Abstract
Background
Outcomes related to prophylactic catheter ablation (PCA) for ventricular tachycardia (VT) before implantable cardioverter-defibrillator (ICD) implantation in non-ischemic cardiomyopathy (NICM) are not well characterized. We assessed the efficacy of single endocardial PCA in NICM patients.
Methods
We retrospectively analyzed 101 consecutive NICM patients with sustained VT. We compared clinical outcomes of patients who underwent PCA (ABL group) with those who did not (No ABL group). Successful PCA was defined as no inducible clinical VT. We also compared the clinical outcomes of patients with successful PCA (PCA success group) with those of the No ABL group. Endpoints were appropriate ICD therapy (shock and anti-tachycardia pacing) and the occurrence of electrical storm (ES).
Results
PCA was performed in 42 patients, and it succeeded in 20. The time to ES occurrence was significantly longer in the ABL group than in the No ABL group (p=0.04). The time to first appropriate ICD therapy and ES occurrence were significantly longer in the PCA success group than in the No ABL group (p=0.02 and p<0.01, respectively).
Conclusion
Single endocardial PCA can decrease ES occurrence in NICM patients. However, high rates of VT recurrence and low success rates are issues to be resolved; therefore, the efficacy of single endocardial PCA is currently limited.
Abbreviations: VT, ventricular tachycardia; ICM, ischemic cardiomyopathy; NICM, non-ischemic cardiomyopathy; ICD, implantable cardioverter-defibrillator; ES, electrical storm; PCA, prophylactic catheter ablation
Keywords: Catheter ablation, Ventricular tachycardia, Implantable cardioverter-defibrillator, Non-ischemic cardiomyopathy, Electrical storm
1. Introduction
The use of implantable cardioverter-defibrillators (ICDs) is the gold standard for primary and secondary prevention of sudden cardiac death [1,2]. However, shocks increase the risk of all-cause mortality, even if the shocks are inappropriate [3]. Recent studies have reported the efficacy of prophylactic catheter ablation (PCA) before ICD implantation in patients with ventricular tachycardia (VT) and ischemic cardiomyopathy [4–6]. Some studies report the efficacy of catheter ablation with a combination endocardial and epicardial approach in patients with non-ischemic cardiomyopathy (NICM) [7,8]. However, the role of PCA before ICD implantation in NICM patients has not been well described. Results from the few published studies on the subject vary, but no prospective studies have compared clinical outcomes of patients with or without prophylactic endocardial catheter ablation before cardioverter-defibrillator implantation.
Therefore, this study aimed to compare clinical outcomes of NICM patients with or without prophylactic endocardial catheter ablation. Specifically, we investigated the effect of single endocardial PCA on the reduction of appropriate ICD therapy and electrical storm (ES).
2. Material and methods
2.1. Study population
This retrospective analysis included 101 consecutive patients with sustained VT and NICM who received cardioverter-defibrillator implantation at either the Hospital of Kobe University or the Hyogo Brain and Heart Center between January 2005 and May 2012. NICM included such non-ischemic diseases as idiopathic dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC), cardiac sarcoidosis, valvular heart disease, congenital heart disease, and others. Patients with polymorphic VT or ventricular fibrillation (VF) were excluded. Patient selection for PCA was based on the physician׳s discretion, but was primarily chosen for patients with several sustained episodes of VT admitted in the later years of the study, when it became a widely accepted therapy. Oral beta-blockers and/or amiodarone or sotalol were the primary drugs for chronic management after cardioverter-defibrillator implantation.
2.2. Electrophysiological study and VT ablation
All patients who underwent catheter ablation provided written informed consent for electrophysiological study and catheter ablation. Methods for mapping and ablation were identical to those reported previously [9–11]. Standard multielectrode catheters were placed in the high right atrium, His-bundle region, coronary sinus, and right ventricular apex.
Left and/or right ventricular mapping was performed using a 4-mm and/or 8-mm non-irrigated tip or a 3.5-mm irrigated tip ablation catheter. Initial mapping was performed using the CARTO system (Biosense Webster, Diamond Bar, CA, USA) or the EnSite system (EnSite 3000 with Precision Software, Endocardial Solutions, Inc., St. Paul, MN, USA) during sinus rhythm or right ventricular pacing. A 3-dimensional voltage map of the left ventricle (LV) and/or right ventricle (RV) was obtained. An area with low amplitude (<1.5 mV) and fractionated or late potential was considered an abnormal substrate. Dense scar was identified when no local capture on bipolar pacing was observed at 9.9 V and 2-ms pulse width. Pace mapping was used to identify the critical components of the VT circuit [11].
A programmed stimulation protocol (S1: 600 and 400 ms, with up to 2 extra stimuli with a minimum 200-ms interval) from the 2 sites (i.e., right/left ventricular apex and right ventricular outflow tract) was performed to induce clinical VT.
Simultaneous recordings of ventricular electrograms (bandpass filtered 30–500 Hz) and 12-lead surface electrocardiograms (ECGs) were stored digitally (Prucka Cardiolab, GE Medical Systems, Milwaukee, WI, USA; Bard LabSystem PRO EP Recording System, C. R. Bard, Inc. Lowell, MA, USA).
When hemodynamically tolerated VT was induced, VT activation mapping was performed in the CARTO or EnSite system, and radiofrequency (RF) current was applied at the central to exit zones of the circuit as identified using entrainment pacing during VT [12,13]. If hemodynamically unstable VT was induced, substrate-based ablation was performed. RF ablation lesions were created within the region identified to be critical for sustaining clinical VT based on substrate mapping [11]. In cases in which we could not detect an abnormal substrate, RF current was applied at the earliest activation site of induced VT or at the exit zone detected on pace mapping.
The standard ablation setting of a non-irrigation catheter consisted of a tip temperature of 60 °C and 30–40 W power for 4-mm tips or 40–50 W for 8-mm tips. When an irrigation catheter was employed, a catheter tip temperature of 43 °C, a 35 W power, and a 30 mL/min flow rate were used.
At the end of the ablation procedure, VT induction was performed with up to double extra stimuli and rapid pacing (minimum pacing cycle at 240 ms, 15 beats) from the 2 sites. Patients without induced VT were classified to the “No VT” group and patients without induced clinical VT (documented on a 12-lead ECG before ablation) were classified to the “No clinical VT” group. In this study, the successful ablation (PCA success) group was composed of the “No VT” and the “No clinical VT” groups. Patients in whom clinical VT was induced at the end of the procedure were classified to the “PCA failure” group.
2.3. Implantation of the cardioverter-defibrillator
Implantation of the cardioverter-defibrillator was performed for all 101 patients. Patients in the ABL group received cardioverter-defibrillator implantation after catheter ablation. The device programming consisted of a VF zone with a cut-off rate of 200–220 beats per minute and a VT zone with a cut-off cycle length of at least 40 ms above the cycle length of documented VT and anti-tachycardia pacing (ATP) followed by shock therapy.
2.4. Follow-up
Patients were evaluated in outpatient clinics at 3-month intervals. Analysis of VT recurrence depended on clearly differentiating VT/VF events from supraventricular tachycardia events. For reliably identifying VT, ICD diagnosis was confirmed by two or more physicians examining stored electrograms. VT recurrence was defined as appropriate ICD therapy, including shock and ATP. ES was defined as ≥3 separate episodes of ventricular arrhythmia during a 24-h period. Drug management during follow-up was used at each physician׳s discretion.
2.5. Statistical analysis
Continuous variables are expressed as mean±standard deviation; they were compared using two-sample t-tests. Categorical variables were compared using the chi-squared test. Time-to-event curves describing event-free survival of patients during follow-up were calculated using the Kaplan–Meier method and compared using the log-rank test. Statistical analyses were performed using the SPSS statistical software program (version 20.0, SPSS, Inc., Chicago, IL, USA). A two-sided p<0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
A total of 101 consecutive NICM patients with sustained VT were retrospectively analyzed (66 men; mean age, 63.0±12.2 years; mean left ventricular ejection fraction, 45.2±14.2%; mean left ventricular end-diastolic diameter [LVDd], 54.6±9.1 mm). PCA was performed in 42 (41.6%) patients (ABL group). The remaining 59 (58.4%) patients were classified as the No ABL group (Fig. 1).
Fig. 1.
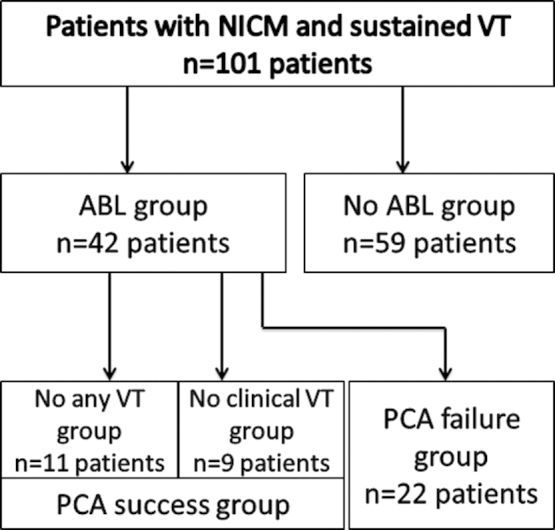
Patient classification. Prophylactic catheter ablation (PCA) was performed in 42 of 101 patients, classified as the ABL group. The remaining 59 patients were classified as the No ABL group. Patients with no induced VT were classified as the “No VT” group and patients with no induced clinical VT were classified as the “No clinical VT” group. These two groups comprised the PCA success group. Patients in which clinical VT was induced at the end of procedure were classified to the “PCA failure” group.
There were no significant group differences in demographic or clinical characteristics, except for LVDd, number of documented VT episodes before cardioverter-defibrillator implantation, prevalence of dyslipidemia and ARVC (ABL group vs. No ABL group: 51.7±8.2 mm vs. 56.6±9.8 mm, p=0.010; 2.26±1.25 vs. 1.49±0.84, p<0.001; 5 of 42 [11.9%]) vs. 17 of 59 [40.5%], p=0.048; and 13 of 42 [31.0%] vs. 1 of 59 [1.7%], p<0.001, respectively) (Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| ABL group | No ABL group | pValue | |
|---|---|---|---|
| (n=42) | (n=59) | ||
| Mean follow-up duration (months) | 41.5±24.2 | 38.7±23.6 | 0.567 |
| Age (years) | 60.4±14.4 | 64.7±10.7 | 0.083 |
| Sex (male) | 32 (76.2%) | 34 (57.6%) | 0.053 |
| NYHA class I | 9 (21.4%) | 9 (15.3%) | 0.424 |
| NYHA class II | 26 (61.9%) | 34 (57.6%) | 0.666 |
| NYHA class III | 7 (16.7%) | 16 (27.1%) | 0.217 |
| NYHA class IV | 0 | 0 | NA |
| HTN | 9 (21.4%) | 19 (45.2%) | 0.233 |
| DM | 5 (11.9%) | 13 (31.0%) | 0.19 |
| DL | 5 (11.9%) | 17 (40.5%) | 0.048 |
| AF/AT | 11 (26.2%) | 24 (57.1%) | 0.132 |
| LVEF (%) | 46.1±12.7 | 44.5±15.2 | 0.576 |
| LVDd (mm) | 51.7±8.2 | 56.6±9.8 | 0.01 |
| Serum creatinine (mg/dl) | 1.06±0.95 | 1.25±1.22 | 0.392 |
| Amiodarone | 7 (16.7%) | 20 (47.6%) | 0.054 |
| Sotalol | 13 (31.0%) | 10 (16.9%) | 0.098 |
| β blocker | 23 (54.8%) | 40 (67.8%) | 0.183 |
| Number of VT episodes | 2.26±1.25 | 1.49±0.84 | <0.001 |
| Cycle length of the clinical VT (ms) | 326.4±81.5 | 344.6±74.7 | 0.248 |
| Structural heart diseases | |||
| DCM | 10 (23.8%) | 22 (37.3%) | 0.151 |
| HCM | 5 (11.9%) | 13 (22.0%) | 0.19 |
| ARVC | 13 (31.0%) | 1 (1.7%) | <0.001 |
| Cardiac sarcoidosis | 8 (19.0%) | 10 (16.9%) | 0.786 |
| VHD | 3 (7.1%) | 7 (11.9%) | 0.434 |
| CHD | 0 (0%) | 3 (5.1%) | 0.138 |
| Others | 3 (7.1%) | 3 (5.1%) | 0.666 |
AF/AT=atrial fibrillation/atrial tachycardia, ARVC=arrhythmogenic right ventricular cardiomyopathy, CHD=congenital heart disease, DCM=dilated cardiomyopathy, DL=dyslipidemia, DM=diabetes mellitus, HCM=hypertrophic cardiomyopathy, HTN=hypertension, LVEF=left ventricular ejection fraction, LVDd=left ventricular end-diastolic diameter, Number of VT episodes=Number of VT episodes before cardioverter-defibrillator implantation, NYHA class=New York Heart Association classification, VHD=valvular heart disease, VT=ventricular tachycardia.
3.2. Electrophysiological findings and catheter ablation data
The procedure was performed predominantly under local anesthesia. A 4-mm tip non-irrigated ablation catheter was used in 31 (81.0%) patients (Navistar; Biosense Webster in 29 patients; Ablaze Fantasista 4-mm distal tip; Japan Lifeline Co., Ltd., Tokyo, Japan in 5 patients); a 4-mm and an 8-mm tip non-irrigated ablation catheter (Ablaze Fantasista 8-mm distal tip; Japan Lifeline), in 3 patients; and a 3.5-mm tip open-irrigated ablation catheter, in 8 (19.0%) patients (Navistar Thermocool; Biosense Webster, in 5 patients; Coolpath Duo; St. Jude Medical, St. Paul, MN, USA in 3 patients).
Electroanatomical mapping was performed using the CARTO system in 37 (88.1%) patients and EnSite system was in 5 (11.9%). An analysis of electroanatomical mapping in the endocardium revealed the prevalence of a low voltage area or fractionated or late potential as an arrhythmogenic substrate in 32 (76.2%) of 42 patients in the ABL group.
The mean number of induced VT morphology was 1.7 per patient (range 0–5; 72 VTs). In 2 patients, a programmed stimulation failed to induce VT. Clinically documented VT was induced in 39 patients. The underlying arrhythmia mechanism of 51 VTs was presumed to be myocardial reentry on entrainment pacing and/or activation mapping, recording mid-diastolic potential. The activation mapping of 11 VTs demonstrated a focal pattern. The mechanism of the remaining 10 VTs was undetermined because of hemodynamic instability, poor re-inducibility, or spontaneous changes from one to another.
RF current was applied in 14 patients during 18 hemodynamically tolerated VTs, which resulted in VT termination in 14 cases. In 22 patients, RF current was applied for substrate modification during sinus rhythm. In 10 patients without low voltage areas or fractionated or late potential as an arrhythmogenic substrate, RF current was applied at the earliest activation site of induced VT or at the exit zone detected on pace mapping.
After ablation, VT failed to be induced in 11 (26.2%) patients (the “No VT” group) and clinical VT was not induced in 9 (21.4%) patients (the “No clinical VT” group). RF ablation lesions were created within the region identified to be abnormal by using substrates and at the exit zone as detected on pace mapping in 2 patients in whom programmed stimulation failed to induce VT. After substrate-based ablation eliminated abnormal potentials, VT was not induced, and the patients were included in the No VT group. These two groups were classified to the PCA success group. In the remaining 22 patients (52.4%), endocardial catheter ablation failed (the “PCA failure” group) (Fig. 1).
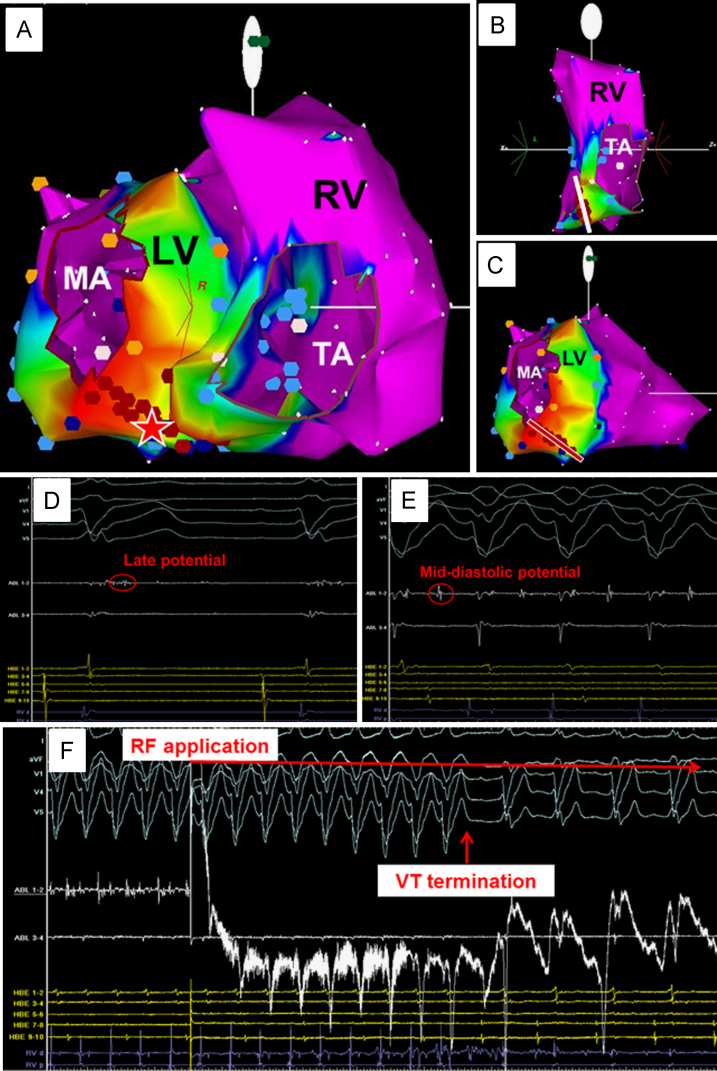
Epicardial mapping and ablation were not performed in any patients at the index procedure. However, the arrhythmogenic substrate of clinical VT was presumed to involve the epicardium in 20 patients, because the endocardial activation sequence did not fulfill the cycle length of clinical VT or clinical VT was re-induced after elimination of the endocardial arrhythmogenic substrate (2 patients in the “No VT” group, 4 in the “No clinical VT” group, and 14 in the “PCA failure” group). Fig. 2 shows a representative case of successful endocardial PCA. This patient had cardiac sarcoidosis and clinical hemodynamically unstable VT represented in the left bundle branch block morphology with a cycle length of 320 ms. The procedure was performed with the CARTO system navigation. The substrate map of the RV and LV revealed an abnormal substrate (an area with low amplitude [<1.5 mV]) in the RV and LV basal septa (Fig. 2A–C). RF lesions were created to abolish the abnormal substrate in the RV septum (white line in Fig. 2B). However, clinical VT remained. The late potential during sinus rhythm was recorded in the LV abnormal substrate (red star in Fig. 2A and D), and mid-diastolic potential was recorded at this site during clinical VT (Fig. 2E). RF application at this site terminated the VT (Fig. 2F), and RF lesions were created from the mitral annulus to a normal voltage area (>1.5 mV) including the site (red line in Fig. 2C). No VT was induced at the end of the procedure.
Fig. 2.
A representative case of successful endocardial prophylactic catheter ablation. This patient had cardiac sarcoidosis and clinical hemodynamically unstable ventricular tachycardia (VT). (A) Voltage map of the right ventricle (RV) and left ventricle (LV) in the right anterior oblique (RAO) view. The red star indicates where late potential during sinus rhythm and mid-diastolic potential during clinical VT were recorded. Colors represent bipolar electrogram peak-to-peak amplitude, with purple being normal (>1.5 mV), and blue, green, yellow, and red regions with progressively lower amplitude. MA and TA indicate mitral annulus and tricuspid annulus, respectively. (B) Voltage map of the RV in the left posterior oblique view. Low voltage area as an abnormal substrate existed in the lower basal-septum of RV. The white line indicates radiofrequency (RF) lesions that were created to abolish the RV abnormal substrate. (C) Voltage map of LV in the RAO view. The red line indicates RF lesions that were created from the MA to the normal voltage area, including the site of the red star in panel A. (D) Intra-cardiac electrogram during sinus rhythm at the site of the red star in panel A. The late potential was recorded at this site. ABL indicates ablation catheter electrogram. HBE indicates His-bundle electrogram. RVd and RVp indicate RV distal and RV proximal bipolar electrograms. (E) Intra-cardiac electrogram during clinical VT at the site of the red star in panel A. The mid-diastolic potential was recorded at this site. (F) Clinical VT termination during RF application at the site of the red star in panel A.
To determine in which patients endocardial PCA is most likely to be successful, we analyzed differences in patients׳ baseline or VT characteristics and in procedural details between successful and unsuccessful PCA patients (Table 2). There were no differences in baseline characteristics or underlying structural heart disease. However, the PCA success group had higher rates of endocardial substrate presence, and the PCA failure group had higher rates of the presumption of epicardial substrate involvement and an undetermined VT mechanism.
Table 2.
Comparison of baseline demographic and clinical VT characteristics and procedural details between patients with successful PCA and those with unsuccessful PCA.
| PCA success group | PCA failure group | pValue | |
|---|---|---|---|
| (n=20) | (n=22) | ||
| Baseline characteristics | |||
| Age (years) | 62.3±13.6 | 58.6±15.2 | 0.424 |
| Sex (male [%]) | 15 (75.0%) | 17 (77.3%) | 0.863 |
| NYHA class ≥II (N [%]) | 15 (75.0%) | 18 (81.8%) | 0.591 |
| HTN | 5 (25.0%) | 4 (18.2%) | 0.591 |
| DL | 1 (5.0%) | 4 (18.2%) | 0.188 |
| DM | 1 (5.0%) | 4 (18.2%) | 0.188 |
| AT/AF | 7 (35.0%) | 4 (18.2%) | 0.216 |
| LVEF (%) | 43.6±13.9 | 48.5±11.4 | 0.215 |
| LVDd (mm) | 53.5±7.8 | 50.1±8.5 | 0.186 |
| Serum creatinine (mg/dl) | 1.21±1.34 | 0.93±0.29 | 0.683 |
| Structural heart disease | |||
| DCM | 3 (15.0%) | 7 (31.8%) | 0.201 |
| HCM | 3 (15.0%) | 2 (9.1%) | 0.555 |
| ARVC | 7 (35.0%) | 6 (27.3%) | 0.588 |
| Cardiac sarcoidosis | 3 (15.0%) | 5 (22.7%) | 0.524 |
| VHD | 2 (10.0%) | 1 (4.5%) | 0.493 |
| CHD | 0 (0%) | 0 (0%) | NA |
| Others | 2 (10.0%) | 1 (4.5%) | 0.493 |
| Medication | |||
| Amiodarone | 3 (15.0%) | 4 (18.2%) | 0.782 |
| Sotalol | 4 (20.0%) | 9 (41.0%) | 0.143 |
| β blocker | 13 (65.0%) | 10 (45.5%) | 0.204 |
| Clinical VT characteristics | |||
| CL of the clinical VT | 318.0±58.2 | 334.1±98.9 | 0.990 |
| Number of VT episodes | 2.15±1.18 | 2.36±1.33 | 0.653 |
| RBBB type | 12 (60.0%) | 14 (63.6%) | 0.808 |
| Procedural findings | |||
| 3-D navigation system (CARTO [%]) | 17 (85.0%) | 20 (90.9%) | 0.555 |
| Irrigated-tip ablation catheter usage | 4 (20.0%) | 4 (18.2%) | 0.881 |
| Number of induced VT | 1.60±1.19 (total 32 VTs) | 1.82±1.22 (total 40 VTs) | 0.579 |
| Myocardial re-entry | 27 of 32 VTs | 24 of 40 VTs | 0.599 |
| Focal activation pattern | 5 of 32 VTs | 6 of 40 VTs | 0.869 |
| Undetermined mechanism | 0 of 32 VTs | 10 of 40 VTs | 0.007 |
| Presence of endocardial substrate | 18 (90.0%) | 14 (63.6%) | 0.045 |
| VT termination during RF application | 9 (45.0%) | 5 (22.7%) | 0.126 |
| Presumption of epicardial substrate involvement | 6 (30.0%) | 14 (63.6%) | 0.029 |
AF/AT=atrial fibrillation/atrial tachycardia, ARVC=arrhythmogenic right ventricular cardiomyopathy, CHD=congenital heart disease, CL=cycle length, DCM=dilated cardiomyopathy, DL=dyslipidemia, DM=diabetes mellitus, HCM=hypertrophic cardiomyopathy, HTN=hypertension, LVEF=left ventricular ejection fraction, LVDd=left ventricular end-diastolic diameter, RF=radiofrequency, RBBB=right bundle branch block, PCA=prophylactic catheter ablation, Number of VT episodes=Number of VT episodes before cardioverter-defibrillator implantation, VHD=valvular heart disease, VT=ventricular tachycardia.
3.3. Complications
Periprocedural complications were observed in 2 (4.8%) patients. One had an embolism in the superior mesenteric artery with severe abdominal pain immediately after the procedure. The other had a femoral hematoma at the puncture site, requiring transfusion. Both patients fully recovered. No other major adverse events were observed during the periprocedural period.
3.4. Long-term follow-up
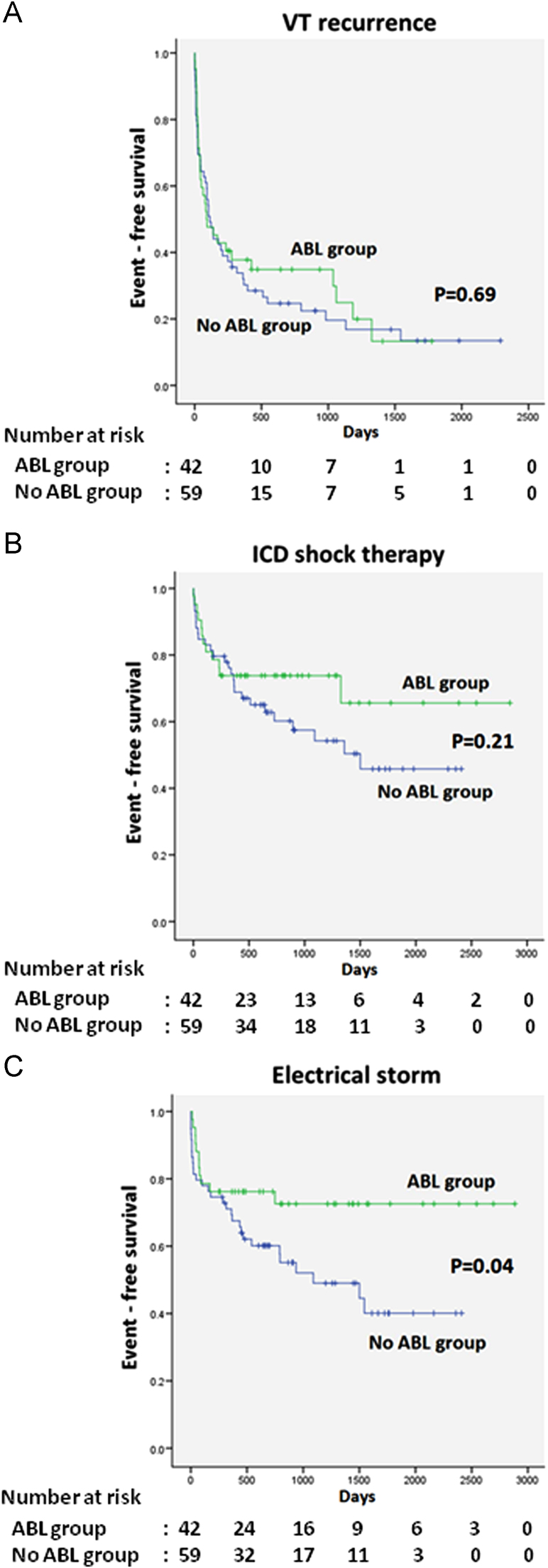
During a follow-up period of 40.1±23.9 months (range, 3–96 months, ABL group vs. No ABL group: 41.5±24.2 vs. 38.7±23.6 months, p=0.567), VT recurrence, defined as appropriate ICD therapy after the index procedure, was found in 31 patients (73.8%) in the ABL group and 48 (81.5%) in the No ABL group. Twelve patients (28.6%) in the ABL group and 26 (44.1%) in the No ABL group received an appropriate shock. In the Kaplan–Meier analysis, there was no significant difference between the ABL and No ABL groups considering time to first VT recurrence or shock therapy during follow-up (p=0.69, p=0.21, respectively). Eleven patients (26.2%) in the ABL group and 29 (49.2%) in the No ABL group had ES. Kaplan–Meier analysis showed a significant difference in time to first ES between the ABL and No ABL groups (p=0.04) (Fig. 3A–C).
Fig. 3.
Kaplan–Meier survival analysis comparing the ABL and no ABL groups. (A) VT recurrence free survival. (B) ICD shock therapy free survival. (C) ES occurrence free survival. The p value was calculated using the log-rank test.
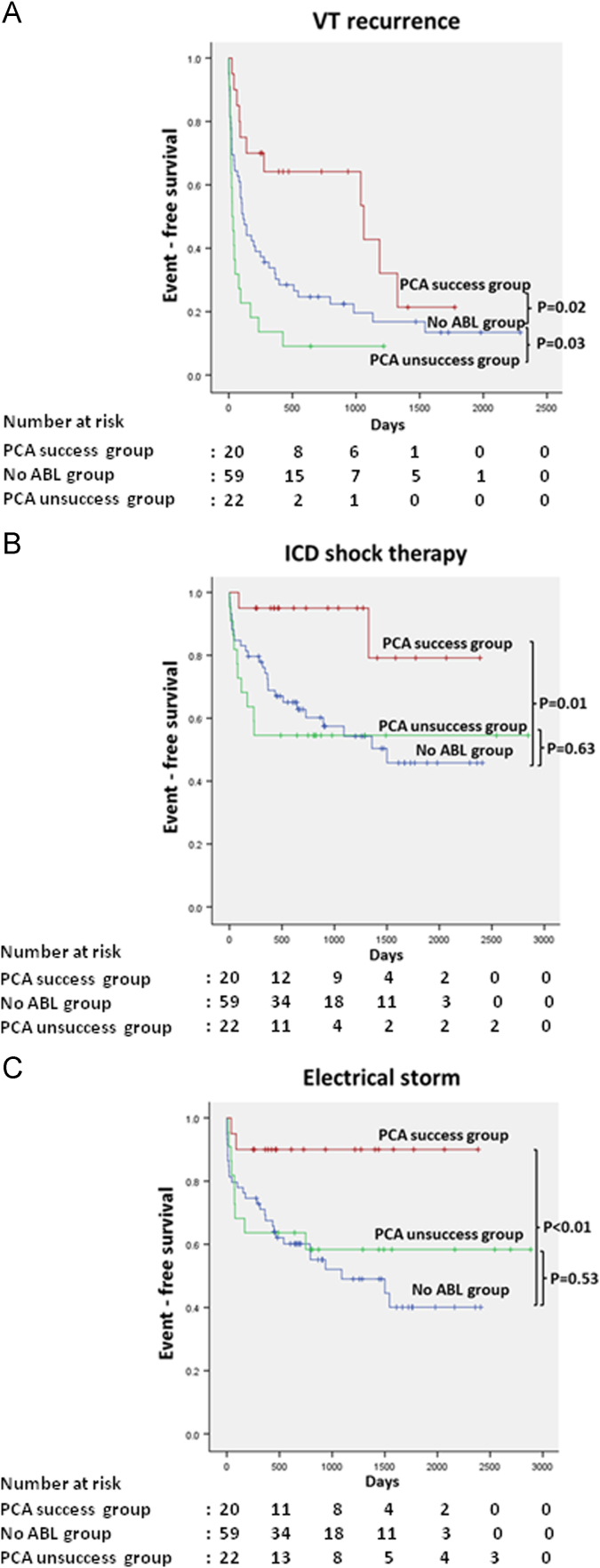
We divided the ABL group into the PCA success and the PCA failure group and compared these groups with the No ABL group (mean follow-up duration: PCA success group vs. No ABL group; 38.2±21.1 vs. 38.7±23.6 months, p=0.920; the PCA success group vs. the PCA failure group; 38.2±21.1 vs. 44.5±26.9 months, p=0.400). VT recurred in 11 patients (55.0%) in the PCA success group and 20 (90.9%) in the PCA failure group. Two patients (10.0%) in the PCA success group and 10 (45.5%) in the PCA failure group received an appropriate shock. Two patients (10.0%) in the PCA success group and 9 (40.9%) in the PCA failure group had ES. By definition, patients in the PCA success group had a lower incidence of ICD shocks and ES when compared with the No ABL group. In the Kaplan–Meier analysis, the time to first VT recurrence, shock therapy, and ES occurrence were significantly longer in the PCA success group than in the No ABL group (p=0.02, p=0.01 and p<0.01, respectively) (Fig. 4A–C).
Fig. 4.
Kaplan–Meier survival analysis comparing the PCA success group, the PCA failure group, and the No ABL group. (A) VT recurrence free survival. (B) ICD shock therapy free survival. (C) ES occurrence free survival. The p value was calculated using the log-rank test.
At least 1 repeated catheter ablation was needed for ES in 4 of 42 patients (9.5%) in the ABL group (1 in the PCA success group and 3 in the PCA failure group). Two patients required an epicardial approach. Catheter ablation was needed for ES and/or frequent ICD shock therapy in 6 of 59 patients (10.2%) in the No ABL group. Two patients in the No any VT group had VT recurrence at >3 years after PCA. In these cases, the cycle length of recurring VTs (210 ms and 360 ms) was faster than those initially documented (260 ms and 400 ms).
All-cause death occurred in 1 patient (2.4%) in the ABL group and in 13 (22.0%) in the No ABL group (p<0.01). Death due to a cardiovascular event occurred in 6 patients (1 patient [2.4%] in the ABL group and 5 [8.5%] in the No ABL group, p=0.13). Causes of non-cardiovascular death in 8 patients included cancer, infection, and interstitial pneumonia.
4. Discussion
4.1. Major findings
Major findings of this retrospective cohort study are as follows:
-
(1)
There were no significant differences in VT recurrence or ICD shock therapy between the ABL and No ABL groups. However, there was a notable reduction in ES occurrence in the ABL group.
-
(2)
PCA success correlated with prolonging the time to VT recurrence and shock therapy, ES. However, the success rate was low (52.4%) although our procedural endpoint was not failure to induce “any VT” but failure to induce “clinical VT.”
4.2. Comparison to previous studies, procedural endpoints and clinical outcomes
Several studies have described long-term results of catheter ablation for VT with different ablation strategies and procedural endpoints in patients with structural heart diseases [4,5,12,14–16]. Catheter ablation performed to abolish all inducible VT in patients with DCM has been reported to reduce mortality as well as VT recurrence and ES [14,17]. Arya et al. reported that, during a median follow-up of 23 months, 87.5% of patients with DCM and ES who underwent complete VT elimination were alive compared with 40% who were alive after partial success [14]. The study population in Arya et al.׳s report was limited to those with VT and ES, and 30.8% of patients needed a second procedure with endocardial and epicardial ablation. The rate of VT recurrence was relatively high in our study, which may have been because clinical outcomes were based on a single endocardial PCA procedure. Clinical outcomes for cardiovascular death and all-cause mortality were similar between our study and the previous study although the procedural endpoint in our study was not the inability to induce “any VT” but rather an inability to induce “clinical VT.” PCA for clinical VT may modify the VT substrate and strengthen ATP therapy׳s efficacy, which might result in reduced ICD shocks, ES, and mortality. This suggests that inability to induce clinical VT may be an acceptable endpoint for prophylactic ablation. However, our observed high rate of VT recurrence after a single endocardial PCA procedure and low success rate are issues to be resolved. These results indicate the need for additional epicardial ablation. However, procedures using the epicardial approach are still associated with a relatively high rate of complications, periprocedural mortality, and difficulty during repeat procedures because of epicardial adhesions [8,15,18], all of which make us hesitant to employ epicardial ablation as a prophylactic therapy before cardioverter-defibrillator implantations.
4.3. Arrhythmogenic substrate and patient selection
In our study, prophylactic endocardial ablation for targeted clinical VT failed in 22 (52.4%) of 42 patients. Elimination of all inducible VTs is difficult in patients with NICM because the arrhythmogenic substrate may frequently include an epicardial scar [19–22]. Bogun et al. reported that delayed-enhancement magnetic resonance imaging (DE–MRI) could identify the location of the VT substrate in 3 dimensions and that only 36% of patients with NICM had a predominant endocardial scar [19]. In our study, an analysis of electroanatomical mapping revealed the prevalence of arrhythmogenic substrates in the endocardium in 76.2% of patients in the ABL group. This discrepancy might be explained by the different assessment modalities used. In our cases, both an endocardial and an epicardial substrate might exist, while in the study by Bogun et al., DE-MRI might have underestimated the endocardial scar. Recently, Bala et al. investigated whether intracardiac echocardiography (ICE) imaging could identify abnormal epicardial substrates in patients with NICM and VT. In their study, the epicardial scar identified by electroanatomical mapping correlated with the echogenic area identified on ICE imaging [23]. Therefore, modern technologies, such as DE-MRI and ICE imaging, facilitate improvements in substrate-specific strategies and help guide decision-making regarding utilization of the endocardial and/or epicardial approach.
4.4. Role of PCA and mechanisms of VT recurrence
Our study suggests that PCA with non-inducible clinical VT can provide a long-lasting reduction in VT recurrence and ES, particularly within the first 3 years after the procedure. The re-entry circuit of clinical VT appears to be important for maintaining spontaneously occurring VT, and modifying the re-entry circuit might suppress VT occurrence or make it more vulnerable to termination. We speculate that those VT recurrences result from the formation of new arrhythmogenic substrates.
4.5. Study limitations
First, this study involved a relatively small sample size. Second, our findings are based on a retrospective analysis. In this study, patients were not randomly assigned to therapeutic strategies (i.e., with or without PCA), and various antiarrhythmic drugs were used, which could lead to biases in the occurrence of endpoints. Third, this study had a heterogeneous population, including patients with various non-ischemic structural heart diseases. The clinical condition of ARVC patients is different from other non-ischemic diseases and may have affected our results. However, one-third of them had reduced LV function, indicating ARVC progression involving LV myocardium, and the underlying structural heart diseases did not differ between the ABL success and the ABL unsuccess groups. Finally, “non-irrigated” tip ablation catheters were used in the majority of patients (81.0%) because irrigated catheters were unavailable in Japan during most of the study period. Use of an irrigated catheter may improve outcomes. Further prospective, randomized controlled studies are required to clarify this issue.
5. Conclusions
Prophylactic endocardial catheter ablation can decrease ES occurrence in NICM patients. However, our high rate of VT recurrence and low success rate are issues to be resolved, and the efficacy of a single endocardial catheter ablation was limited. These results indicate the need for additional epicardial ablation.
Conflict of interest
Section of Arrhythmia, Division of Cardiovascular Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine is financially supported by Medtronic and St. Jude Medical. Medtronic and St. Jude Medical had no any involvement in this study.
References
- 1.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.van Rees J.B., Borleffs C.J., de Bie M.K. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. doi: 10.1016/j.jacc.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Reddy V.Y., Reynolds M.R., Neuzil P. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuck K.H., Schaumann A., Eckardt L. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 6.Carbucicchio C., Santamaria M., Trevisi N. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 7.Dukkipati S.R., d׳Avila A., Soejima K. Long-term outcomes of combined epicardial and endocardial ablation of monomorphic ventricular tachycardia related to hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:185–194. doi: 10.1161/CIRCEP.110.957290. [DOI] [PubMed] [Google Scholar]
- 8.Sacher F., Roberts-Thomson K., Maury P. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 9.Marchlinski F.E., Callans D.J., Gottlieb C.D. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 10.Strickberger S.A., Knight B.P., Michaud G.F. Mapping and ablation of ventricular tachycardia guided by virtual electrograms using a noncontact, computerized mapping system. J Am Coll Cardiol. 2000;35:414–421. doi: 10.1016/s0735-1097(99)00578-1. [DOI] [PubMed] [Google Scholar]
- 11.Volkmer M., Ouyang F., Deger F. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation. Europace. 2006;8:968–976. doi: 10.1093/europace/eul109. [DOI] [PubMed] [Google Scholar]
- 12.Calkins H., Epstein A., Packer D. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000;35:1905–1914. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 13.Soejima K., Suzuki M., Maisel W.H. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104:664–669. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 14.Arya A., Bode K., Piorkowski C. Catheter ablation of electrical storm due to monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy: acute results and its effect on long-term survival. Pacing Clin Electrophysiol. 2010;33:1504–1509. doi: 10.1111/j.1540-8159.2010.02835.x. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson W.G., Wilber D.J., Natale A. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 16.Tung R., Josephson M.E., Reddy V. Influence of clinical and procedural predictors on ventricular tachycardia ablation outcomes: an analysis from the substrate mapping and ablation in Sinus Rhythm to Halt Ventricular Tachycardia Trial (SMASH-VT) J Cardiovasc Electrophysiol. 2010;21:799–803. doi: 10.1111/j.1540-8167.2009.01705.x. [DOI] [PubMed] [Google Scholar]
- 17.Kozeluhova M., Peichl P., Cihak R. Catheter ablation of electrical storm in patients with structural heart disease. Europace. 2011;13:109–113. doi: 10.1093/europace/euq364. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B., Chun K.R., Baensch D. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Heart Rhythm. 2010;7:1746–1752. doi: 10.1016/j.hrthm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Bogun F.M., Desjardins B., Good E. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53:1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bakker J.M., Stein M., van Rijen H.V. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm. 2005;2:777–779. doi: 10.1016/j.hrthm.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A., Azevedo C.F., Cheng A. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-David E., Arenal A., Rubio-Guivernau J.L. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol. 2011;57:184–194. doi: 10.1016/j.jacc.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Bala R., Ren J.F., Hutchinson M.D. Assessing epicardial substrate using intracardiac echocardiography during VT ablation. Circ Arrhythm Electrophysiol. 2011;4:667–673. doi: 10.1161/CIRCEP.111.963553. [DOI] [PMC free article] [PubMed] [Google Scholar]