Abstract
Background
Esophageal injury following catheter ablation of atrial fibrillation (AF) is reported to occur in 35% of patients. Even with a low energy setting (20–25 W), lesions develop in 10% of patients. Body mass index (BMI) has been reported to be a predictor of esophageal injury, indicating that patients with a low BMI (<24.9 kg/m2) are at a higher risk. We hypothesized that catheter ablation with a lower energy setting of 20 W controlled by esophageal temperature monitoring (ETM) at 39 °C could prevent esophageal injury even in patients with a BMI <24.9 kg/m2.
Methods
Twenty patients with AF were included (age, 63±8 years; BMI, 22.9±1.3 kg/m2, left atrium diameter, 44±11 mm). If the esophageal temperature probe registered a temperature of >39 °C, radiofrequency (RF) application was stopped immediately. RF application could be performed in a “point by point” manner for a maximum of 20 s. Endoscopy was performed 1–5 days after ablation.
Results
Esophageal mucosal injury was not observed in any patient in the study.
Conclusions
Catheter ablation using ETM reduced the incidence of esophageal injuries, even in patients with a low BMI.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; RF, radiofrequency
Keywords: Esophageal injury, Pulmonary vein isolation, Atrial fibrillation
1. Introduction
Catheter ablation (CA) is a treatment option for patients with atrial fibrillation (AF). However, interventional AF treatment has been associated with dangerous complications such as atrio-esophageal fistula [1–3]. The mechanism most likely leading to atrio-esophageal fistula after radiofrequency (RF) ablation is thermal esophageal injury during the ablation procedure [4,5]. The incidence of esophageal injury after RF ablation of AF has been reported to range from 5% to 50% depending on the RF power settings and ablation strategies [6–8]. To avoid this complication, esophageal temperature probes, inserted nasally and advanced to the level of the left atrium (LA) under fluoroscopic guidance, are used to mark the location of the esophageal lumen and permit real-time intraluminal esophageal temperature monitoring (ETM) during ablation [9–11]. Furthermore, body mass index (BMI) has been reported to be a predictor of esophageal injury, indicating that patients with a lower BMI (<24.9) are at a higher risk [12]. However, the incidence of esophageal injury in patients with a low BMI has not been well scrutinized.
We hypothesized that CA with a lower energy setting (20 W) and strict monitoring of esophageal temperature at 39 °C may prevent esophageal injury, even in patients with a BMI <24.9. The first goal of the current study was to investigate the incidence of esophageal injury after a single procedure using ETM in patients with a BMI <24.9. A secondary goal was to assess the rhythm outcome after a single procedure using ETM as compared to the conventional procedure without the use of ETM.
2. Methods
2.1. Patient selection
The current study examined 20 consecutive patients with highly symptomatic, medically refractory AF who were treated with pulmonary vein isolation (PVI) using ETM between October 2012 and January 2013. Patients with a BMI >24.9 were excluded. Patients with AF recurrence after a single procedure either with or without the use of ETM were compared with 20 retrospectively recruited, case-matched patients who were treated with PVI without the use of ETM between June 2012 and September 2012. This study was approved by the ethics committees of Himeji Cardiovascular Center (approval date is October 16, 2012: approval number is 12). A written informed consent to participate was obtained from all patients.
2.2. Mapping and ablation procedure
Prior to the procedure, transesophageal echocardiography was performed to exclude thrombus formation. Patients were studied under deep propofol sedation while breathing spontaneously. Standard electrode catheters were placed in the right ventricular apex and the coronary sinus after which a single transseptal puncture was performed. Unfractionated heparin was administered in bolus form before the transseptal puncture to maintain an activated clotting time of >300 s. If AF occurred, internal electrical cardioversion was performed to restore the sinus rhythm.
Mapping and ablation were performed using the CARTO3 system (Biosense Webstar, Diamond Bar, CA, USA) as a guide after integration of a three-dimensional (3D) model of the anatomy of the LA and PV obtained from pre-interventional computed tomography (CT). Prior to ablation, circular mapping catheter- (Lasso, Biosense Webster, Diamond Bar, CA) and ablation catheter-reconstructed LA posterior anatomies were aligned with the CT image [13,14]. Fine adjustment of image integration was achieved through 3 additional landmarks (top of the left superior PV, right superior PV, and bottom of the left inferior PV), with the tip of the ablation catheter (Thermocool, Biosense Webster) at the landmarks according to fluoroscopy and electrogram information.
RF alternating current was delivered in a unipolar mode between the irrigated tip electrode of the ablation catheter and an external back-plate electrode. The initial RF generator setting consisted of an upper catheter tip temperature of 43 °C, a maximal RF power of 30 W, and an irrigation flow rate of 17 mL/min. In patients requiring RF application to the posterior wall, the initial RF generator setting consisted of a maximal RF power of 20 W. All patients underwent PVI. RF application could be performed in a “point by point” manner. The level of ablation was chosen at the atrial side of the PV antrum and depended primarily on the operator׳s decision. The maximum time spent at the anterior and posterior walls was 40 and 20 s, respectively. RF energy was routinely reduced by 10 W when ablating the posterior wall according to the esophageal temperature measured with an esophageal temperature probe (SensiTherm, St. Jude Medical) [15]. If the esophageal temperature rose to >39 °C, the ablation was stopped immediately and the energy was further reduced. After the esophageal temperature decreased to the normal range (37 °C), RF application was resumed. If ablation could not be performed with 20 W, line placement was performed either more antral or closer to the PV, depending on the individual׳s anatomical characteristics [16]. Catheter navigation was performed with a non-steerable sheath (Preface, Multipurpose, Biosense Webster) (Fig. 1A).
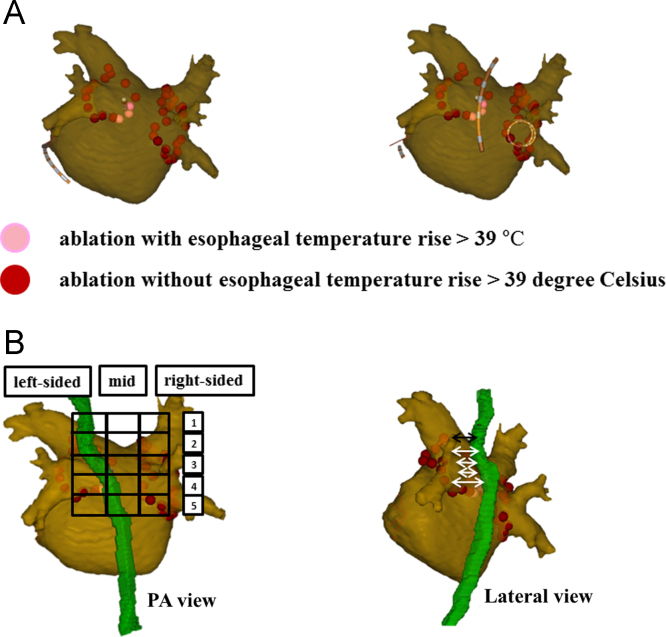
Fig. 1.
(A) Catheter ablation with esophageal temperature monitoring. Pink three-dimensional dots represent sites where the esophageal temperature increased to >39 °C, even with the use of a lower energy setting (20 W). (B) The location of the esophagus close to left atrium posterior wall. Superimposition of a grid with vertical columns (left, mid, right) and horizontal rows 1–5 (left panel). The minimum distance between the left atrium posterior wall and the esophagus was measured at each segment (right panel).
The procedural endpoint was considered to be the electrophysiologically proven bidirectional block for the PV-encircling ablation lines confirmed with a circular mapping catheter. After proving bidirectional block of the PV, we performed a stimulation protocol (burst pacing from CS with 300 ms, 250 ms, and 200 ms for 10 s each) to test inducibility. In cases when AF was induced, patients were cardioverted and the procedure ended. A pharmacological test consisting of high-dose isoproterenol infusion (20 µg/min) was performed to identify non-PV-triggers. Ablation of the cavotricuspid isthmus (CTI) was performed only if the typical right atrial flutter was either documented previously or induced by burst pacing at the end of the procedure. Patients were started on proton pump inhibitors on admission and continued for 4 weeks; esophagoscopy was repeated after 3–4 days if mucosal ulcerations >10 mm were observed initially [7].
2.3. Validation of the esophageal course and the proximity to the LA posterior wall
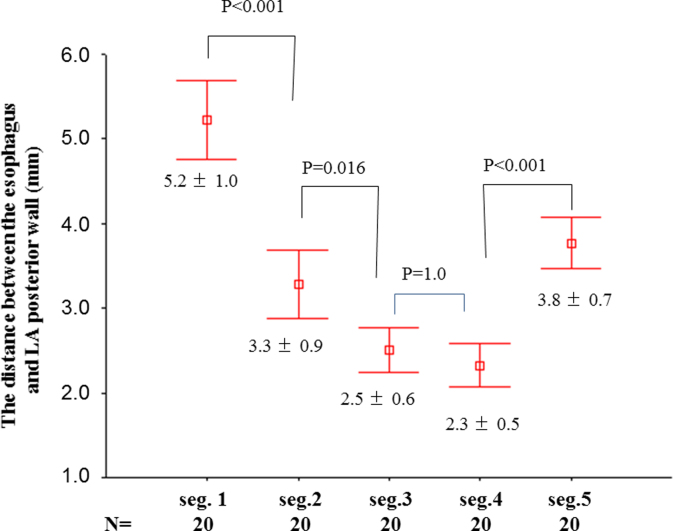
After the circular mapping catheter- (Lasso, Biosense Webster) and ablation catheter-reconstructed LA posterior anatomies were aligned with the CT image, the course of the esophagus was categorized into 15 segments: vertically into 3 columns (left-sided area, mid-area, and right-sided area); and horizontally into 5 rows (segments 1–5) [16]. Row 1 indicated the posterior wall behind the LA roof; row 2 indicated the posterior wall behind the left superior pulmonary vein (LSPV); row 3 indicated the posterior wall behind the carina between the LSPV and the left inferior pulmonary vein (LIPV); row 4 indicated the posterior wall behind the LIPV; and row 5 indicated the posterior wall behind the bottom of the LA. As for the proximity to LA posterior wall, we measured the distance between the esophagus and the LA posterior wall at each segment (Fig. 1B).
2.4. Post-interventional management and follow-up
Anti-arrhythmic treatment was discontinued postinterventionally and beta-blockers were administered to all patients. After treatment, patients were administered oral anticoagulants for ≥6 months (target international normalized ratio: 2.0–3.0), depending on the individual stroke risk based on the CHADS2 score. In all patients, 24-h Holter electrocardiography (SCM-6600; FUKUDA DENSHI, Tokyo, Japan) was performed after 3, 6, and 12 months. If symptoms occurred outside of the recording period, patients were requested to contact our center or the referring physician to obtain electrocardiogram documentation. AF and/or macro-re-entrant atrial tachycardia episodes lasting >30 s were considered as recurrences [17].
2.5. Statistics
Data assessed using the Kolmogorov–Smirnov test are presented as mean±standard deviation for normally distributed variables. Median and quartile values are given for non-normally distributed variables. Categorical variables are expressed as number and percentage of patients. Continuous and categorical variables were analyzed using the Student׳s t test and Fisher׳s exact test, respectively. Continuous variables grouped by location (segments 1–5) were compared using 1-way analysis of variance followed by post-hoc analysis with a Bonferroni correction for multiple data comparisons if normally distributed, or a Kruskal–Wallis test if skewed. Differences were considered statistically significant at a value of P<0.05. All statistical analyses were performed with SPSS, Release 11.0.
3. Results
3.1. Patient characteristics
Patient characteristics are displayed in Table 1. Patients treated with the procedure using ETM were compared with the same number of patients treated with the conventional procedure without the use of ETM. The groups were comparable with the exception of the prevalence of atrial hypertension (50% vs. 10%, P<0.014). A total of 20 patients in the ETM group with symptomatic AF and a median AF history of 45 months (1st–3rd quartile, 14–72) were included in the ETM group. Their mean age was 63±8 years and 14 patients (70%) were men. The mean LA diameter was 44±10 mm and the left ventricular ejection fraction was 56±11%. Hypertension was noted in 10 patients (50%), and the mean BMI was 22.9±1.3 kg/m2.
Table 1.
Clinical characteristics of the patient population.
| Patients (using ETM) (n=20) | Patients (without using ETM) (n=20) | P-value | |
|---|---|---|---|
| Age (years) | 63±8 | 62±10 | 0.68 |
| Male (n – %) | 14 (70) | 14 (70) | 1.0 |
| Paroxysmal AF (n – %) | 14 (70) | 16 (80) | 0.71 |
| AF history (months) | 45 (14; 72) | 35 (7; 48) | 0.27 |
| Antiarrhythmic drugs (n) | 1 (0; 1) | 0 (0; 1.75) | 0.42 |
| Arterial hypertension (n – %) | 10 (50) | 2 (10) | 0.014 |
| Coronary artery disease (n – %) | 0 (0) | 0 (0) | 1.0 |
| Diabetes mellitus (n – %) | 1 (5) | 0 (0) | 0.50 |
| Lone AF, (n – %) | 5 (25) | 8 (40) | 0.56 |
| Left atrial diameter (mm) | 44±10 | 43±4 | 0.45 |
| LV ejection fraction (%) | 56±11 | 56±7.9 | 0.26 |
| BMI (per 1 kg/m2) | 22.9±1.3 | 22.5±1.2 |
AF, atrial fibrillation; LAA, left atrial appendage; LV, left ventricle; BMI, body mass index.
3.2. The proximity and course of the esophagus to the LA posterior wall
Esophageal courses differed significantly among patients. In majority of the patients (15/20, 75%), the esophageal courses were in the left-sided LA posterior wall (LAPW). Esophageal courses were in the mid-area and the right-sided LAPW in 4 patients (20%) and 1 (5%) patient, respectively.
Regarding proximity of the esophagus to the LAPW, the distance between them was shorter behind the posterior/inferior areas (rows 3 and 4) of the LA than that behind the superior areas of the LA (Fig. 2; P<0.001).
Fig. 2.
The minimum distances between the esophagus and the left atrium posterior wall at each segment.
3.3. Incidence of esophageal temperature increases to >39 °C and the maximum temperature of the esophagus
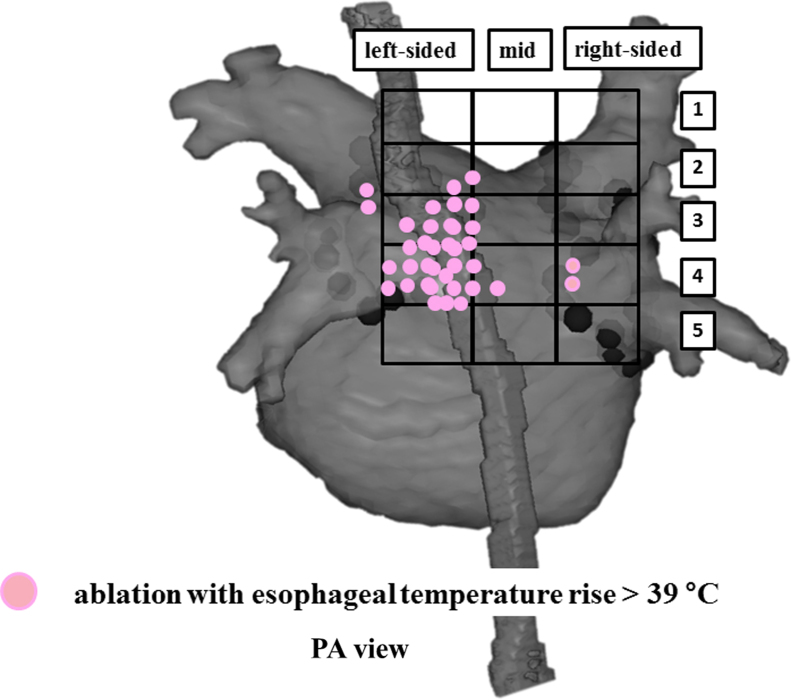
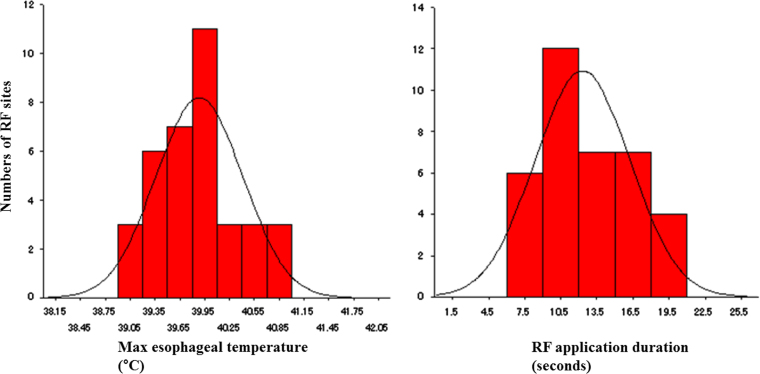
At 36 RF application sites in 14 of 20 (70%) patients, esophageal temperatures rose to >39 °C during RF application to the LAPW (Fig. 3). Of those, the mean RF duration and maximum esophageal temperature measured were 12±4 s and 39.9±0.6 °C, respectively. It was noteworthy that the maximum esophageal temperature did not reach 41 °C in any of the patients (Fig. 4).
Fig. 3.
RF application sites with esophageal temperature increases to >39 °C are expressed with pink dots. Two sites with esophageal temperature increases to >39 °C were found at the carina region between the left superior pulmonary vein and the left inferior pulmonary vein.
Fig. 4.
The distribution of the maximum esophageal temperature and RF duration at the sites with esophageal temperature increases. RF, radiofrequency.
3.4. Incidence of esophageal injury
Esophageal lesions were not found in any of the patients studied.
3.5. Rhythm outcome and procedural parameters
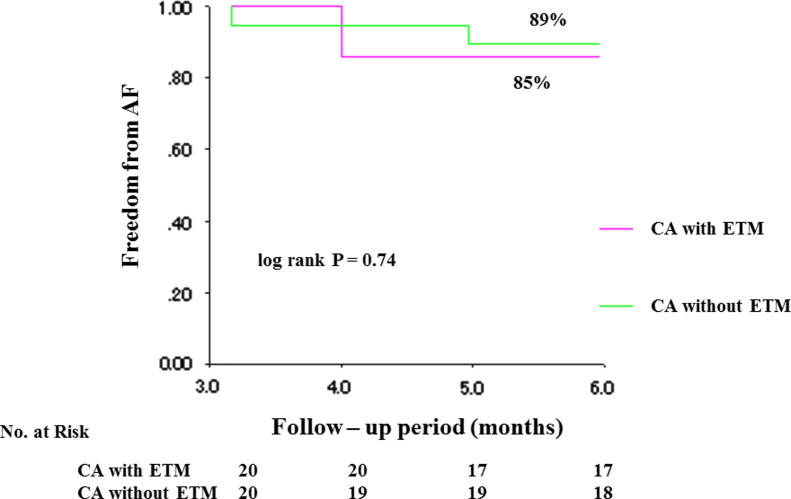
Ablation was performed by 3 experienced electrophysiologists. Complete PVI was achieved in all patients. After PVI, additional ablations, consisting of both the LA roof line and mitral isthmus line (n=2) or the roof line only (n=1) were performed. Ablation for CTI (n=6), non-PV foci (n=3), and superior vena cava isolation (n=1) was performed. Mean procedure time was 210±57 min, mean fluoroscopy time was 63±14 min, and median RF energy application was 0.67±0.28×105 J. The fluoroscopy time and RF energy application did not differ significantly between patients in the ETM group and the 20 case-matched patients who did not undergo ETM. The procedure time was longer for those in the ETM group (210±57 min vs. 182±44 min), but the difference did not reach statistical significance (P=0.098; Table 2). Additionally, the rhythm outcome (AF recurrence) at 6 months did not differ significantly (15% vs. 10%, P=0.74) between the 2 groups (Fig. 5).
Table 2.
Rhythm outcome and procedural parameters.
| CA using ETM (n=20) | CA without using ETM (n=20) | P value | |
|---|---|---|---|
| Procedure time (min) | 210±57 | 182±44 | 0.098 |
| Fluoroscopy time (min) | 63±14 | 62±15 | 0.94 |
| RF energy application (J) | 0.67±0.28×105 | 0.72±0.23×105 | 0.50 |
| AF recurrence at 6 month FU | 3 (15%) | 2 (10%) | 0.74 |
CA, catheter ablation; ETM, esophageal temperature monitoring; RF, radiofrequency; AF, atrial fibrillation; FU, follow-up.
Fig. 5.
Kaplan–Meier curve of freedom from AF after a single procedure with or without ETM. AF, atrial fibrillation; CA, catheter ablation; ETM, esophageal temperature monitoring.
4. Discussion
4.1. Main findings
The results of the current study demonstrated that CA controlled by ETM at a 39 °C setting reduced the incidence of esophageal injury, even in patients with a low BMI. Furthermore, study results indicated that the procedure time was relatively longer when using ETM, however, this difference was not statistically significant and had no bearing on the excellent rhythm outcome at the 6 month follow-up.
4.2. Incidence of esophageal injury after CA controlled by ETM with a 39 °C setting in patients with a low BMI
In the largest study published to date regarding the significance of ETM, it was shown that maximal measured esophageal temperature was highly predictive of esophageal injury. Study results revealed that no esophageal lesions were observed when the maximal intraluminal temperature was <41.8 °C during manually driven CA supported by steerable sheath technology [7]. In their series of more than 1500 procedures with temperature probes placed in the esophagus, however, fatal atrio-esophageal fistula occurred in 1 patient without a documented significant temperature increase during the ablation procedure. A recent multivariate analysis comparing data from patients with and without esophageal lesions revealed that patients with a BMI <26 displayed an increased risk for esophageal injury [12].
The incidence of esophageal injury after AF ablation has been described previously and varied among studies. An incidence of 0% has been reported in some studies [9], whereas others have reported an incidence of esophageal injury as high as 40% [18]. Contreras-Valdes et al. reported 37.4% of patients (82 of 219) with esophageal intraluminal temperatures >39 °C [6]. Twenty-two patients (10%) were identified with esophageal injury. In their study, Contreras-Valdes et al. reported that the power of RF application and duration were limited to 25 W and 20–30 s, respectively. Although these settings were similar to those used in the current study, the incidence of esophageal injury was higher. This difference may have been attributable to the usage of a steerable sheath, which may strengthen the intensity of the ablation electrode to tissue contact, a crucial determinant of lesion depth that can result in a high incidence of esophageal injury [19]. Tilz et al. have also reported that a high incidence of thermal esophageal injury was noted following robotic PVI using 30 W along the LA posterior wall. It should be noted that the remote robotic catheter navigation system allows for intense contact between the ablation electrode and left atrial tissue [18].
Comparing data from patients with and without esophageal lesions in a multivariate analysis revealed that patients with BMI <26 had an increased risk for esophageal injury. Yamasaki et al. concluded that asymptomatic esophageal injuries were not rare, even with a low energy setting, in patients below normal weight. Furthermore, they demonstrated that the distance between the esophagus and the LA correlated positively with BMI. The distance was reported to be approximately 3 mm in patients with a BMI of 23 kg/m2. Consistent with their results, the mean distance in the current study was 2.3±0.5 mm. Therefore, tailored energy settings based on the patient׳s BMI may be required when performing PVI [12]. An additional study reported that deep-ulcerated lesions with a hemorrhagic appearance or fibrinoid material covering seemed to represent a more severe injury to the esophagus [20], with a longer healing time than superficial lesions. In McCall׳s studies, the mean BMI in patients with deep-ulcerated lesions was 26 kg/m2. In contrast, the mean BMI in patients with superficial lesions, characterized by mucosal erythema or shallow desquamation of the upper layers of the esophageal mucosa, was 29 kg/m2. In the current study, the mean BMI was 23 kg/m2. Contreras-Valdes et al. reported that 22 (27%) of 82 patients with esophageal temperature increases to >39 °C were identified with esophageal injury [6]. Their ETM was similar to that employed in the current study, however, the initial power setting of 25 W and RF duration of 20–30 s differed from those used in our study. In our cases, mean RF duration for the sites with temperature increases was 12±4 s. Therefore, the maximal power was also limited to 8–20 W (data not shown). Our power setting and RF duration were dramatically lower and shorter, respectively, than those used in previous studies. Results from the current study indicated that our RF application setting controlled by ETM was safe, even in patients with a low BMI.
4.3. Location and vicinity of the esophagus to the LAPW in patients with a low BMI
Previous studies have demonstrated that esophageal courses behind the left-sided or mid-LA were more frequent than the right-sided LA [16,21,22]. In the current study, the esophagus was observed to descend strictly posterior to the LA behind the superior and mid-areas of the LA and then bend anteriorly behind the posterior/inferior areas of the LA. Therefore, esophagus–LA contact was observed in those areas. Our results, even in patients with a low BMI, were completely consistent with those of previous studies. The distances between the LA and esophagus, specifically between the endocardial sites of the LAPW and the epicardial sites of the esophagus as assessed by 3D CT, were measured as only 2.5 mm. In those areas, the epicardial sites of the esophagus are considered to have strong contact with the epicardial sites of the LAPW with no pericardial fat to serve as a thermal insulator. Therefore, RF application with lower settings such as 20–25 W in those regions can induce an increase in esophageal temperature with ease. It should also be noted that RF application at the carina induced an increase in esophageal temperature. ETM may represent a prudent solution to preventing the unexpected increase in esophageal temperature.
4.4. The difficulty of PVI controlled by ETM
In the current study, the procedure time for conventional PVI using ETM was relatively longer than that without ETM. However, the total RF energy application was similar regardless of ETM usage. This indicates that esophageal temperature increases to >39 °C limits the continuous RF applications. In cases with a cessation of RF application for 5 s due to increased esophageal temperature, RF application to the same site was required 4 times after the esophageal temperature decreased to the normal range (37 °C) to achieve the same level of energy application using the 20 W and 20 s settings. To prevent esophageal injury, prolongation of procedure time up to 15% is considered acceptable [23]. Furthermore, prolongation of procedure time in the current study decreased during CA in the last 10 patients possibly due to a learning curve.
4.5. The 39 °C setting and the maximum esophageal temperature in patients with a small BMI
Halm et al. have reported that esophageal lesions after CA did not occur below an esophageal temperature of 41 °C [7]. Furthermore, they determined that for every 1 °C increase in esophageal temperature, the odds of developing an esophageal lesion were increased by a factor of 1.36. It is important to note that Kuwahara et al. detected gradual increases in esophageal temperature after the cessation of RF energy application and found that increases of 1–3 °C within 10–20 s of cessation were common before temperatures returned to control levels [11]. Therefore, the cessation of RF energy at a 39 °C should be considered ideal to prevent reaching the maximum esophageal temperature of 41 °C. In the current study, all maximum esophageal temperatures were measured at <41 °C, which should and did result in a 0% incidence of esophageal injury.
4.6. Study limitations
Our study had 2 major limitations. First, the sample size was rather small. Second, this study was nonrandomized and not designed to make clear comparisons with the incidence of esophageal injury and procedural outcomes obtained with other methods. Our findings should therefore be verified in a randomized prospective study.
5. Conclusion
CA using ETM may reduce the incidence of esophageal injuries, even in patients with a BMI <24.9 kg/m2, without compromising the rhythm outcome of the procedure.
Disclosures
None.
Conflict of interest
None.
Acknowledgments
None.
References
- 1.Pappone C., Oral H., Santinelli V. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 2.Dagres N., Hindricks G., Kottkamp H. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol. 2009;20:1014–1019. doi: 10.1111/j.1540-8167.2009.01493.x. [DOI] [PubMed] [Google Scholar]
- 3.Eitel C., Rolf S., Zachaus M. Successful non-surgical treatment of esophagopericardial fistulas following atrial fibrillation catheter ablation: a case series. Circ Arrhythm Electrophysiol. 2013;6:675–681. doi: 10.1161/CIRCEP.113.000384. [DOI] [PubMed] [Google Scholar]
- 4.Gilcrease G.W., Stein J.B. A delayed case of fatal atrioesophageal fistula following radiofrequency ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:708–711. doi: 10.1111/j.1540-8167.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 5.Tancevski I., Hintringer F., Stuehlinger M. Atrioesophageal fistula after percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2012;125:966. doi: 10.1161/CIRCULATIONAHA.111.044438. [DOI] [PubMed] [Google Scholar]
- 6.Contreras-Valdes F.M., Heist E.K., Danik S.B. Severity of esophageal injury predicts time to healing after radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm. 2011;8:1862–1868. doi: 10.1016/j.hrthm.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Halm U., Gaspar T., Zachaus M. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105:551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M., Nolker G., Marschang H. Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace. 2008;10:205–209. doi: 10.1093/europace/eun001. [DOI] [PubMed] [Google Scholar]
- 9.Leite L.R., Santos S.N., Maia H. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011;4:149–156. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 10.Carroll B.J., Contreras-Valdes F.M., Heist E.K. Multi-sensor esophageal temperature probe used during radiofrequency ablation for atrial fibrillation is associated with increased intraluminal temperature detection and increased risk of esophageal injury compared to single-sensor probe. J Cardiovasc Electrophysiol. 2013;24:958–964. doi: 10.1111/jce.12180. [DOI] [PubMed] [Google Scholar]
- 11.Kuwahara T., Takahashi A., Kobori A. Safe and effective ablation of atrial fibrillation: importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol. 2009;20:1–6. doi: 10.1111/j.1540-8167.2008.01280.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki H., Tada H., Sekiguchi Y. Prevalence and characteristics of asymptomatic excessive transmural injury after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2011;8:826–832. doi: 10.1016/j.hrthm.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T., Sasaki S., Kimura M. Three-dimensional cardiac image integration of electroanatomical mapping of only left atrial posterior wall with CT image to guide circumferential pulmonary vein ablation. J Interv Card Electrophysiol. 2010;29:167–173. doi: 10.1007/s10840-010-9521-5. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang F., Bansch D., Ernst S. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi K., Kircher S., Watanabe N. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol. 2012;5:667–675. doi: 10.1161/CIRCEP.111.969923. [DOI] [PubMed] [Google Scholar]
- 16.Kottkamp H., Piorkowski C., Tanner H. Topographic variability of the esophageal left atrial relation influencing ablation lines in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:146–150. doi: 10.1046/j.1540-8167.2005.40604.x. [DOI] [PubMed] [Google Scholar]
- 17.Calkins H., Kuck K.H., Cappato R. 2012 h/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Tilz R.R., Chun K.R., Metzner A. Unexpected high incidence of esophageal injury following pulmonary vein isolation using robotic navigation. J Cardiovasc Electrophysiol. 2010;21:853–858. doi: 10.1111/j.1540-8167.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- 19.Piorkowski C., Eitel C., Rolf S. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol. 2011;4:157–165. doi: 10.1161/CIRCEP.110.957761. [DOI] [PubMed] [Google Scholar]
- 20.McCall R., Thomas S.P. Esophageal hematoma complicating catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:221–223. doi: 10.1111/j.1540-8167.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- 21.Bunch T.J., May H.T., Crandall B.G. Intracardiac ultrasound for esophageal anatomic assessment and localization during left atrial ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:33–39. doi: 10.1111/j.1540-8167.2012.02441.x. [DOI] [PubMed] [Google Scholar]
- 22.Bahnson T.D. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2009;32:248–260. doi: 10.1111/j.1540-8159.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinek M., Bencsik G., Aichinger J. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol. 2009;20:726–733. doi: 10.1111/j.1540-8167.2008.01426.x. [DOI] [PubMed] [Google Scholar]