholar]
- 28.Dalal D., Jain R., Tandri H. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2007;50:432–440. doi: 10.1016/j.jacc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Verma A., Kilicaslan F., Schweikert R.A. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111:3209–3216. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 30.Satomi K., Kurita T., Suyama K. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2006;17:469–476. doi: 10.1111/j.1540-8167.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Nogami A., Sugiyasu A., Tada H. Changes in the isolated delayed component as an endpoint of catheter ablation in arrhythmogenic right ventricular cardiomyopathy: predictor for long-term success. J Cardiovasc Electrophysiol. 2008;19:681–688. doi: 10.1111/j.1540-8167.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 32.Bai R., Di Biase L., Shivkumar K. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4:478–485. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 33.Berruezo A., Fernandez-Armenta J., Mont L. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–121. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 34.Philips B., Madhavan S., James C. High prevalence of catecholamine-facilitated focal ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2013;6:160–166. doi: 10.1161/CIRCEP.112.975441. [DOI] [PubMed] [Google Scholar]
- 35.Hsia H.H., Callans D.J., Marchlinski F.E. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 36.Soejima K., Stevenson W.G., Sapp J.L. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Nazarian S., Bluemke D.A., Lardo A.C. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacher F., Tedrow U.B., Field M.E. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008;1:153–161. doi: 10.1161/CIRCEP.108.769471. [DOI] [PubMed] [Google Scholar]
- 39.Tokuda M., Tedrow U.B., Kojodjojo P. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol. 2012;5:992–1000. doi: 10.1161/CIRCEP.112.971341. [DOI] [PubMed] [Google Scholar]