Abstract
Currently available commercial vaccines against porcine circovirus strain 2 (PCV2) solely target the PCV2a genotype. While PCV2 vaccines are highly effective in preventing clinical signs, PCV2b has dominated over the PCV2a genotype in prevalence, corresponding with the introduction of PCV2a vaccines. A recently emerged PCV2b recombinant with an additional amino acid in the capsid protein, designated the mutant PCV2b (mPCV2b), is cause for concern due to its increased virulence and rapid spread. The accumulation of recent evidence for the increased genetic diversity in PCV2 suggests that current vaccines against PCV2a may be inducing selection pressure and driving viral evolution. In this study, the hypothesis that differences in key immune epitopes between the PCV2a vaccine strains, a classical PCV2b strain called PCV2b 41513 obtained from a vaccine-failure case, and mPCV2b strains could promote vaccine escape was tested using immuno-informatic tools. In the major viral proteins, 9 of the 18 predicted swine leukocyte antigens (SLA) class-I epitopes, 8 of the 22 predicted SLA class-II epitopes, and 7 of the 25 predicted B cell epitopes varied between the vaccine and field strains. A majority of the substitutions in both the T- and B-cell epitopes were located in the capsid protein. Some B- and T-cell epitopes that were identified as immunogenic in the vaccine strain were not identified as epitopes in the field strains, indicating a subtle shift in the antigenic profile of the field strains. Several nonconserved epitopes had both predicted B- and T-cell functions. Therefore, substitutions in the dual epitopes could affect both arms of the immune response simultaneously, causing immune escape. Our findings support further rational design of PCV2 vaccines to increase the current threshold of protection.
Keywords: porcine circovirus strain 2 (PCV2), post-weaning multisystemic wasting syndrome (PMWS), epitope
Introduction
Porcine circovirus (PCV) is a 1.7 kb, nonenveloped virus belonging to the Circoviridae family with a circular, single-stranded DNA genome. Porcine circoviruses consist of two major types, PCV1 and PCV2. PCV1 is considered to be nonpathogenic.1 However, PCV2 is a substantial problem for the global swine industry as the cause of post-weaning multisystemic wasting syndrome (PMWS),2 which manifests as severe wasting in weaning piglets. With time, several other disease manifestations, which include cutaneous, reproductive, and respiratory signs, have emerged and are now collectively known as porcine circovirus-associated disease (PCVAD).3–5
The circular genome of PCV2 contains three major open reading frames: ORF1, ORF2, and ORF3. ORF1 encodes the replicase proteins that are necessary for the replication of the virus. The sequence for the binding sites of Rep and Rep’ are located within the origin of replication.6 ORF2 encodes the capsid protein that is responsible for viral structure and protective immunity. Thus, ORF2 is often used as a phylogenic and epidemiological marker for PCV2.7 ORF3, while not essential for replication, has been found to have a role in apoptotic activity and may potentially regulate virulence.8,9 Recently, ORF 4 was discovered within an overlapping region of the ORF3. Experimental analysis has proposed that ORF4 plays a role in suppressing caspase activity as well as regulating the production of CD4+ and CD8+ T cells.10
There are two major subtypes of PCV2 that are commonly prevalent in swine: PCV2a and PCV2b.11,12 From the late 1990s to about 2006, PCV2a was predominant in the United States, until commercial vaccines against PCV2 were introduced in 2006. All of the current vaccines contain the PCV2a capsid protein as the primary immunogen. Corresponding with the introduction of the PCV2a vaccines, there was a global shift in the prevalence of genotypes from PCV2a to PCV2b, associated with severe clinical manifestations in vaccinated herds.13 PCV2b is now the predominant subtype all over the world.14–16 It is well recognized that coinfections with other pathogens such as swine influenza virus and the porcine reproductive and respiratory syndrome virus exacerbate PCVAD.17 Additionally, over 90% of farmed swine are coinfected with both PCV2a and b subtypes.18,19 Coinfection can promote homologous recombination between PCV2a and PCV2b strains.20 Mutation also plays a role in viral evolution. Mutated forms of the viral antigens, including extension of the capsid by one or two amino acids, have been described.7 PCV2 has evolved rapidly since its discovery. Viral variants that are composed of recombined genomes containing new mutations have increased the probability of altered immunogenicity.21–25 The recent emergence of a virulent recombinant form of PCV2b with an additional amino acid in the C terminus of the capsid protein, called the mutant PCV2b (mPCV2b), is of additional concern, as it was isolated from vaccine-failure cases all over the world.26–29 Similar to the previous type-switching event with the classical PCV2b, the new variant is spreading rapidly and globally, and is believed to be a new subtype that could be designated as PCV2d.30
Accumulating evidence points toward the increased virulence of the emerging subtypes and vaccine-induced selection pressure in driving viral evolution. However, experimental evidence for the same remains variable,26,31,32 possibly due to the limitations of experimental models in reproducing virulence. The demonstrated cross-protection induced by PCV2a vaccines indicates that the commonality in shared epitopes between PCV2a and PCV2b allows protection against clinical signs induced by both subtypes. However, it is also likely that suboptimal immunity against subtype-specific epitopes could induce selection pressure and account for the recent acceleration in PCV2’s genetic diversification. The sensitivity of current serological methods precludes the detection of epitope-specific immunity. Continued evolution of PCV2 could allow the evasion of vaccine immunity as well as increase in virulence, resulting in economic losses to the pork industry.
To determine whether subtle changes to T- or B-cell epitopes could cause vaccine escape, we have predicted and compared putative antigenic epitopes in the PCV2a vaccine strain, a classical PCV2b strain isolated and characterized in this study, as well as the newly recognized mPCV2 strains, using immuno-informatic tools. We have also compared our findings with published information about experimentally validated PCV2 epitopes. We expect that our findings will advance PCV2 vaccine design at the epitope, rather than protein, level.
Materials and Methods
Clinical Samples
Lung and liver samples were obtained from two piglets from a case of PCV2 vaccine failure, which was submitted to the North Dakota State Veterinary Diagnostic Laboratory. All samples were confirmed as PCV2 positive by immunohistochemistry and polymerase chain reaction (PCR) using standard diagnostic lab protocols. Samples were processed and stored at −80 °C until needed.
Infectious clone
Genomic DNA was extracted from the lung tissue of one piglet using the QIAamp DNA mini kit (Qiagen) following the manufacturer’s protocol. PCV2 genomic DNA was amplified and cloned as previously described.33 Briefly, the forward primer (5′- GAACCGCGGGCTGGCTGAACTTTTGAAAGT-3′) and the reverse primer (5′-GCACCGCGGAAATTTCTGACAAACGTTACA-3′) with flanking unique SacII restriction sites were used to amplify the PCV2 genome with a product of size 1.7 kb. The PCR protocol consisted of an initial denaturation step at 95 °C for two minutes, followed by denaturation at 95 °C for 30 seconds, annealing at 50 °C for 30 seconds, extension at 72 °C for two minutes, and final extension at 72 °C for 10 minutes. The PCR product was purified using QIAamp gel extraction kit (Qiagen), subjected to restriction digestion by the Sac-II enzyme (New England Biolabs), and ligated into a commercial plasmid pBluescript SK II+ (Agilent Technologies). Onegenomic clone was sequenced and identified as a classical PCV2b strain. It was named as PCV2b 41513. Sequences were assembled using the Lasergene 9 suite (DNASTAR Inc.). The sequence information was deposited in GenBank with the accession number KR816332. The cloned genome was extracted from pBlueScript SK II+ and self-ligated at 37 °C for 7 minutes to form tandem dimers. The dimers were ligated into pBluescript SK II+ and used for transfection.33
Rescue and immunoreactivity of the infectious clone
The dimerized infectious clones were used to transfect PK15 N (PK15 cells free of PCV1 contamination) (NVSL labs). Liopfectamine LTX (Life technologies) was used for transfections, according to the manufacturer’s instructions. After 24 hours of incubation at 37 °C in a CO2 incubator, the cells sheets were fixed using a mixture of methanol/acetone in the ratio of 1:1. The immunoreactivity of the cloned PCV2b capsid protein to anti-PCV2a anti-serum was verified by an indirect immunofluorescence assay (IFA). The fixed cell sheets were stained with polyclonal and swine anti-PCV2a serum from experimentally infected pigs,34 followed by an FITC-conjugated anti-swine IgG secondary antibody (KPL) and counter-stained with DAPI (Life technologies). The stained cells were viewed using a fluorescent microscope for evidence of PCV2 infection.
Phylogenetic analysis
Pairwise comparisons of the PCV2b 41513 isolate with a prototype PCV2b strain NC 16845 (EU340258.1)31 and a PCV2a strain representing the vaccine strain PCV2a 40895 (AF264042.1)33 were carried out using the Clustal-W program. The Jalview program and Clustal web service35 was used for multiple sequence alignment. For phylogenetic analysis, ORFs 1, 2, and 3 of PCV2b 41513 were aligned against representative sequences of PCV2a, PCV2b, and mPCV2b strains obtained from GenBank. The MegAlign Pro software (DNASTAR Inc.) of the LaserGene core suite 9.0 was used to generate phylogenetic trees by the neighbor-joining method. Corresponding sequences from PCV1 were used as the “outgroup”.
Prediction of swine leukocyte class I (SLA Class-I) epitopes
The NetMHCpan server version 2.836,37 was used to predict SLA Class-I epitopes in PCV2’s ORF1, 2, and 3 proteins. Alleles SLA1:-0401, SLA2:-0401, and SLA3:-0401 were selected for analysis, as they represent the most common SLA class-I haplotypes present in the United States.38 Prediction results for 9 mer epitopes were ranked according to binding affinity, with the cutoff for a strong binder being a% rank of 0.5 or an IC50 of 50. The cutoff for weak binders was set at the default value of a% rank of 2 or IC50 of 500. Strong binding peptides with a score of 0.5 or less are listed in Table 2. PCV2a strain 40895 (AF264042.1) was used to represent the vaccine strain for comparison of changes to the predicted epitopes in the field strain PCV2b 41513 (KR816332). The mPCV2b strain was represented by GenBank accession number JX535297.
Table 2.
Identification of predicted conserved and variable MHC-I epitopes in the major PCV2 proteins.
| ALLELE | POSITION | VACCINE STRAIN PCVa AF264042.1 | PCV2b 41513 KR816332 | mPCV2b JX535297 |
|---|---|---|---|---|
| ORF1 | ||||
| SLA-1:0401 | 87 | GTDQQNKEY | ||
| 186 | FADPETTYW | |||
| SLA-2:0401 | 67 | QTFNKVKWY | ||
| SLA-3:0401 | 274 | YRRIFLVF | YRRITSLVF | YRRITSLVF |
| 275 | RRITFLVFW | RRITSLVFW | RRITSLVFW | |
| ORF2 | ||||
| SLA-1:0401 | 132 | ATALTYDPY | ATALTYDPY | ANALTYDPYa |
| 165 | VLDSTIDYF | VLDSTIDYF | VLDRTIDYF | |
| 198 | GTAFENSIY | |||
| SLA-2:0401 | 129 | VTKATALTY | VTKATALTY | VTKANALTYa |
| 132 | ATALTYDPY | ATALTYDPY | ANALTYDPY | |
| 147 | HTIPQPFSY | HTITQPFSY | HTITQPFSY | |
| SLA-3:0401 | 46 | TRLSRTFGY | TRLSRTFGY | TRLSRTIGYa |
| 48 | LSRTFGYTV | LSRTFGYTI | LSRTIGYTVb | |
| 58 | ATTVTRPSWb | RTTVRTPSW | KTTVRTPSWa | |
| 145 | SRHTIPQPF | SRHTITPF | SRHTITQPF | |
| 178 | KRNQLWMRL | |||
| ORF3 | ||||
| SLA-3:0401 | 86 | SRQVTPLSL | SRQVTPLSL | SREVTPLSLb |
| 95 | RSRSSTFNK | RSRSTLNQ@ | RSRSSTFYQb | |
Notes:
Weak binders with a% greater than 0.5 and less than 2.0.
Not identified −% rank greater than 2.0.
Prediction of swine leukocyte class II (SLA Class-II) epitopes
As SLA class-II prediction servers for swine are not available, potential epitopes were predicted using the PROPRED MHC II prediction server for all the 51 DRB1 or DRB 5 alleles listed on the server.39 The predicted SLA class-II epitopes in ORF1, 2, and 3 of the field strains were compared to the vaccine strain (accession numbers as described above) to determine sequence differences that could lead to vaccine escape. A default threshold setting of 3 was used to reduce the chances of obtaining false positives. Results were narrowed by default to peptides to the top 10% scored peptides. Anchor residues M, F, I, L, V, W, and Y, which are essential at the first position for high-affinity binding,40 are identified by small letters in Table 3.
Table 3.
Identification of predicted conserved and variable MHC-II epitopes in the major PCV2 proteins.
| START | VACCINE STRAIN PCV2a AF26 4 042.1a | PCV2b 41513 KR816332a | mPCV2b JX535297a |
|---|---|---|---|
| ORF1 | |||
| 17 | wVFTLNNPS | ||
| 37 | iSLFDYFIVV | ||
| 64 | fVKKQTFNKvKWYIGARCHIEE | ||
| 143 | fvRNfRGLAELIKVSGKMQK | ||
| 206 | yHGEEVvVIDDFUGW | ||
| 217 | fYGWLPWDDIIRLCDRYPL | ||
| 246 | fLARSIIITSNQTPLEwYSSTAVPA | ||
| 274 | lyRRITFLVfWKNATEQS | ||
| ORF2 | |||
| 8 | yRRRRHRPR | ||
| 22 | iIRRRPWLvHPRHR yRwRRKNGI | ||
| 45 | fNTRSLSRTF | fNTRLSRTF | fNTRLSRTI |
| 74 | fNIDDFVPP | fNINAFLPP | fNINDFLPP |
| 95 | yYRIRKVKVE | ||
| 123 | vILDDNFVT | ||
| 141 | yVNYSSRHT | ||
| 161 | fTPKPVLDS | fTPKPVLDS | fTPKPVLDR |
| 174 | fQPNNKKRQI wmRLQTSRNV | fQPNNKRNQL wIRLQTAGNV | yFQPNNKRNQL wIRLQTTGNV |
| 211 | yNIRVTMYVqf refNLKDPPLK | yNIRVTMYVqf refNLKDPPLN | yNIRITMYVqf refNLKDPPLN |
| ORF3 | |||
| 1 | mVTIPPIvSRWfPVCGFRVCKISSP | ||
| 40 | iGLPITLLHFPAH fQKFSQPAE | iSLPITPLHFPAH fQKFSQPAE | iRLPITLLHFPAH fQKFSQPAE |
| 67 | yRVllcngh | ||
| 90 | vTPISIRSRSSTFN | vTPISLIRSRSSTLH | vTPISIRSRSSTFY |
Note:
Anchor residues that are essential for high affinity binding are represented in small letters.
Prediction of B cell epitopes
To determine whether possible changes in the B-cell epitopes of the field strains could lead to immune escape, the Kolaskar and Tongaonkar antigenicity index tool from the Immune Epitope Database (IEDB) was used to predict and compare potential B-cell epitopes.41 Since antibody responses to ORF3 have not been detected in infected animals thus far, the B-cell epitope analysis was confined to ORF1 and 2 of the three selected PCV2 subtypes (accession numbers as described above).
Experimentally validated epitopes
Published biomedical literature on PCV2 epitopes was mined for information about experimentally validated PCV2 epitopes using the NCBI PubMed database and the Boolean search terms “PCV2” and “epitope”. Findings from published studies were compared to the immuno-informatic predictions to discuss the validity and functionality of the predictions.
Results
Phenotypic characterization of the PCV2b 41513 field isolate
Recombinant PCV2 virus was successfully rescued upon transfection of PK-15 cells with the infectious clone (Fig. 1). As expected, the rescued virus was clearly detected using anti-serum from pigs infected with a PCV2a strain,34 indicating a strong cross-reactivity with vaccine-induced antibodies.
Figure 1.
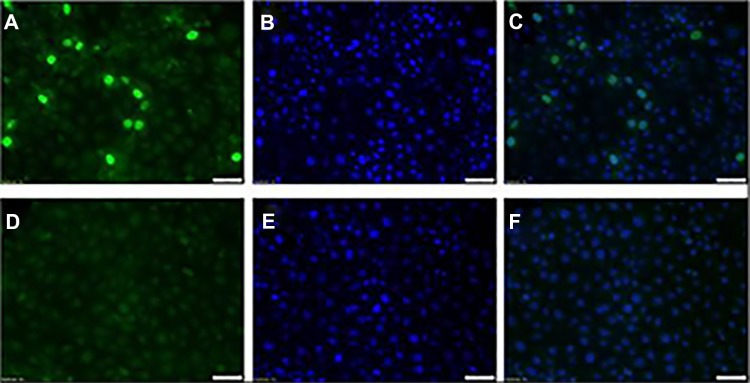
Indirect Immunofluorescence (IFA) image of the rescued PCV2b 41513 field virus. PK-15 N cells transfected with the dimerized infectious clones of PCV2b 41513. Cells were stained at 24 hours post transfection with anti-sera from swine infected with PCV2a strain and detected by an FITC-tagged secondary anti-swine IgG antibody. The nuclei were counter-stained with DAPI (blue). (A) Transfected PK-15 cells stained with an FITC-tagged secondary anti-swine IgG antibody. (B) Transfected PK-15 cells stained with DAPI (C) Overlay of (A) and (B). (D) Untransfected PK-15 cells stained with an FITC-tagged secondary anti-swine IgG antibody. (E) Untransfected PK-15 cells stained with DAPI. (F) Overlay of (D) and (E). Positive cells show an apple green nuclear florescence, typical of PCV2.
Genotypic characterization of the PCV2b 41513 field isolate
To determine whether the field strain isolated from the vaccine failure case was unique, its genetic and amino acid sequences were compared to the vaccine strain PCV2a 40895 and a classical prototype PCV2b strain. The field strain PCV2b 41513 shared a total genome identity of 95.7% with the PCV2a vaccine strain. The genome length of the PCV2b strain (1767 bp) was 1 bp shorter than that of the PCV2a vaccine isolate.11 ORF1 was 98.73% similar, and the ORF2 showed the greatest number of changes, with a nucleotide identity of 93.13% and 16 amino acid changes, some of which were in the previously identified immunogenic regions.42,43 ORF3 differed by seven nucleotides and six amino acids including the C-terminal amino acids PNK 102–104 LHQ. ORF4s differed by two bases and one amino acid C34Y (Table 1).
Table 1.
Pairwise comparison of percentage nucleotide and amino acid identity.
| ORF | PCV1c | 40895-PCV2aa | 16845-PCV2bb |
|---|---|---|---|
| A. Nucleotide sequence comparison | |||
| NDSU 41513-ORF1 | 82.95% | 97.88% | 99.68% |
| NDSU 41513-ORF2 | 67.52% | 92.31% | 99.57% |
| NDSU 41513-ORF3 | 80.95% | 9 8.10% | 100.00% |
| NDSU 41513-ORF4 | 86.67% | 98.33% | 100.00% |
| B. Amino acid sequence comparison | |||
| NDSU 41513-ORF1 | 82.80% | 98.73% | 99.36% |
| NDSU 41513-ORF2 | 66.67% | 93.13% | 99.14% |
| NDSU 41513-ORF3 | 80.95% | 97.12% | 100.00% |
| NDSU 41513-ORF4 | 86.67% | 98.31% | 100.00% |
Notes:
Representative vaccine strain PCV2a 40895 (AF264042.1).
Prototype PCV2b field strain (EU340258.1).
When compared to the prototype PCV2b 16845 sequence, the overall nucleotide identity was 99.7% with three and two point mutations in the ORF2 and ORF1 genes, respectively. The changes to the ORF2 gene resulted in two amino acid changes, D79A and E210G, while in ORF1 the change was manifested as N41D. Changes to the nucleotide sequences of ORF3 and ORF4 did not result in amino acid changes (Table 1). Therefore, the PCV2b 41513 isolate was largely comparable to circulating classical PCV2b strains.
Identification of variable amino acid residues
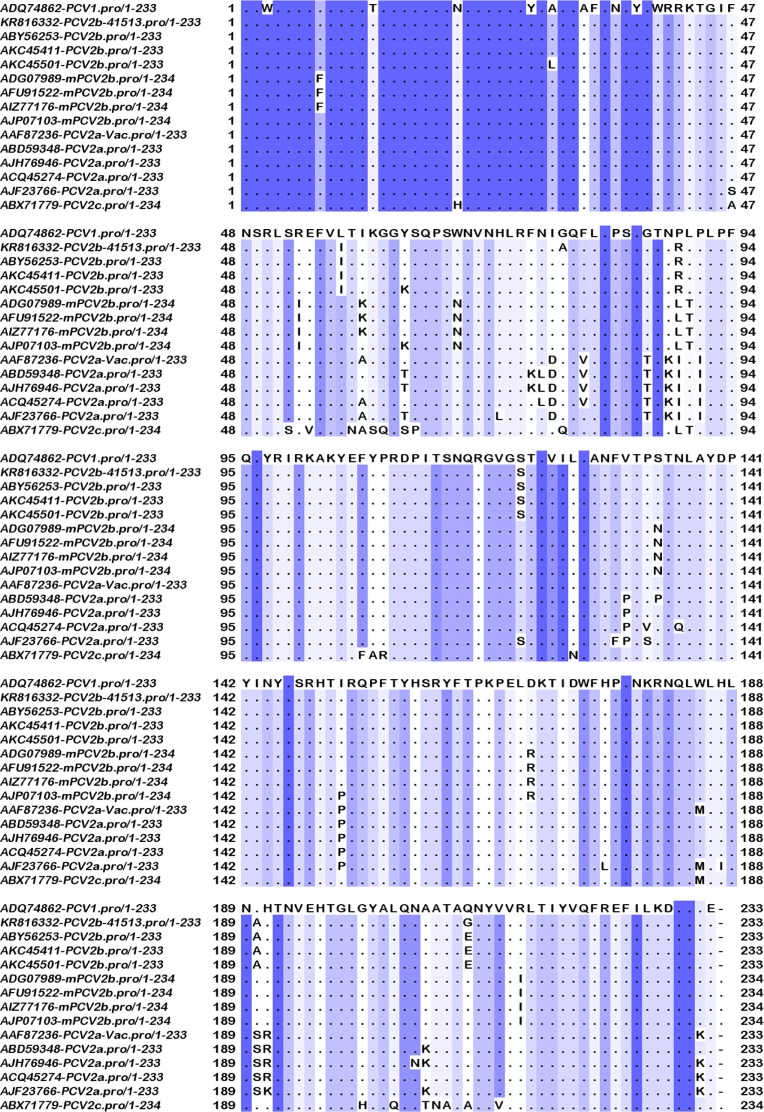
Amino acid sequence differences in the capsid proteins of the three subtypes indicated that substitutions were present in all the four experimentally characterized major immunogenic regions.43,44 Conserved residues, which are likely responsible for cross-protection, were also identified. Differences between the vaccine strain and the classical PCV2b strains were present at residues S57I, I89R, T121S, S190A, and K232 N. The PCV2b 41513 isolate had two unique changes at residues D80A and E210G, which distinguished it from all the other strains examined. While change at residue 80 was located in the first immunogenic region, the change at residue 210 was not, although it was adjacent to predicted SLA class-II and B-cell epitopes (Tables 3 and 4). Differences between the vaccine strain and the newly evolved mPCVb strain (Fig. 2) were present at residues F55I, A68N, I89L, S90T, T134N, S169R, V215I, and the additional K at position 234.
Table 4.
Predicted differences in linear B-cell epitopes using the Kolaskar and tongaonkar antigenicity index tool.
| STARTa | VACCINE STRAIN PCV2a AF264042.1 | PCV2b 41513 KR816332 | mPCV2b JX535297 | |
|---|---|---|---|---|
| ORF1 | ||||
| 15 | KRWVFTL | |||
| 33 | RELPISLFDYFIVG | RDLPISLFDYFIVG | RELPISLFDYFIVG | |
| 56 | PHLQGFANFVKKQ | |||
| 73 | GARCHIE | |||
| 94 | KEYCSKE | |||
| 102 | NLLIECGAP | |||
| 120 | STAVSTLLESGSLVTVAEQHPVTFV | |||
| 150 | LAELLKVS | |||
| 168 | NVHVIVGPPGCG | |||
| 208 | GEEVVVIDD | |||
| 223 | WDDLLRLCDRYPLTVE | |||
| 243 | TVPFLARSILITS | |||
| 265 | STAVPAVEALYRRITFLVFWK | |||
| 298 | FVTLSPPCPEFP | |||
| ORF2 | ||||
| 16 | RSHLGQI | |||
| 26 | RPWLVHPRH | |||
| 54 | GYTVKAT | Not detected | GYTVKKT | |
| 62 | VRTPSWA | RTPSWA | VRTPSWNb | |
| 77 | DDFVPPG | NAFLPPG | NDFLPPG | |
| 90 | SIPFEYYRIRKVKVEFWPCSPI | RSVPFEYYRIRKVKVEFWPCSPI | PLTVPFEYYRIRKVKVEFWPCSPI | |
| 120 | STAVUILDDN | GSSAVILDDN | STAVILDDN | |
| 130 | VTKATALTYDPYVNYS | |||
| 147 | RHTIPQPFSYHSRYFTPKPVLDSTIDYF | ITQPFSYHSRYFTPKPVLDSTIDYF | ITQPFSYHSRYFTPKPVLDRT | |
| 193 | VDHVGLG | |||
| 212 | NIRVTMYVQFR | |||
Notes:
Start position in the vaccine strain.
Not predicted.
Figure 2.
Multiple sequence alignment of the capsid proteins. Depiction of conserved and variable residues in the capsid protein of PCV2 isolates representative of the major subtypes PCV2a, classical PCV2b, and the recently identified mutant PCV2b. The diagram was generated using Jalview 2.4 software. Conserved residues are represented by dots and a sliding blue color scale, with the darkest residues being the most conserved. The experimentally validated antigenic regions are demarcated by solid boxes.
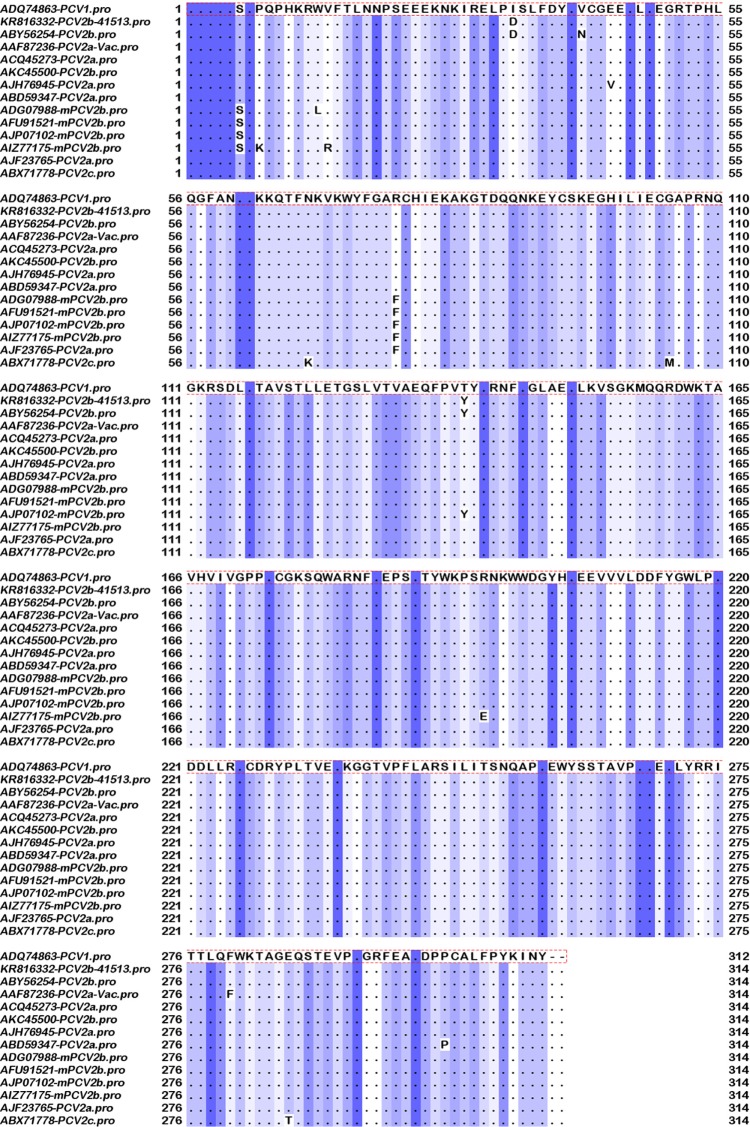
As expected, the replicase gene was largely conserved. Differences that were conserved between the all the classical PCV2b strains examined and the vaccine strains were not detected. The selected mPCVb isolates showed conserved differences at residues 6 (S) and 77 (F) when compared to the other two subtypes (Fig. 3).
Figure 3.
Multiple sequence alignment of the replicase proteins. Depiction of conserved and variable residues in the replicase protein of PCV2 isolates representative of the major subtypes PCV2a, classical PCV2b, and the recently identified mutant PCV2b, generated using Jalview 2.4 software. Conserved residues are represented by dots and a sliding blue color scale, with the darkest residues being the most conserved.
Phylogenetic analysis
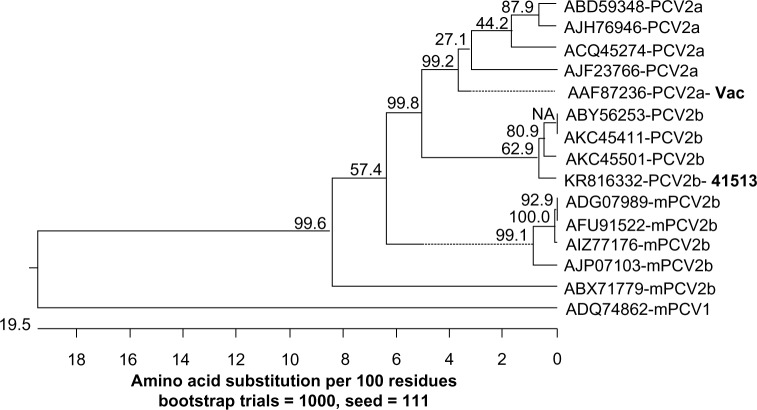
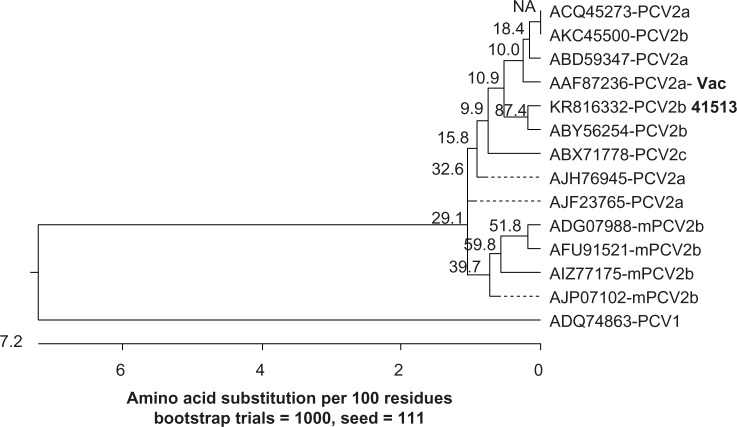
When the amino acid sequences of the capsid protein were analyzed, the PCV2b 41513 isolate clustered in the same branch as the other classical PCV2b strains. The mPCV2b strains clustered together in a distinct branch and showed the maximum distance from the vaccine PCV2a strain (Fig. 4). As expected, the analysis with the replicase protein did not show a distinct clustering of the PCV2a and classical PCV2b isolates, due to the more conserved nature of the replicase gene. However, the mPCV2b strains clustered in a separate branch (Fig. 5).
Figure 4.
Phylogenetic analysis of the PCV2 capsid protein. Amino acid sequences representative of the PCV2a vaccine strain, classical PCV2b strains, and the newly identified mutant PCV2b isolates are represented. The tree was constructed using the MegAlign software by the neighbor-joining method with 1000 bootstrap replicates. Bootstrap values are indicated above the branches. The length of the branches denotes the extent of differences between sequences. A PCV1 capsid sequence was used as the outgroup.
Figure 5.
Phylogenetic analysis of the PCV2 replicase proteins. Amino acid sequences representative of the PCV2a vaccine strain, classical PCV2b strains, and the mPCV2b strains are depicted. The tree was constructed using the MegAlign software by the neighbor-joining method with 1000 bootstrap replicates. Bootstrap values are indicated above branches. The length of the branches denotes the extent of differences between sequences. A PCV1 replicase protein sequence was used as the outgroup.
Variable SLA class-I epitopes predicted by NetMHCpan
When SLA class-I epitopes for alleles SLA1-: 0401, SLA2-: 0401 and SLA3-: 0401 were predicted for the replicase proteins of the three subtypes using the NetMHCpan tool, five strong binding peptides were identified. Two adjacent peptides at positions 274 and 275 varied from the vaccine strain by one amino acid in both the field strains, while all the other peptides were conserved (Table 2).
In ORF2, 11 strong binding peptides were predicted for the above-mentioned alleles. Only two peptides at position 178 and 198 were conserved in all three strains. Of the 11 predicted SLA class-I epitopes, 4 and 7 epitopes varied between the PCV2a vaccine strain and PCV2b 41513 or mPCV2b strain, respectively. All variations comprised single amino acid changes. Interestingly, of the seven predicted SLA class-I epitopes that varied between the vaccine strain and mPCV2b, four were identified as weak binders and one was not identified as a binder. Two peptides at position 132 and 147 were identified as binding to both SLA1-: 0401 and SLA2-: 0401 (Table 2).
In ORF3, two strong binding peptides were identified for the SLA3-:0401 allele. One of them was conserved between the PCV2b 41513 and PCV2a vaccine strains. Neither of them was conserved in the mPCV2b strain, nor were they identified as binders in the analysis of the mPCV2b ORF3 protein (Table 2).
Variable SLA class-II epitopes predicted by ProPred MHC-II
Eight, 10, and 4 SLA class-II epitopes were predicted by the Propred MHC-II server in the ORFs 1, 2, and 3 respectively; with some of them overlapping two or three other possible epitopes. In ORF1, only one of the predicted epitopes varied between the vaccine strain and the mPCV2b field strain. In ORF2, three predicted epitopes varied between the PCV2b 41513 and the vaccine strain, while five epitopes varied in the mPCV2b strain. One substitution at position 174 was conserved between the two field strains. In ORF3, the predicted SLA class-II epitopes at positions 41 and 90 varied between all three strains. The epitope at position 90 included the last three C-terminal amino acids, which vary between all the three strains (Table 3). A majority of the epitopes were highly promiscuous, as they were predicted as binding to several of the 51 alleles examined.
Variable B-cell epitopes predicted by the Kolaskar and Tongaonkar antigenicity index tool
In ORF1, 14 linear B-cell epitopes were predicted for the three PCV2 viral strains examined. Except for one amino acid change at position 34, all epitopes were conserved among the vaccine and field strains. Eleven linear epitopes were predicted in the ORF2 proteins. Four of them varied between the vaccine strain and the PCV2b 41513 strain, while five varied between the mPCV2b strain and the vaccine strain. None of the varying epitopes was conserved between the two PCV2b strains, indicating that existing immunity against the circulating classical PCV2b may not guarantee complete protection against the mPCV2b. One epitope that was present in the vaccine strain at position 54 was not predicted for the PCV2b 41513 strain, and two epitopes at positions 62 and 77 were not predicted in the mPCV2b strain ORF2 (Table 4). The positions of the amino acids that differed between the strains were plotted on the crustal structure 3R0R from the protein database (Fig. 6). Except for the residue in position 120, all other changes were located on the surface.
Figure 6.
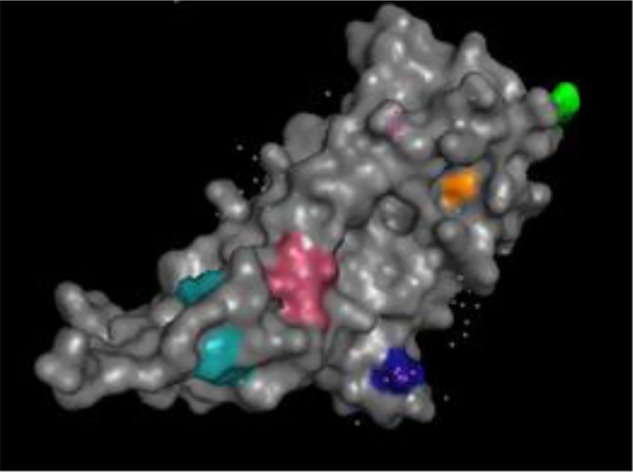
Location of amino acid changes in B-cell epitopes. The amino acids that differed between the vaccine PCV2a strain and the field strains within the predicted B-cell epitopes listed in Table 4 are indicated on the crystal structure. The diagram was generated using Pymol 1.3, and structure ID 3ROR from the protein database. Numbers indicate the positions and the color the differing residue: 54 – yellow, 62 – orange, 77 – pink, 90 – cyan, 120 – not exposed, 147 – dark blue.
Experimentally validated epitopes
Information about experimentally validated T-cell epitopes was limited to one publication45 describing two epitopes in ORF1 and one epitope in ORF3. In the study by Stevenson et al, although the authors tested several peptides from each protein, only three showed broad immunogenicity in the test system. The major immune-dominant regions depicted in Figure 2 and the epitopes within these regions are described by several authors.42–44,46,47 Additionally, one epitope was identified in the N-terminal nuclear localization signal of the ORF2,48 which does not fall under the experimentally validated immunogenic regions. Functionally, conformational neutralizing residues and epitopes have been identified in positions 59 and 60 as well as the last four C-terminal amino acids.42,44,49,50 An immunodominant decoy epitope is reported to span residues 169–18051 and hypothesized to play a role in immune subversion.
Discussion
Current commercial vaccines against PCV2 have demonstrated success in preventing clinical signs induced by both the PCV2a and b subtypes. However, PCV2 has continued to evolve rapidly after the introduction of commercial vaccines. Although the PCV2b 41513 strain described in this study was isolated from a case of vaccine failure, it was unexpectedly very similar to circulating PCV2b strains, both antigenically and genetically (Table 1). While there is a possibility that improper adherence to vaccination protocols, rather than genetic diversity, could have accounted for the failure, the mechanisms of immune escape for PCV2 or vaccine-induced selection pressure in driving viral evolution most likely involve very subtle changes to the immunogenic epitopes due to the small size of the virus. Indeed, it has been shown that even a minor change of two amino acids can result in a phenotypic change,52 prompting the detailed examination of differences in the putative B- and T-cell epitope sequences of the three circulating PCV2 subtypes in this study, with the ultimate goal of understanding the possible antigenic basis of vaccine escape.
Although the replicase and capsid proteins of the selected strains had a high level of amino acid sequence identity overall, conserved substitutions between the PCV2a vaccine strain and the field strains, which could translate to subtle antigenic changes, were predicted by the immuno-informatic tools used. ORF1 and ORF3 are well-characterized nonstructural proteins of PCV2. While there were no major amino acid sequence differences between the ORF1 of the vaccine and PCV2b 41513 strains, two conserved changes in the mPCV2b strains at positions 6 and 77 distinguished the mPCV2b strains from the circulating PCV2a and b strains and led to clustering on a separate branch on the phylogenetic tree (Figs. 3 and 5). The change at position 77 was a part of a predicted MHC-II epitope and an overlapping B-cell epitope located close to an experimentally validated T-cell epitope at position 81.45 While further experimental work is required to determine the significance of this change, it could be speculated that it plays a role in the increased virulence or immune escape of the mPCV2b. Since antibody responses to ORF3 have not been detected in infected animals so far, B-cell epitope analysis for ORF3 has not been carried out. However, overlapping MHC-I and MHC-II epitopes were predicted at the C-terminal amino acids of ORF3 (Tables 2 and 3). These epitopes vary between the three subtypes and could therefore play a role in virulence and T-cell-mediated immunity. Moreover, a predicted MHC-II epitope in the ORF3 at position 40 corresponded to an experimentally validated epitope.45 The role of ORF1 and 3 in inducing protective immunity against PCV2 is not well characterized. Attempts to map T-cell epitopes in the three major ORFs revealed that only three peptides in ORF1 or 3 were immunodominant in a majority of the animals from which primed peripheral blood mononuclear cells (PBMCs) were derived for the analysis.45 The lack of reactivity can be justified by the genetic diversity of conventional outbred pigs as well as by the fact that PCV2 is largely immunosuppressive.4 However, based on our in silico analysis, ORF1 and 3 likely influence the T-cell-mediated immune response to PCV2 and should be considered in vaccine design.
Viruses commonly achieve increased fitness due to beneficial mutations in their receptor binding sites.53 The putative receptor binding site of the PCV2 capsid protein is believed to be located between residues 99 and 104.54 However, the strict conservation of the putative receptor binding site between the three subtypes examined in this study precludes the possibility that the newly emerging PCV2 variants achieve increased fitness due to positive selection in their receptor binding site. As expected, several conserved differences in sequence were detected between the vaccine strain and the field strains in the capsid protein sequences (Fig. 2). Of the four conserved differences between the vaccine strain and the PCV2b 41513 strain detected in ORF2, three were located in the experimentally validated immunogenic regions. A motif of six amino acids in length, which distinguishes the PCV2 subtypes, is located at positions 86 to 91.11 However, the motif region has not been experimentally recognized as immunogenic thus far, except for the last three residues,55 but could form as-yet-unidentified conformational epitopes. The motif region was predicted as a possible B-cell epitope in our analysis (Table 4). One conserved change at position 89 also overlapped a predicted SLA class-II epitope. Interestingly, the epitope including residue 89 was not recognized as immunogenic in mPCV2b by the prediction algorithm, indicating a possible change in the antigenicity of mPCV2b (Table 3) when compared to the vaccine and classical PCV2b strains.
Although computational prediction of B-cell epitopes is known to be less reliable than that of T-cell epitopes,56 the Kolaskar and Tongaonkar antigenicity index tool is reported to have a 70% accuracy in prediction, as it is trained using experimentally validated epitopes.41 Of the eight conserved differences in mPCV2b ORF2 when compared to the vaccine, strain 6 localized to the experimentally validated immunogenic regions and epitopes (Fig. 2, Table 5). Overlapping and nonconserved MHC-I, MHC-II, and B epitopes were predicted at residues 53, 134, and 169 for the mPCV2b ORF2. The differences could affect both B- and T-cell immunity against the field strains and promote immune escape. The last four amino acids of ORF2 have been experimentally validated as neutralizing residues42,44 and differ among all three subtypes. In fact, the additional K at the C terminus of the mPCV2b strain is hypothesized to play a role in its increased virulence.26,57 However, the C-terminal amino acids were not predicted as immunogenic by the Kolaskar and Tongaonkar antigenicity index tool, nor were they predicted to contain T-cell epitopes. A decoy B-cell epitope located at position 169–18051 was completely conserved in all the three subtypes, and hence is probably not involved in differential immunity.
Table 5.
Experimentally validated B- and T-cell epitopes.
| ORF | POSITION | SEQUENCE | REFERENCE |
|---|---|---|---|
| B-cell epitopes | |||
| 1 | 39–46 | LDFDFIVG | 46 |
| 1 | 99–106 | KEGNLLIE | 46 |
| 2 | 69–83 | VDMMRFNINDFLPPG | 43 |
| 2 | 113–127 | QGDRGVGSSAVILDD | 43,47 |
| 2 | 169–183 | FTIDYFWPNNKRNQL | 43 |
| 2 | 169–180 | STIDYFQPNNKRa | 51 |
| 2 | 156–162 | YHSRYFT | 44 |
| 2 | 175–192 | QPNNKRNQLWLRLQTAGN | 44,42 |
| 2 | 195–202 | HVGLGTAF | 44 |
| 2 | 231–233 | LNPb | 44 |
| 2 | 62–73 | VRTPSWAVDMMR | 55 |
| 2 | 79–84 | FLPPGG | 55 |
| 2 | 86–93 | SNPRSVPF | 55 |
| 2 | 102–107 | KVEFWP | 55 |
| 2 | 119–128 | GSSXXXLDDN | 55 |
| 2 | 229–223 | PPLNPb | 55,42 |
| 2 | 59,60 | A/R, A/Tc | 49,50,42 |
| 2 | 26–36 | RPWLVHPRHRY | 48 |
| T-cell epitopes | |||
| 1 | 81–200 | CHIEKAKGTDQQNKEYCSKE | 45 |
| 1 | 201–220 | KWWDGYHGEEVVVIDDFYGW | 45 |
| 3 | 31–50 | PRWPHNDVYIGLPITLIHFP | 45 |
Notes:
Decoy epitope.
Neutralizing epitope.
Critical residues in a type-specific, neutralizing, conformational epitope.
Based on the analysis with monoclonal antibodies, residue 59 is believed to form a conformational epitope, which confers type-specific neutralizing properties.49,50 Multiple sequence alignment confirmed that residue 59 was substituted from an A to R in classical PCV2b strains when compared to the vaccine strains and from an A to K in mPCV2b strains (Fig. 2). Hence, it is very likely that the substitution at residue 59 plays an important role in antibody-mediated immune escape. Residue 59 was also predicted as part of a linear B-cell epitope (Table 4, Fig. 6). However, the corresponding epitope was not predicted as immunogenic for the mPCV2b. Similarly, other MHC-I and B-cell epitopes, which were predicted to be immunogenic in the vaccine strain, were either identified as weak binders or nonbinders in the field strains (Tables 2 and 4), especially for the mPCV2b, indicating subtle changes in antigenicity of the mPCV2b. Over 95% of conventional swine are infected with the classical PCV2b and very likely have immunity against it.16 However, based on our in silico analysis, the number of subtle differences predicted between the T- and B-cell epitopes of the classical PCV2b and mPCV2b (Tables 2–4) suggest that natural immunity against PCV2b is unlikely to completely protect against the mPCV2b. Indeed, the recent and rapid spread of the mPCV2b30 lends credence to our findings. The importance of the capsid protein in inducing protective antibody responses is generally well accepted. However, our in silico analysis suggests that ORF2 plays an equally important role in differential T-cell-mediated immunity due to the sequence diversity and presence of predicted T-cell epitopes (Tables 2 and 3).
While the limitations of immuno-informatic tools for epitope analysis are acknowledged, it is not within the scope of this study to examine specific weaknesses that may prevent obtaining a highly correlated experimental validation, should such an analysis be attempted. The lack of freely available SLA class-II epitope prediction tools is an additional limitation of this study. However, it is known that the SLA class-II and HLA-II regions are very similarly organized. SLA class-II genes exhibit a higher homology to their HLA counterparts than with other SLA class-II genes.58,59 Experimental validation is key to verifying that the predicted findings can translate into practice and also to improve current computational methods. However, our findings emphasize the importance of computational vaccinology in reducing the lead development time by detecting strain-specific differences, which can aid in the development of universal vaccine technologies for genetically diverse pathogens.60
Conclusions
The wide-spread deployment of commercial vaccines after the discovery of PCV2 is associated with the proportionally increasing genetic diversity of PCV2, all over the world. Our analysis of the antigenic basis for possible immune escape from vaccine-derived immunity by the predominant circulating PCV2b subtypes showed three major trends. The first was the presence of conserved single amino acid substitutions in the predicted and experimentally validated immune epitopes, which could lead to partial immune derecognition in vaccine-primed animals. Secondly, some predicted immunodominant T- and B-cell epitopes in the vaccine strain were not identified as epitopes in the field strains by the immuno-informatic tools, indicating a difference in their antigenicity or binding affinity to receptors, which, in turn, would affect the quality of the downstream immune cascade. The third was the presence of several epitopes with overlapping B- and T-cell functions, wherein changes in sequence could cause possible deleterious effects on protective immunity in both the cell-mediated and humoral arms of the host immune response. In conclusion, our findings support the rational design of next-generation PCV2 vaccines at the epitope level.
Acknowledgments
We thank Dr. Anuradha Vegi and Dr. Jeba J. Chelladurai for technical help.
Footnotes
ACADEMIC EDITOR: J.T. Efird, Associate Editor
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,506 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the USDA National Institute of Food and Agriculture (NIFA), Agriculture and Food Research Initiative (AFRI), competitive grant number 2015-67016-23318, under USDA project ND02427 and the research N. Dakota Venture Awards. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. all editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the committee on Publication Ethics (COPE).
Author contributions
Conceived and designed the experiments: SR. Analyzed the data: MC, SR, MS, OK. Wrote the first draft of the manuscript: SR. Contributed to the writing of the manuscript: MC, MS, OK. Agree with manuscript results and conclusions: MC, MS, OK, SR. Jointly developed the structure and arguments for the paper: MC, MS, OK, SR. Made critical revisions and approved final version: SR. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Tischer I, Rasch R, Tochtermann G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol Orig A. 1974;226:153–67. [PubMed] [Google Scholar]
- 2.Harding JC. The clinical expression and emergence of porcine circovirus 2. Vet Microbiol. 2004;98:131–5. doi: 10.1016/j.vetmic.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Ellis J. Porcine circovirus: a historical perspective. Vet Pathol. 2014;51:315–27. doi: 10.1177/0300985814521245. [DOI] [PubMed] [Google Scholar]
- 4.Ramamoorthy S, Meng XJ. Porcine circoviruses: a minuscule yet mammoth paradox. Anim Health Res Rev. 2009;10:1–20. doi: 10.1017/S1466252308001461. [DOI] [PubMed] [Google Scholar]
- 5.Segales J, Kekarainen T, Cortey M. The natural history of porcine circovirus type 2: from an inoffensive virus to a devastating swine disease? Vet Microbiol. 2013;165:13–20. doi: 10.1016/j.vetmic.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK. Comparative analysis of the transcriptional patterns of pathogenic and nonpathogenic porcine circoviruses. Virology. 2003;310:41–9. doi: 10.1016/s0042-6822(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 7.Olvera A, Cortey M, Segales J. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology. 2007;357:175–85. doi: 10.1016/j.virol.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Chen I, Kwang J. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J Virol. 2005;79:8262–74. doi: 10.1128/JVI.79.13.8262-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Zhu Y, Chen I, et al. The ORF3 protein of porcine circovirus type 2 interacts with porcine ubiquitin E3 ligase Pirh2 and facilitates p53 expression in viral infection. J Virol. 2007;81:9560–7. doi: 10.1128/JVI.00681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Cao J, Zhou N, Jin Y, Wu J, Zhou J. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J Virol. 2013;87:1420–9. doi: 10.1128/JVI.01443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AK, Lager KM, Kohutyuk OI, et al. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol. 2007;152:1035–44. doi: 10.1007/s00705-006-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen H-G, Zhou J-Y, Haung Z-Y, et al. Protective immunity against porcine circovirus 2 by vaccination with ORF2-based DNA and subunit vaccines in mice. J Gen Virol. 2008;89:1857–65. doi: 10.1099/vir.0.2008/000125-0. [DOI] [PubMed] [Google Scholar]
- 13.Carman S, Cai HY, DeLay J, et al. The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease–2004–2006. Can J Vet Res. 2008;72:259–68. [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont K, Nielsen EO, Baekbo P, Larsen LE. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case-control study supports a shift in genotypes with time. Vet Microbiol. 2008;128:56–64. doi: 10.1016/j.vetmic.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Wang X, Ma T, Feng Z, Li Y, Jiang P. Genetic analysis of porcine circovirus type 2 (PCV2) strains isolated between 2001 and 2009: genotype PCV2b predominate in postweaning multisystemic wasting syndrome occurrences in eastern China. Virus Genes. 2010;40:244–51. doi: 10.1007/s11262-009-0438-y. [DOI] [PubMed] [Google Scholar]
- 16.Shen HG, Halbur PG, Opriessnig T. Prevalence and phylogenetic analysis of the current porcine circovirus 2 genotypes after implementation of widespread vaccination programmes in the USA. J Gen Virol. 2012;93:1345–55. doi: 10.1099/vir.0.039552-0. [DOI] [PubMed] [Google Scholar]
- 17.Rammohan L, Xue L, Wang C, Chittick W, Ganesan S, Ramamoorthy S. Increased prevalence of torque teno viruses in porcine respiratory disease complex affected pigs. Vet Microbiol. 2012;157:61–8. doi: 10.1016/j.vetmic.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Firth C, Charleston MA, Duffy S, Shapiro B, Holmes EC. Insights into the evolutionary history of an emerging livestock pathogen: porcine circovirus 2. J Virol. 2009;83:12813–21. doi: 10.1128/JVI.01719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaiseb S, Sydler T, Zimmermann D, Pospischil A, Sidler X, Brugnera E. Coreplication of the major genotype group members of porcine circovirus type 2 as a prerequisite to coevolution may explain the variable disease manifestations. J Virol. 2011;85:11111–20. doi: 10.1128/JVI.05156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung AK. Homologous recombination within the capsid gene of porcine circovirus type 2 subgroup viruses via natural co-infection. Arch Virol. 2009;154:531–4. doi: 10.1007/s00705-009-0329-5. [DOI] [PubMed] [Google Scholar]
- 21.Cai L, Han X, Hu D, et al. A novel porcine circovirus type 2a strain, 10JS-2, with eleven-nucleotide insertions in the origin of genome replication. J Virol. 2012;86:7017. doi: 10.1128/JVI.00760-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai L, Ni J, Xia Y, et al. Identification of an emerging recombinant cluster in porcine circovirus type 2. Virus Res. 2012;165:95–102. doi: 10.1016/j.virusres.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Shao M, Xu X, et al. Evidence for different patterns of natural inter-genotype recombination between two PCV2 parental strains in the field. Virus Res. 2013;175:78–86. doi: 10.1016/j.virusres.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Ramos N, Mirazo S, Castro G, Arbiza J. Molecular analysis of porcine circovirus type 2 strains from Uruguay: evidence for natural occurring recombination. Infect Genet Evol. 2013;19:23–31. doi: 10.1016/j.meegid.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Wen L, He K, Li B, et al. In vitro and in vivo isolation of a novel rearranged porcine circovirus type 2. J Virol. 2012;86:13120. doi: 10.1128/JVI.02392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, Fu Y, Wang Y, et al. A porcine circovirus type 2 (PCV2) mutant with 234 amino acids in capsid protein showed more virulence in vivo, compared with classical PCV2a/b strain. PLoS One. 2012;7:e41463. doi: 10.1371/journal.pone.0041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo LJ, Lu YH, Wei YW, Huang LP, Liu CM. Porcine circovirus type 2 (PCV2): genetic variation and newly emerging genotypes in China. Virol J. 2010;7:273. doi: 10.1186/1743-422X-7-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo HW, Park C, Kang I, et al. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch Virol. 2014;159:3107–11. doi: 10.1007/s00705-014-2164-6. [DOI] [PubMed] [Google Scholar]
- 29.Xiao CT, Halbur PG, Opriessnig T. Complete genome sequence of a novel porcine circovirus type 2b variant present in cases of vaccine failures in the United States. J Virol. 2012;86:12469. doi: 10.1128/JVI.02345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao CT, Halbur PG, Opriessnig T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J Gen Virol. 2015 doi: 10.1099/vir.0.000100. pii:vir.0.000100. [DOI] [PubMed] [Google Scholar]
- 31.Opriessnig T, Ramamoorthy S, Madson DM, et al. Differences in virulence among porcine circovirus type 2 isolates are unrelated to cluster type 2a or 2b and prior infection provides heterologous protection. J Gen Virol. 2008;89:2482–91. doi: 10.1099/vir.0.2008/001081-0. [DOI] [PubMed] [Google Scholar]
- 32.Opriessnig T, Xiao CT, Gerber PF, Halbur PG, Matzinger SR, Meng XJ. Mutant USA strain of porcine circovirus type 2 (mPCV2) exhibits similar virulence to the classical PCV2a and PCV2b strains in caesarean-derived, colostrum-deprived pigs. J Gen Virol. 2014;95:2495–503. doi: 10.1099/vir.0.066423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenaux M, Halbur PG, Haqshenas G, et al. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J Virol. 2002;76:541–51. doi: 10.1128/JVI.76.2.541-551.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamoorthy S, Opriessnig T, Pal N, Huang FF, Meng XJ. Effect of an interferon-stimulated response element (ISRE) mutant of porcine circovirus type 2 (PCV2) on PCV2-induced pathological lesions in a porcine reproductive and respiratory syndrome virus (PRRSV) co-infection model. Vet Microbiol. 2011;147:49–58. doi: 10.1016/j.vetmic.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–91. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoof I, Peters B, Sidney J, et al. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics. 2009;61:1–13. doi: 10.1007/s00251-008-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen M, Lundegaard C, Blicher T, et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2:e796. doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho CS, Lunney JK, Ando A, et al. Nomenclature for factors of the SLA system, update 2008. Tissue Antigens. 2009;73:307–15. doi: 10.1111/j.1399-0039.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- 39.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–7. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 40.Sturniolo T, Bono E, Ding J, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–61. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 41.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–4. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 42.Lekcharoensuk P, Morozov I, Paul PS, Thangthumniyom N, Wajjawalku W, Meng XJ. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J Virol. 2004;78:8135–45. doi: 10.1128/JVI.78.15.8135-8145.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahe D, Blanchard P, Truong C, et al. Differential recognition of ORF2 protein from type 1 and type 2 porcine circoviruses and identification of immunorelevant epitopes. J Gen Virol. 2000;81:1815–24. doi: 10.1099/0022-1317-81-7-1815. [DOI] [PubMed] [Google Scholar]
- 44.Shang SB, Jin YL, Jiang XT, et al. Fine mapping of antigenic epitopes on capsid proteins of porcine circovirus, and antigenic phenotype of porcine circovirus type 2. Mol Immunol. 2009;46:327–34. doi: 10.1016/j.molimm.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson LS, Gilpin DF, Douglas A, et al. T lymphocyte epitope mapping of porcine circovirus type 2. Viral Immunol. 2007;20:389–98. doi: 10.1089/vim.2006.0106. [DOI] [PubMed] [Google Scholar]
- 46.Meng T, Jia Q, Liu S, Karuppannan AK, Chang CC, Kwang J. Characterization and epitope mapping of monoclonal antibodies recognizing N-terminus of rep of porcine circovirus type 2. J Virol Methods. 2010;165:222–9. doi: 10.1016/j.jviromet.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 47.Truong C, Mahe D, Blanchard P, et al. Identification of an immunorelevant ORF2 epitope from porcine circovirus type 2 as a serological marker for experimental and natural infection. Arch Virol. 2001;146:1197–211. doi: 10.1007/s007050170115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo L, Lu Y, Huang L, Wei Y, Liu C. Identification of a new antigen epitope in the nuclear localization signal region of porcine circovirus type 2 capsid protein. Intervirology. 2011;54:156–63. doi: 10.1159/000319838. [DOI] [PubMed] [Google Scholar]
- 49.Huang LP, Lu YH, Wei YW, Guo LJ, Liu CM. Identification of one critical amino acid that determines a conformational neutralizing epitope in the capsid protein of porcine circovirus type 2. BMC Microbiol. 2011;11:188. doi: 10.1186/1471-2180-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Liu J, Huang L, Wei Y, et al. Amino acid mutations in the capsid protein produce novel porcine circovirus type 2 neutralizing epitopes. Vet Microbiol. 2013;165:260–7. doi: 10.1016/j.vetmic.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Trible BR, Ramirez A, Suddith A, et al. Antibody responses following vaccination versus infection in a porcine circovirus-type 2 (PCV2) disease model show distinct differences in virus neutralization and epitope recognition. Vaccine. 2012;30:4079–85. doi: 10.1016/j.vaccine.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 52.Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J Virol. 2004;78:13440–6. doi: 10.1128/JVI.78.24.13440-13446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hueffer K, Parker JS, Weichert WS, Geisel RE, Sgro JY, Parrish CR. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J Virol. 2003;77:1718–26. doi: 10.1128/JVI.77.3.1718-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J Virol. 2006;80:3487–94. doi: 10.1128/JVI.80.7.3487-3494.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge M, Yan A, Luo W, et al. Epitope screening of the PCV2 Cap protein by use of a random peptide-displayed library and polyclonal antibody. Virus Res. 2013;177:103–7. doi: 10.1016/j.virusres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Sun P, Ju H, Zhang B, et al. Conformational B-cell epitope prediction method based on antigen preprocessing and mimotopes analysis. Biomed Res Int. 2015;2015:257030. doi: 10.1155/2015/257030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Opriessnig T, Xiao CT, Gerber PF, Halbur PG. Emergence of a novel mutant PCV2b variant associated with clinical PCVAD in two vaccinated pig farms in the U.S. concurrently infected with PPV2. Vet Microbiol. 2013;163:177–83. doi: 10.1016/j.vetmic.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Lunney JK, Ho CS, Wysocki M, Smith DM. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev Comp Immunol. 2009;33:362–74. doi: 10.1016/j.dci.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Smith DM, Lunney JK, Ho CS, et al. Nomenclature for factors of the swine leukocyte antigen class II system, 2005. Tissue Antigens. 2005;66:623–39. doi: 10.1111/j.1399-0039.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 60.Seib KL, Zhao X, Rappuoli R. Developing vaccines in the era of genomics: a decade of reverse vaccinology. Clin Microbiol Infect. 2012;18(suppl 5):109–16. doi: 10.1111/j.1469-0691.2012.03939.x. [DOI] [PubMed] [Google Scholar]