Abstract
This case report describes a 19-year-old man with early repolarization (ER) in the inferolateral leads and a normal QT interval who survived a cardiac arrest that was likely related to polymorphic ventricular tachycardia (VT). Electrocardiograms (ECGs) also showed unifocal premature ventricular beats (PVBs) with a relatively narrow QRS duration. A Holter ECG documented occasional short-coupled PVBs following non-sustained VTs. Pharmacological stress testing was also performed to assess the effects of anti-arrhythmic drugs on ER (the J wave) and PVBs. We performed successful radiofrequency catheter ablation to prevent the recurrence of ventricular fibrillation after cardioverter-defibrillator implantation.
Keywords: Premature ventricular beat, Early repolarization, Short-coupled variant of torsades de pointes, Radiofrequency catheter ablation, Implantable cardioverter-defibrillator
1. Introduction
In 1994, Leenhardt et al. first described electrocardiogram (ECG) findings of torsades de pointes and QT prolongation in patients without demonstrable structural heart disease, with the torsades de pointes initiated by short-coupled ventricular beats [1]. Some recent reports showed that premature ventricular beats (PVBs) from the distal Purkinje system, which occasionally occur with a short coupling interval, triggered polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF) [2,3]. On the other hand, in 2008, Haïssaguerre et al. reported an association between inferolateral early repolarization (ER), also called the J wave, and unexplained sudden cardiac death [4]. Since then, inferolateral ER has been identified as a marker of arrhythmic risk in the general population. However, risk assessment remains to be elucidated, since the prevalence of inferolateral ER in the general population is high and most individuals with this ECG pattern have a benign prognosis. Here, we report a case of idiopathic VF (IVF) with PVBs originating in the Purkinje system, occasionally with a short coupling interval, and ER in the inferolateral leads.
2. Case presentation
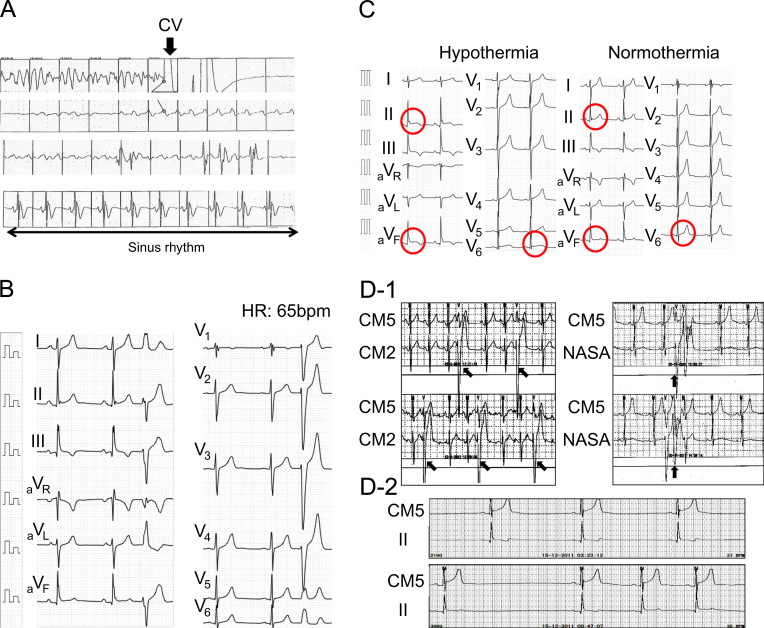
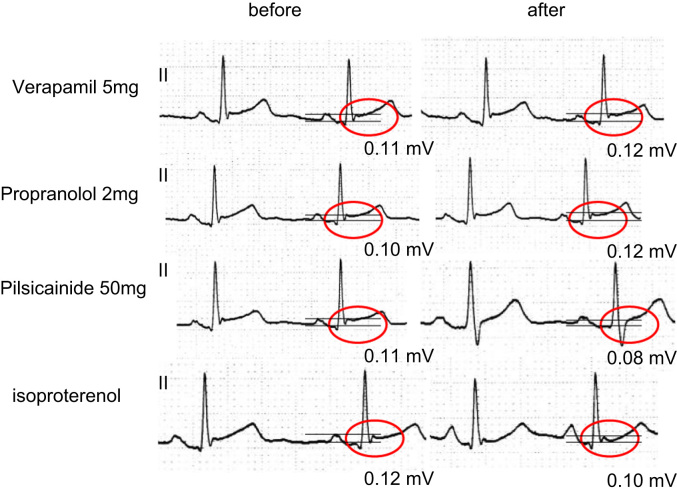
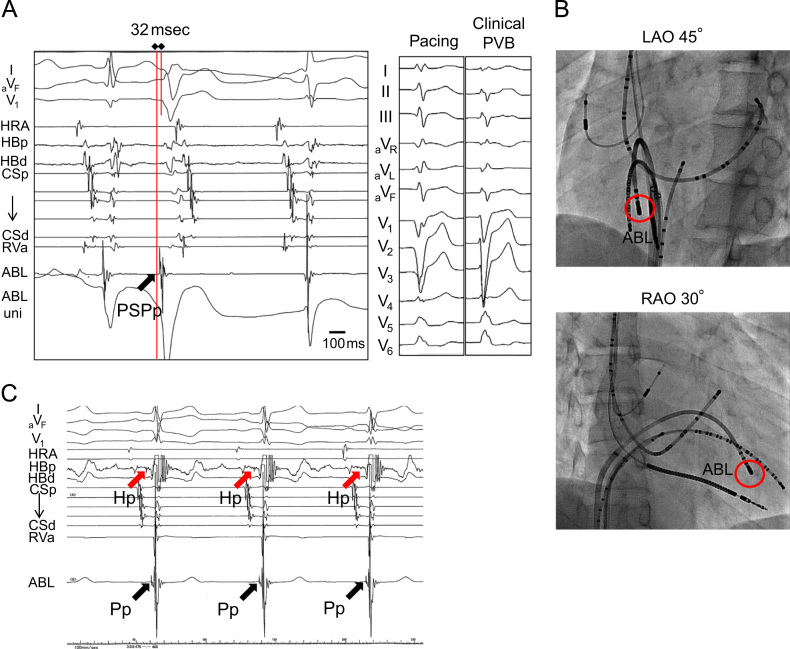
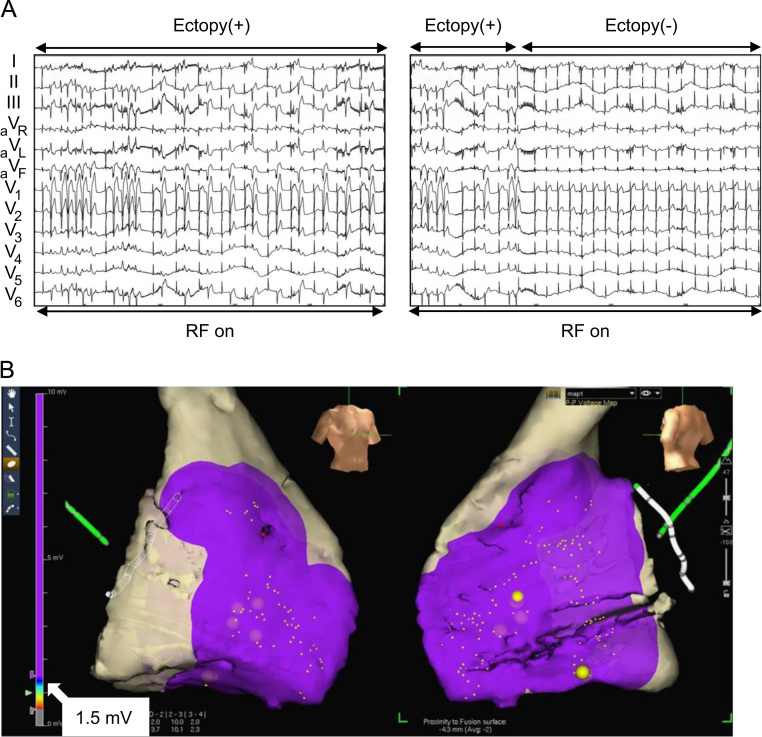
In November 2011, a 19-year-old man with no family history of sudden death and a history of syncope was admitted to our hospital after experiencing a sudden cardiac arrest. He suddenly developed abnormal respiration and subsequently experienced convulsions while watching television. His friend, who was awakened by the unusual noise, found him unconscious, called emergency medical services immediately, and started cardiopulmonary resuscitation. Emergency medical services arrived 16 min later and found that the patient had VF. The patient was successfully converted to sinus rhythm with an automated external defibrillator (Fig. 1A). He was intubated, transported to the intensive care unit, and initiated on a hypothermia protocol because of his decreased level of consciousness. At admission, his ECG showed sinus rhythm with PVBs, ER in the inferolateral leads, and a normal QT interval (Fig. 1B). Although ER in the inferolateral leads became prominent during hypothermic treatment, no arrhythmic event occurred at this time (Fig. 1C). The patient׳s cardiac evaluation during admission included cardiac catheterization, echocardiography, myocardial magnetic resonance imaging (MRI), and myocardial scintigraphy, which demonstrated no organic structural heart disease. In addition, a signal-averaged ECG was negative since all of three standard parameters showed normal values (the filtered QRS vector magnitude: 104 ms (<105 ms), the root mean square voltage of the terminal 40 ms of the vector magnitude: 29.4 μV (>11.0 μV), the low-amplitude signal duration under 40 μV in the terminal portion of the vector magnitude: 32 ms (<44 ms)). However, a Holter ECG documented PVBs and non-sustained VTs (NSVTs), which frequently appeared during the day or during heart rate increases. In addition, PVBs or the first initiating PVBs of NSVTs occasionally appeared with a short coupling interval (Fig. 1D-1). ER in the inferolateral leads diminished at that time, but became obvious during the night or at periods of decreased heart rate (Fig. 1D-2). Pharmacological stress testing was performed to assess the effects of anti-arrhythmic drugs on the J wave (Fig. 2) and PVBs (data not shown). The J points of the inferolateral leads were elevated after verapamil and propranolol infusion and depressed after isoproterenol and pilsicainide infusion. PVBs were increased after isoproterenol infusion and decreased after propranolol and verapamil infusion. An implantable cardioverter-defibrillator (ICD) was placed per current recommendations. After ICD placement, an electrophysiological study and radiofrequency catheter ablation were performed. Programmed ventricular stimulation was performed using a maximum of three extra stimuli (S4) at several different driven cycle lengths (CLs) from the right ventricular apex and outflow tract. However, because neither NSVT nor VF was induced, the stimulation was repeated during isoproterenol infusion (1–3 μg/min). Isoproterenol infusion mildly increased the occurrence of clinical PVBs or NSVT with the same QRS morphology as the spontaneous clinical PVBs. Because the PVBs were not bidirectional and a ryanodine receptor gene mutation was not detected later on, we excluded catecholaminergic polymorphic VT. The clinical PVBs had a left bundle branch block configuration with a left-axis deviation. At the time of PVB, an early activated lesion was explored using an A-20 electrode catheter placed at the right ventricular septum. Then, an earliest activated site (EAS) located at the inferior septum of the right ventricle and preceding QRS onset by 32 ms with presystolic Purkinje potentials and a unipolar QS pattern was detected with an ablation catheter (Fig. 3A and B). Furthermore, paced mapping at the EAS showed a QRS morphology nearly identical to that of the spontaneous PVBs (Fig. 3A). Purkinje potentials during sinus rhythm were also recorded at the EAS (Fig. 3C). Radiofrequency energy was delivered to the EAS in the temperature-controlled mode with a target temperature of 55–60 °C and a maximum power of 45 W using a conventional 4-mm tip ablation catheter. Although ectopic NSVTs were temporally induced just after starting energization, they completely disappeared 30 s later (Fig. 4A). Clinical PVBs remained, but NSVT and the couplets of PVBs completely disappeared and were not inducible by isoproterenol. No low voltage area in the right ventricle was confirmed by electroanatomical mapping (Fig. 4B). Holter monitoring and an exercise stress test after ablation showed no PVB couplets or NSVT. During the 8-month follow-up examination at which time the patient received no drug therapy, no episodes of syncope or VF recurrence were observed.
Fig. 1.
(A) Documented VF recorded in the automated external defibrillator. Return of spontaneous circulation was subsequently documented after cardioversion and the heart rhythm gradually recovered to sinus rhythm (arrow). (B) The 12-lead ECG on admission. ER was observed in the inferior leads. The QRS duration of the PVB was 128 ms (relatively narrow). The QT interval was 410 ms. (C) ER in the inferolateral leads became prominent when the patient underwent hypothermic treatment as compared to the ER during normothermia. (D) Holter ECG monitoring during hospitalization. (D-1) Increased PVBs and NSVTs were documented during the day and occasionally broke out into a short coupling interval (300 ms). (D-2) ER became prominent during the night or during periods of decreased heart rate, and PVBs were rarely documented at those times. CV=cardioversion and HR=heart rate.
Fig. 2.
The changes of ER in ECG lead II before or after pharmacological stress testing with verapamil, propranolol, pilsicainide, and isoproterenol.
Fig. 3.
Catheter mapping for radiofrequency catheter ablation and a 12-lead ECG while delivering radiofrequency energy. (A) Activation mapping for spontaneous trigger PVBs showed the earliest ventricular activation, which included presystolic Purkinje potentials (PSPp) 32 ms prior to the QRS onset. Paced mapping at the right ventricular inferior septum, which was the EAS, showed a QRS morphology nearly identical to that of spontaneous PVBs. (B) A representation of an ablation catheter placed on the EAS and other catheters or leads. (C) A representative intracardiac electrocardiogram recording that recorded Purkinje potentials (Pp, black arrows) at the EAS during sinus rhythm. His potentials (Hp, red arrows) were also recorded at the proximal His bundle (HBp) catheter. PSPp=presystolic Purkinje potential, HRA=high right atrium, HBp=proximal His bundle, HBd=distal His bundle, CSp=proximal coronary sinus, CSd=distal coronary sinus, RVa=right ventricular apex, ABL=ablation catheter, LAO=left anterior oblique, RAO=right anterior oblique, Pp=Purkinje potential, and Hp=His potential.
Fig. 4.
(A) PVBs and NSVTs appeared during ablation because of a possible ectopic mechanism. They completely disappeared 30 s after energization onset. (B) A voltage map obtained during catheter ablation by three-dimensional electroanatomical mapping. The low voltage area was defined as <1.5 mV. The purple area indicates a normal voltage area. Although the voltage of the lateral wall of the right ventricle could not be fully recorded (gray areas do not indicate scar areas, but unrecorded areas), no low voltage area in the right ventricle was confirmed. RF=radiofrequency.
3. Discussion
Haïssaguerre et al. [2] reported that PVBs originating from the distal Purkinje system triggered VF or polymorphic VT in patients without structural heart disease. In this report, the coupling interval of the first initiating PVB of VF was relatively short (297±41 ms), and the PVBs originated from both the left and right ventricles. Several reports described triggered activity, abnormal automaticity, or reentry as possible underlying mechanisms of IVF originating from the Purkinje system [1–3,5–8]. Verapamil is considered effective in preventing such arrhythmic events.
On the other hand, Haïssaguerre et al. also reported that triggered PVBs of IVF with ER in the inferolateral leads, also called the J wave syndrome [9], originated from the left ventricular myocardium or Purkinje system [4]. They further reported that the characteristics of IVF with ER in the inferolateral leads included the following: (1) most of the patients were men; (2) they had a history of unexplained syncope or sudden cardiac arrest during sleep or at night; and (3) exercise testing or isoproterenol infusion consistently reduced or eliminated ER. In particular, during repetitive episodes of VF, isoproterenol infusion eliminated all arrhythmias when the sinus heart rate was increased above 120 beats/min. By contrast, β-blockers accentuated repolarization abnormalities.
Interestingly, in our case, although ER in the inferolateral leads was detected by ECG, other characteristics did not fit with the J wave syndrome [4]. For example, exercise testing and isoproterenol infusion consistently reduced ER but frequently produced PVBs or NSVT, and the VF was initiated in the daytime. Furthermore, triggered PVBs originated from the right ventricular Purkinje system, not the left ventricular Purkinje system.
We consider the underlying etiology of this case to be short-coupled PVB-related VT/VF rather than ER-related VT/VF, because PVBs or NSVTs were decreased by verapamil and propranolol infusion but increased by isoproterenol infusion, indicating triggered activity as the underlying mechanism [8,10]. Reentry was also related to the partial underlying mechanism of this case, as indicated by the complete disappearance of NSVT and PVB couplets despite the remaining trigger PVBs [2,3,8].
We had difficulty in differentiating the short-coupled variant of torsades de pointes from ER syndrome in this case because VF initiation was not documented. When episodes of VT/VF recurrence are documented by ICD recordings during future follow-up visits, the form of VT/VF initiation will provide us with essential evidence needed to decide the exact etiology of this case.
4. Limitations
Our case report has some limitations. First, we cannot completely exclude the possibility of ARVD or myocarditis because myocardial biopsy was not performed. However, we believe it is unlikely that the etiology of this patient is ARVD or myocarditis because he did not have symptoms of the common cold and his echocardiography, myocardial MRI, and myocardial scintigraphy demonstrated no organic structural heart disease. Second, because no exact definitions of short-coupled PVBs exist, it remains controversial to define a PVB with a 300-ms coupling interval during exercise or isoproterenol infusion as a short-coupled PVB.
Conflict of interest
The authors state that they have no conflict of interest.
References
- 1.Leenhardt A., Glaser E., Burguera M. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M., Shoda M., Jaïs P. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 3.Nogami A., Sugiyasu A., Kubota S. Mapping and ablation of idiopathic ventricular fibrillation from the purkinje system. Heart Rhythm. 2005;2:646–649. doi: 10.1016/j.hrthm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Haïssaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 5.Gilmour R.F., Moïse N.S. Triggered activity as a mechanism for inherited ventricular arrhythmias in german shepherd dogs. J Am Coll Cardiol. 1996;27:1526–1533. doi: 10.1016/0735-1097(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.H., Xie F., Yashima M. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation. 1999;100:1450–1459. doi: 10.1161/01.cir.100.13.1450. [DOI] [PubMed] [Google Scholar]
- 7.Mérot J., Probst V., Debailleul M. Electropharmacological characterization of cardiac repolarization in german shepherd dogs with an inherited syndrome of sudden death: Abnormal response to potassium channel blockers. J Am Coll Cardiol. 2000;36:939–947. doi: 10.1016/s0735-1097(00)00811-1. [DOI] [PubMed] [Google Scholar]
- 8.Nogami A. Purkinje-related arrhythmias part ii: Polymorphic ventricular tachycardia and ventricular fibrillation. Pacing Clin Electrophysiol. 2011;34:1034–1049. doi: 10.1111/j.1540-8159.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- 9.Antzelevitch C., Yan G.X. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheinman M.M. Role of the his-purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm. 2009;6:1050–1058. doi: 10.1016/j.hrthm.2009.03.011. [DOI] [PubMed] [Google Scholar]