Abstract
A 74-year-old woman with takotsubo cardiomyopathy developed polymorphic ventricular tachycardia during the acute phase. She exhibited prominent J-wave and T-wave alternans preceding ventricular tachycardia. These abnormalities disappeared after recovery from myocardial stunning.
Keywords: Takotsubo cardiomyopathy, QT-interval prolongation, J-wave alternans, T-wave alternans, Mechanical alternans
1. Introduction
Takotsubo cardiomyopathy (TC) is a reversible myocardial stunning condition that mimics acute myocardial infarction [1,2]. Patients with TC generally have good prognoses, but some cases are complicated by ventricular arrhythmias associated with prolongation of QT interval [3]. T-wave alternans (TWA) has been reported in patients with pathological conditions who show QT interval prolongation, including long-QT syndrome, myocardial ischemia, and electrolyte disturbances, and is closely associated with the development of ventricular arrhythmias [4].
Here, we describe a patient with TC and hypokalemia who had significant QT interval prolongation, as well as alternans of the J- and T-wave amplitudes preceding ventricular arrhythmias.
2. Case report
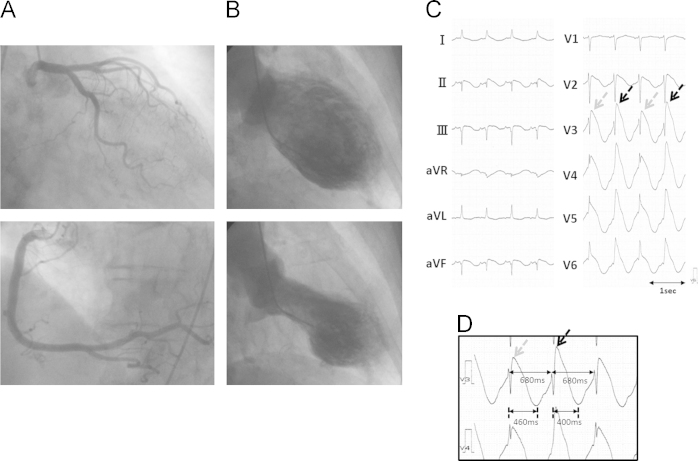
The patient was a 74-year-old woman who was transported to the emergency room of our hospital after a transient loss of consciousness. Her electrocardiogram (ECG) showed QT interval prolongation and incessant polymorphic ventricular tachycardia (VT). Blood test results revealed hypokalemia (K+=2.2 mEq/L) and cardiac enzyme elevation (troponin I=2.25 μg/mL). The patient was administered a slow infusion of magnesium and potassium. Her coronary angiography showed no significant stenosis (Fig. 1A), but ventriculography revealed left ventricular dysfunction with akinesis of the apical wall and compensatory hyperkinesis of the basal wall (Fig. 1B). We diagnosed the patient with TC.
Fig. 1.
(A) Coronary angiogram showing no significant stenosis in the major coronary artery. (B) Left ventriculography revealing akinesis of the apical left ventricle and hyperkinesis of the basal left ventricle. (C and D) ECG recorded in the catheterization room. Despite stable RR intervals (680 ms), prominent J-wave and T-wave alternans were observed. The QT interval could not be measured precisely due to overlap of the terminal T wave with the following QRS. (D) shows the ECG records in V3 and V4 with an enlarged scale. The beats with higher J-wave amplitude (black arrows) had a shorter QT peak interval (400 ms) than (460 ms) beats with lower J-wave elevation (gray arrows), resulting in longer preceding diastolic interval in the latter.
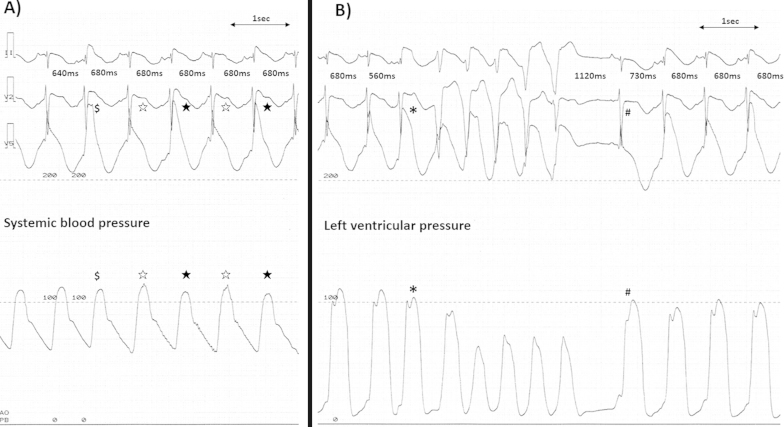
In the catheterization room, the frequency of polymorphic VT decreased and her ECG showed significant QT prolongation and beat-to-beat alternans of the J- and T-wave amplitudes with a constant RR interval (Fig. 1C). Prominent J-wave alternans was apparent in leads V2–V6, which lead positions corresponded to the area of akinetic left ventricular segments (Fig. 1C). The beats with higher J-wave amplitude had a shorter QT peak interval than the beats with lower J-wave amplitude with a longer QT peak interval (Fig. 1D). Because of the constant R–R interval with alternating QT intervals, the beats with higher J-wave amplitude had shorter preceding diastolic intervals (DIs) and those with lower J-wave amplitude had longer preceding DIs. The beats with higher J-wave amplitude induced lower systolic blood pressure than those with lower J-wave amplitude (Fig. 2A). A beat with a short coupling interval that showed higher J-point elevation and induced lower ventricular systolic pressure than the preceding beats initiated non-sustained VT (Fig. 2B). This sequence of VT initiation was repeatedly observed. After termination of VT, a beat after a prolonged DI started with a low amplitude J wave and high ventricular pressure followed by alternans of the J wave, TWA, and mechanical alternans (Fig. 2B).
Fig. 2.
(A) The polygraphs showing simultaneous ECG recordings (II, V1, and V5) and aortic pressure. Despite a constant RR interval (680 ms), the fourth and sixth beats (black stars) had higher J-wave amplitude and lower systolic blood pressure than the third and fifth beats (white stars). The second beat ($), which had a shorter coupling interval (640 ms) had higher J-point elevation than the fourth and sixth beats (black stars). (B): The third beat (⁎), which occurred at a short coupling interval (560 ms), had prominent J-point elevation and was followed by non-sustained VT. The ninth beat (#) developed after a long coupling interval (1120 ms) and showed no J-point elevation, but induced a high ventricular pressure followed by electrical and mechanical alternans.
Despite potassium administration, her serum potassium concentration did not normalize until 56 h after admission because she had taken hydrocortisone for pituitary adrenal insufficiency after surgery for a pituitary tumor. Under the persistent hypokalemic condition (K+=2.5–2.64 mEq/L), alternans of J-wave amplitude and prominent TWA gradually decreased over time and disappeared after 38 h (Fig. 3). Echocardiography performed 5 days after admission confirmed that left ventricular function had normalized. She was discharged without complications 18 days after admission. At the 1-month follow-up examination, she had hypokalemia (K+=2.6 mEq/L) because she had not been taking a potassium supplement after discharge. Nevertheless, her ECG demonstrated an almost normal pattern (Fig. 3).
Fig. 3.
Serial changes in the patient׳s ECG (V1–V6) after admission. Prominent TWA and J-wave alternans were apparent until day 3 at 18:00. These changes subsequently subsided.
3. Discussion
Both hypokalemia and TC are known to cause QT prolongation and TWA. In spite of prolonged hypokalemia, the alternans of J-wave amplitude and TWA changed dramatically with the time course of the patient׳s recovery from myocardial stunning, which suggested that hypokalemia was not likely a cause of the J-wave and T-wave dynamic change. The J-wave alternans was prominently recorded in leads V2–V6, which lead positions were closely adjoining the area of the akinetic left ventricular segments. We hypothesize that myocardial stunning due to TC played a pivotal role in the J-wave alternans and TWA. To the best of our knowledge, this is the first report of a patient with TC who exhibited alternans of J-wave amplitude and TWA.
Although QT-interval prolongation is relatively prevalent among patients with TC, they generally have good prognoses; however, development of torsade de pointes has been reported [3]. Patients with TC show QT-interval prolongation with a deep negative T-wave, the mechanism of which has not been explored in detail. A recent study indicated that a dynamic negative T wave and QT interval prolongation reflect edema-induced transient inhomogeneity and dispersion of repolarization between the apical and basal left ventricular regions [5]. This result may not be consistent with the fact that QT interval prolongation and deep negative T wave in patients with TC are observed broadly in the precordial leads rather than in the inferior leads, which reflect the apico-basal dispersion of repolarization. On the other hand, precordial leads, because of their proximity to the area, reflect the transmural potential gradient of the apical LV during the repolarization phase [6].
Visible TWA has been reported in patients with ischemia, long-QT syndrome, electrolyte disturbances, and post-tachycardia conditions, and is associated with the development of ventricular arrhythmias. It has been suggested that TWA results from alternans of the temporal dispersion of the action potential duration (APD), which is facilitated by the mechanism of steep APD restitution and calcium handling. APD restitution expresses the relationship between the APD and the preceding DI: a shorter DI is followed by a shorter APD. If APD restitution is steep, a small change in the DI causes large APD fluctuations and TWA. The alternans of cytosolic calcium also causes fluctuations in the APD; hence, TWA can be caused by calcium accumulation [7–9] and is linked with mechanical alternans [10,11]. A possible mechanism of TC is catecholamine-mediated myocardial stunning. Catecholamines induce myocardial injury via calcium overload [12], which may be related to alternans of cytosolic calcium.
In addition to TWA, prominent J-wave alternans and mechanical alternans were observed in this case. The beats with shorter preceding DIs produced higher-amplitude J waves associated with lower systolic blood pressure, and the beats with longer preceding DIs produced lower-amplitude J waves associated with higher blood pressure (Fig. 2A). Further, a beat after a long pause showed an abbreviated J wave with increased ventricular pressure (Fig. 2B). With regard to the mechanism of J wave, the transient outward current (Ito) was assumed to play an important role. Ito is most prominently expressed in the ventricular epicardial cells and least in the endocardial cells. The prominent Ito-mediated action potential in the epicardium against the least developed one in the endocardium causes a transmural voltage gradient and produces a notch at the early phase of ventricular repolarization that registers as a J-wave on ECG [13]. The Ito current is reduced in a beat with a short coupling interval because of its slow recovery from inactivation [14]. According to the Ito theory, the beats with short coupling intervals or preceding DIs should cause small amplitude of J waves. In this case, the beats with shorter preceding DIs or coupling intervals had higher J-wave amplitude than those with longer preceding DIs, which does not agree with the above explanation of the Ito-mediated mechanism. During the repolarization phase of ventricular action potential, however, several different outward and inward currents overlap. The balance between them can determine the configuration of action potential repolarization and APD. Among these overlapped currents, the L-type calcium current (ICaL), being one of the most important contributors for repolarization, also displays a recovery from inactivation during the diastolic period with a different time course from that of Ito. The balance between the recovery of Ito and ICaL was shown to determine the action potential configuration and APD [15].
The beats with high-amplitude J wave with short preceding DIs induced low systolic blood pressure, indicating weak cardiac contractility due to decreased calcium influx through ICaL and calcium release during ventricular systole. In contrast, the beats with low-amplitude J wave preceded by long DIs induced increased systolic blood pressure. The stunned myocardium resulting from TC may cause damage to the properties of ICaL and cause its slow recovery from inactivation. If ICaL recovery is sufficiently lower than Ito recovery, a high-amplitude J wave can be expected after a short DI, while ICaL recovery progresses in longer DIs to overcome Ito recovery. Hence, the amplitude of the J wave in the beats with longer DIs somewhat decreases compared with that in the beats with shorter DIs. Therefore, we hypothesize that the balance between Ito and ICaL may determine the amplitude of the J wave, depending on the preceding DI.
4. Conclusions
The patient in this study had prominent J-wave alternans and TWA associated with mechanical alternans during cardiac stunning in TC. The beats with short preceding DI caused high-amplitude J waves, whereas those with long preceding DIs produced low-amplitude J waves. The electrical and mechanical alternans disappeared with the improvement of myocardial stunning, suggesting the involvement of ICaL. The balance between Ito and ICaL may determine the amplitude of the J wave.
Conflict of interest
There are no conflicts of interest to disclose.
References
- 1.Tsuchihashi K., Ueshima K., Uchida T. Angina pectoris-myocardial infarction investigations in Japan: transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 2.Wittstein I.S., Thiemann D.R., Lima J.A. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 3.Samuelov-Kinori L., Kinori M., Kogan Y. Takotsubo cardiomyopathy and QT interval prolongation: who are the patients at risk for torsades de pointes? J Electrocardiol. 2009;42:353-7.e1. doi: 10.1016/j.jelectrocard.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Cutler M.J., Rosenbaum D.S. Explaining the clinical manifestations of T wave alternans in patients at risk for sudden cardiac death. Heart Rhythm. 2009;6:S22–S28. doi: 10.1016/j.hrthm.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perazzolo Marra M., Zorzi A., Corbetti F. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens׳ ECG pattern) in takotsubo cardiomyopathy. Heart Rhythm. 2013;10:70–77. doi: 10.1016/j.hrthm.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Furushima H., Chinushi M., Sanada A. Ventricular repolarization gradients in a patient with takotsubo cardiomyopathy. Europace. 2008;10:1112–1115. doi: 10.1093/europace/eun166. [DOI] [PubMed] [Google Scholar]
- 7.Laurita K.R., Katra R., Wible B. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 8.Pruvot E.J., Katra R.P., Rosenbaum D.S. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 9.Bayer J.D., Narayan S.M., Lalani G.G. Rate-dependent action potential alternans in human heart failure implicates abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–1101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama M., Kato K., Hirono S. Linkage between mechanical and electrical alternans in patients with chronic heart failure. J Cardiovasc Electrophysiol. 2004;15:295–299. doi: 10.1046/j.1540-8167.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- 11.Selvaraj R.J., Suszko A., Subramanian A. Microscopic systolic pressure alternans in human cardiomyopathy: noninvasive evaluation of a novel risk marker and correlation with microvolt T-wave alternans. Heart Rhythm. 2011;8:236–243. doi: 10.1016/j.hrthm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Mann D.L., Kent R.L., Parsons B. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 13.Antzelevitch C., Yan G.X. J-wave syndromes. from cell to bedside. J Electrocardiol. 2011;44:656–661. doi: 10.1016/j.jelectrocard.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubart M., Lopshire J.C., Fineberg N.S. Changes in left ventricular repolarization and ion channel currents following a transient rate increase superimposed on bradycardia in anesthetized dogs. J Cardiovasc Electrophysiol. 2000;11:652–664. doi: 10.1111/j.1540-8167.2000.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka M., Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ Res. 1987;60:14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]