Abstract
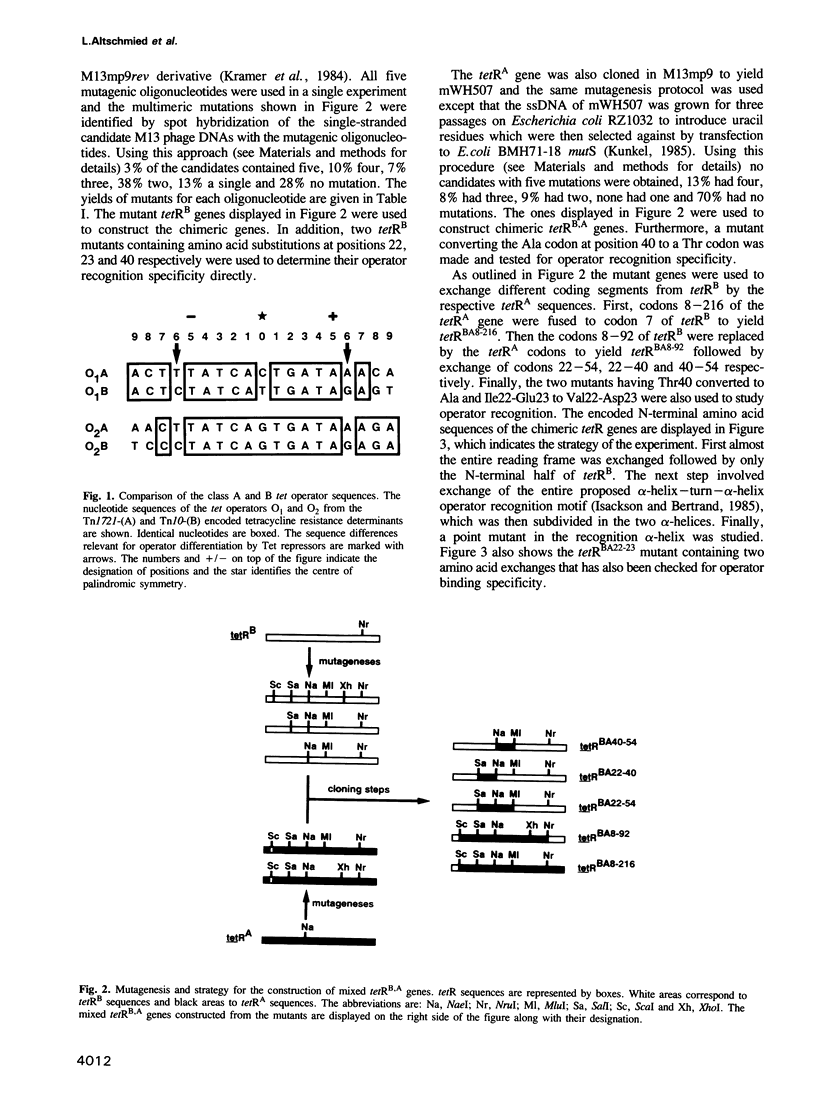
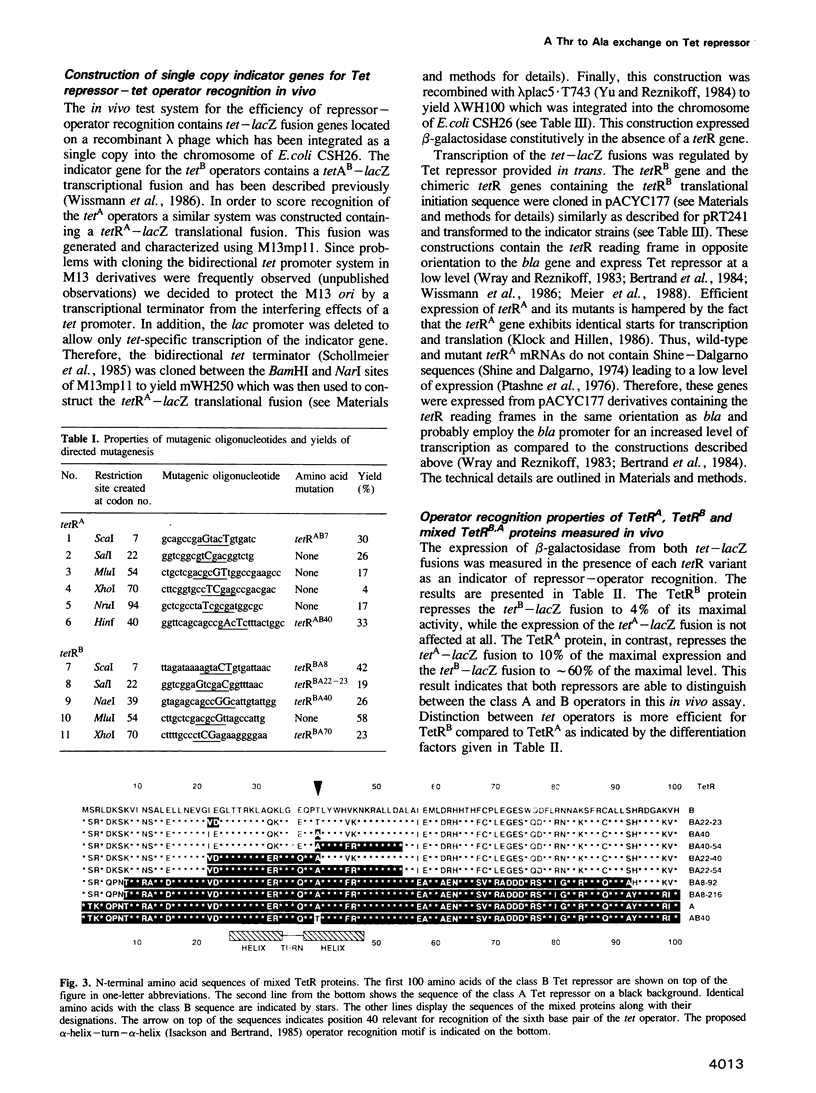
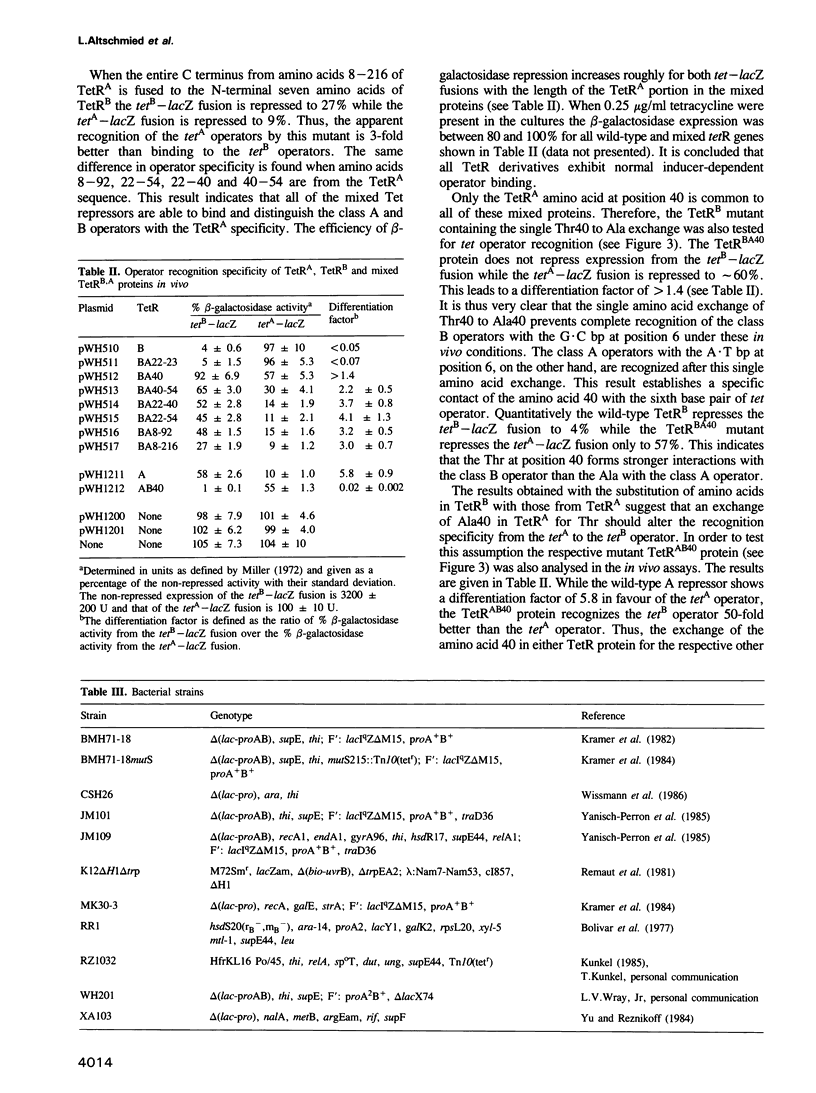
The tet operators of two naturally evolved tetracycline resistance determinants differ by a G.C to A.T transition at the sixth base pair. This mutation prevents heterologous recognition of these tet operators by their respective two Tet repressor proteins. The amino acid side chains responsible for this sequence-specific distinction of operators were determined. For this purpose in vitro recombinants of the two tetR genes were constructed. Restriction sites were introduced by oligonucleotide-directed mutagenesis in both genes followed by the exchange of different coding segments between them. The encoded chimeric Tet repressor proteins were expressed and their operator recognition specificity was scored in vivo. Exchanging gradually smaller coding segments led finally to a single amino acid exchange in both genes at position 40 of the primary structures. Each Tet repressor containing Thr at this position recognizes the G.C operator while those with Ala recognize the A.T operator regardless of the rest of the sequences. This result demonstrates clearly that the amino acid 40 of Tet repressor contacts and recognizes base pair 6 of tet operator. Sterical interference of the large Thr side chain with the methyl group of A.T and a possible involvement of the hydroxyl in hydrogen bonding to the operator are discussed as the molecular basis of this differentiation between A.T and G.C base pairs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984 Jun;158(3):910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Altschmied J., Hillen W. Cloning and expression of the Acinetobacter calcoaceticus mutarotase gene in Escherichia coli. J Bacteriol. 1986 Oct;168(1):31–39. doi: 10.1128/jb.168.1.31-39.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer C., Hickman R. K., Curiale M. S., Hillen W., Levy S. B. Constitutive expression of tetracycline resistance mediated by a Tn10-like element in Haemophilus parainfluenzae results from a mutation in the repressor gene. J Bacteriol. 1987 Mar;169(3):990–994. doi: 10.1128/jb.169.3.990-994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson P. J., Bertrand K. P. Dominant negative mutations in the Tn10 tet repressor: evidence for use of the conserved helix-turn-helix motif in DNA binding. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6226–6230. doi: 10.1073/pnas.82.18.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt C., Tovar K., Hillen W., Porschke D. Dynamics of repressor-operator recognition: the Tn10-encoded tetracycline resistance control. Biochemistry. 1988 Feb 23;27(4):1094–1104. doi: 10.1021/bi00404a003. [DOI] [PubMed] [Google Scholar]
- Klock G., Hillen W. Expression, purification and operator binding of the transposon Tn1721-encoded Tet repressor. J Mol Biol. 1986 Jun 20;189(4):633–641. doi: 10.1016/0022-2836(86)90493-6. [DOI] [PubMed] [Google Scholar]
- Klock G., Unger B., Gatz C., Hillen W., Altenbuchner J., Schmid K., Schmitt R. Heterologous repressor-operator recognition among four classes of tetracycline resistance determinants. J Bacteriol. 1985 Jan;161(1):326–332. doi: 10.1128/jb.161.1.326-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Schughart K., Fritz H. J. Directed mutagenesis of DNA cloned in filamentous phage: influence of hemimethylated GATC sites on marker recovery from restriction fragments. Nucleic Acids Res. 1982 Oct 25;10(20):6475–6485. doi: 10.1093/nar/10.20.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Meier I., Wray L. V., Hillen W. Differential regulation of the Tn10-encoded tetracycline resistance genes tetA and tetR by the tandem tet operators O1 and O2. EMBO J. 1988 Feb;7(2):567–572. doi: 10.1002/j.1460-2075.1988.tb02846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Oehmichen R., Klock G., Altschmied L., Hillen W. Construction of an E. coli strain overproducing the Tn10-encoded TET repressor and its use for large scale purification. EMBO J. 1984 Mar;3(3):539–543. doi: 10.1002/j.1460-2075.1984.tb01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Nguyen T. T., Bertrand K. P. Nucleotide sequence of the repressor gene of the TN10 tetracycline resistance determinant. Nucleic Acids Res. 1984 Jun 25;12(12):4849–4863. doi: 10.1093/nar/12.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Schollmeier K., Gärtner D., Hillen W. A bidirectionally active signal for termination of transcription is located between tetA and orfL on transposon Tn10. Nucleic Acids Res. 1985 Jun 25;13(12):4227–4237. doi: 10.1093/nar/13.12.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger B., Becker J., Hillen W. Nucleotide sequence of the gene, protein purification and characterization of the pSC101-encoded tetracycline resistance-gene-repressor. Gene. 1984 Nov;31(1-3):103–108. doi: 10.1016/0378-1119(84)90199-9. [DOI] [PubMed] [Google Scholar]
- Unger B., Klock G., Hillen W. Nucleotide sequence of the repressor gene of the RA1 tetracycline resistance determinant: structural and functional comparison with three related Tet repressor genes. Nucleic Acids Res. 1984 Oct 25;12(20):7693–7703. doi: 10.1093/nar/12.20.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann A., Meier I., Hillen W. Saturation mutagenesis of the Tn10-encoded tet operator O1. Identification of base-pairs involved in Tet repressor recognition. J Mol Biol. 1988 Aug 5;202(3):397–406. doi: 10.1016/0022-2836(88)90273-2. [DOI] [PubMed] [Google Scholar]
- Wissmann A., Meier I., Wray L. V., Jr, Geissendörfer M., Hillen W. Tn10 tet operator mutations affecting Tet repressor recognition. Nucleic Acids Res. 1986 May 27;14(10):4253–4266. doi: 10.1093/nar/14.10.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray L. V., Jr, Reznikoff W. S. Identification of repressor binding sites controlling expression of tetracycline resistance encoded by Tn10. J Bacteriol. 1983 Dec;156(3):1188–1191. doi: 10.1128/jb.156.3.1188-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]