Abstract
A 15-year-old asymptomatic male patient presented with an electrocardiographic abnormality and left ventricular (LV) dysfunction (left ventricle ejection fraction of 40%) in a physical examination performed 2 years previously. LV dysfunction did not improve despite optimal medical therapy for dilated cardiomyopathy. Twelve-lead electrocardiography revealed a normal PR interval (138 ms) with a small delta-like wave in V2, but not a typical diagnostic wave that could be diagnosed as Wolff–Parkinson–White (WPW) syndrome by an electrocardiogram auto-analysis. Transthoracic echocardiography showed a remarkable asynchronous septal motion. An electrophysiological study was performed to exclude WPW syndrome. An accessory pathway (AP) was revealed on the lateral wall of the right ventricle, and radiofrequency catheter ablation was successfully performed to disconnect the AP. Thereafter, the dyssynchrony disappeared, and LV function improved. The intrinsic atrioventricular nodal conduction was very slow (A-H, 237 ms). The results of electrocardiogram auto-analysis could not be used to confirm the diagnosis of WPW syndrome because of the atypical delta wave. Conduction via the right lateral AP caused electrical dyssynchrony in the LV. This case suggests that atypical delta waves should be evaluated without depending on electrocardiographic auto-analyses in patients with LV dysfunction accompanied by dyssynchrony.
Keywords: Accessory pathway, Dyssynchrony, Ablation, Atypical delta wave
1. Introduction
Twelve-lead electrocardiography (ECG) in patients with Wolff–Parkinson–White (WPW) syndrome demonstrates typical delta waves and a short PQ interval, suggesting the presence of a manifest accessory pathway (AP), the so-called manifest WPW syndrome. Approximately 30–50% of patients with APs do not have delta waves because they have only a retrograde electrical connection [1–4]. In contrast, several studies have reported left ventricular (LV) dysfunction in patients with manifest WPW syndrome without supraventricular tachycardia, and the LV dysfunction is primarily associated with a remarkable dyssynchrony caused by a septal AP [5–10].
In the present report, we describe a case of a young WPW syndrome patient with atypical 12-lead ECG characteristics and remarkable LV dyssynchrony.
2. Case report
A 15-year-old male patient was found to have an electrocardiographic abnormality during a physical examination. LV dysfunction was detected by echocardiography at another hospital. However, the patient was asymptomatic, with no abnormalities observed in any of the other examinations, including coronary angiography, enhanced cardiac magnetic resonance imaging, and I-123-β-methyl-p-iodophenyl-pentadecanoic acid myocardial single-photon-emission computed tomography. Furthermore, no arrhythmic events were detected on Holter ECG. He was diagnosed with dilated cardiomyopathy (DCM) and underwent medical therapy for 2 years. However, his LV dysfunction did not improve. Thus, he was referred to our hospital and admitted for further examination.
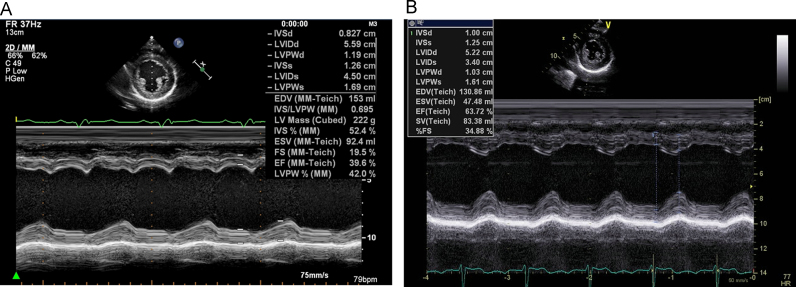
In a 12-lead ECG, the PR interval was not short (138 ms), and no typical delta waves were detected by the auto-analysis (Fig. 1A). Chest radiography did not indicate any cardiac enlargement. The plasma brain natriuretic peptide level was <5 pg/mL. Fig. 2A shows the results of M-mode transthoracic echocardiography. The LV ejection fraction (LVEF) measured by the bi-plane Simpson׳s method was 43%. The LV diameter was slightly dilated, and the LV diastolic and systolic diameters (LVDd and LVDs) were 56 mm and 45 mm, respectively. However, no LV wall thinning was noted (the interventricular septum and posterior wall thickness were 0.82 cm and 1.19 cm, respectively). Remarkable LV dyssynchrony was observed between the septal and lateral walls. There were no abnormalities of tricuspid valve attachment such as an Ebstein׳s anomaly.
Fig. 1.
Surface 12-lead electrocardiogram with an auto-analysis. (A) Before catheter ablation, the heart rate is 66 bpm; PR interval, 138 ms; and QRS duration, 192 ms. (B) After catheter ablation, the heart rate is 72 bpm; PR interval, 266 ms; and QRS duration, 96 ms.
Fig. 2.
M-mode echocardiograms. (A) Before ablation, dyssynchrony is observed between the septal wall and inferior walls. (B) After the ablation, the dyssynchrony disappeared.
The results of the 12-lead ECG were carefully analyzed. In lead II, a small rise, similar to a delta wave, above the baseline, was noted. In leads V1 and V2, the PR interval was slightly short. Because these findings indicated the possibility of WPW syndrome we decided to perform an electrophysiological study and a radiofrequency catheter ablation (RFCA), if necessary, obtaining written informed consent.
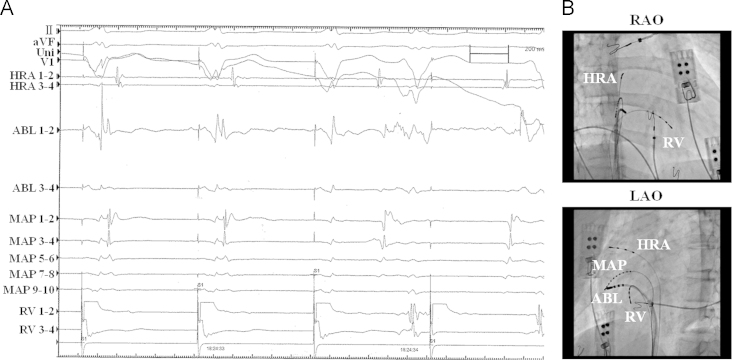
Three quadripolar electrode catheters were placed in the high right atrium (HRA), in the right ventricle, and at the bundle of His via the femoral vein. During right ventricular pacing, the earliest atrial activation was recorded at HRA 1–2, on the lateral side of the right atrium (Fig. 2). We diagnosed the patient with WPW syndrome type B and performed an RFCA of the AP in consecutive sessions. The catheters placed in the HRA and at the bundle of His were extracted. We placed a decapolar electrode catheter around the lateral side of the tricuspid valve annulus and used an 8-mm-tip ablation catheter, placed with a steerable introducer, to map the ablation site of the right lateral AP. The shortest atrioventricular (A-V) interval was observed above the ablation catheter on the lateral annulus of the tricuspid valve. During right ventricular pacing, retrograde conduction via the AP was blocked 2.8 s into RFCA application (Fig. 3A). After RFCA was completed, both the antegrade and retrograde conduction via the AP had disappeared. The QRS during the sinus rhythm became narrow.
Fig. 3.
Successful ablation site. (A) Electrophysiological study during right ventricular pacing. The retrograde conduction via the accessory pathway is blocked in the third beat in the recording. (B) The successful ablation site in the right anterior oblique view. (C) The successful ablation site in the left anterior oblique view. The successful ablation site is in the 8 o′clock position on the tricuspid valve annulus.
In the 12-lead ECG after successful RFCA (Fig. 1B), the QRS duration had decreased from 194 ms to 84 ms, and a significant first degree A-V block (PR interval, 274 ms) was noted. The AH interval was 237 ms as measured by the catheter placed at the bundle of His.
On ECG performed the next day, the remarkable LV dyssynchrony had disappeared, and the LVEF had improved from 43% to 53%. Moreover, ECG performed 6 months after the RFCA showed that the LVEF had improved further (63%). The LVDd and LVDs decreased to 52 mm and 34 mm, respectively.
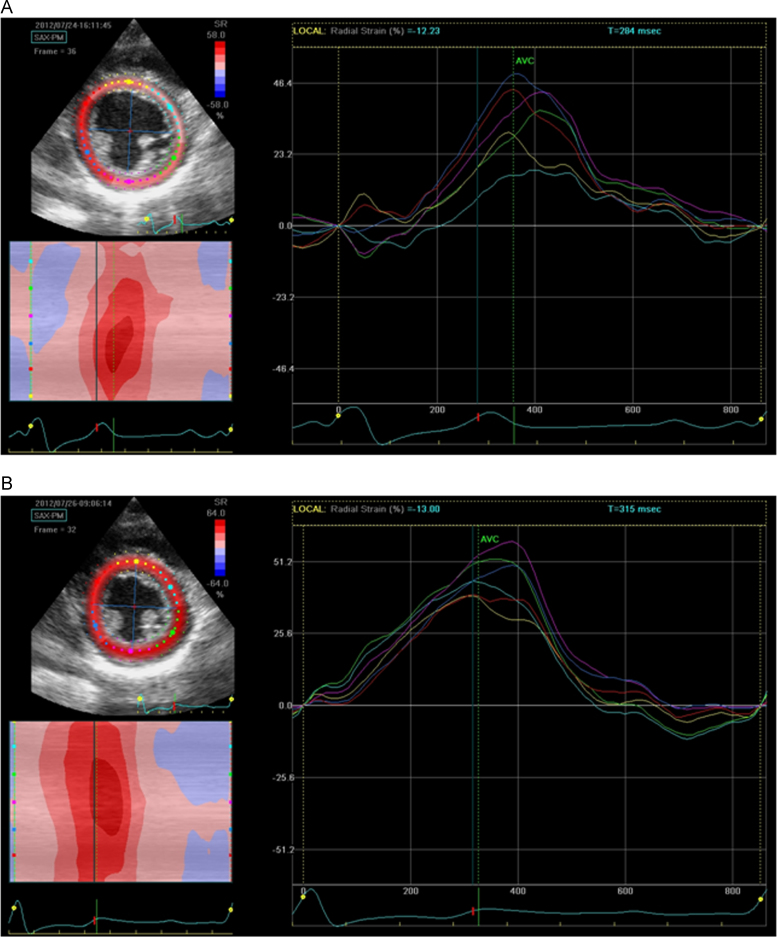
Comparisons were made between the LV dyssynchrony before and after the RFCA by using a two-dimensional (2-D) speckle-tracking analysis. Radial strain images were measured with 2-D speckle-tracking software (Echopac PC Ver.112, GE, Vingmed). The 2-D short axis view at the level of the papillary muscle was used to calculate the time to the peak radial strain in each of the 6 segments of the segmental radial strain over the cardiac cycle. The time to the peak radial strain in each segment before the RFCA is shown in Fig. 4A. The time from QRS onset to the peak strain measurement was taken over 6 segments. The maximum difference in the time to peak radial strain was noted between the septum and posterior wall (86 ms), as shown in the radial strain curves. The radial strain curves after the RFCA are shown in Fig. 4B. The maximum difference in the time to the peak radial strain between the septum and posterior wall became shorter (50 ms) after RFCA. Prior to RFCA, the anterior (red) and antero-septal (yellow) segments contracted during early systole, and the posterior (green) and inferior (purple) segments dilated. However, this asynchronous wall motion disappeared after RFCA. The radial strain curves of the 6 segments after RFCA became more synchronous compared with those before RFCA. Medical treatment for LV dysfunction has been gradually reduced.
Fig. 4.
Radial (2-D) strain by speckle-tracking echo in the short axis view at the level of the papillary muscle. The color cording of the strain curves refers to the respective left ventricular segments: (A) before catheter ablation and (B) after catheter ablation.
3. Discussion
Delta waves and a short PR interval on the 12-lead ECG are the most well-known characteristics of WPW syndrome. In the present case, those findings were not clearly identifiable, and the ECG auto-analysis did not suggest WPW syndrome on the report sheet. These reasons likely explain why we were unable to make an early diagnosis.
We hypothesize that the atypical delta waves occurred because the first potential of the right ventricle as a component of the first QRS wave was not large enough. A close-up analysis of the 12-lead ECG in the present study indicated the possibility of WPW syndrome and ultimately resulted in improved LV function. In this context, remarkable LV dyssynchrony and an LV wall without thinning in young patients might indicate the possibility of WPW syndrome rather than DCM.
Previous studies have also reported that treatment of APs with RFCA results in the disappearance of LV dyssynchrony and improved LV function [5–10]. However, most of those studies reported that septal APs induce dyssynchrony and a reduced LV function. Conduction via the septal AP initially caused the septal myocardium to contract and then gradually caused a contraction of the myocardium toward the lateral side. LV dyssynchrony with a septal AP can be explained by the mechanism described in those previous studies [5–7]. Long-term LV dyssynchrony induced mechanical remodeling and reduced LV function. In this case, the AP presented on the right lateral ventricular wall. Reports regarding right lateral APs with LV dysfunction are rare [11,12]. Almost all the patients with right lateral APs and LV dyssynchrony have been reported to have first degree A-V block after RFCA [12]. In the present case, first-degree A-V block due to an AH block was similarly noted after RFCA. We present a possible explanation for LV dyssynchrony with right lateral APs and first degree A-V block. In type-B WPW syndrome patients without conduction delay in the normal A-V conduction system, the septal myocardium is contracted by the fused conduction of the right lateral AP and normal A-V conduction system. The LV dyssynchrony is not remarkable, and LV dysfunction is rarely induced. However, in type-B WPW syndrome patients with conduction delay in the normal A-V conduction system, such as in the present case, the septal myocardium is contracted by conduction via the right AP, and delayed conduction via the normal A-V conduction system does not contribute to the contraction of the septal myocardium. Thus, a remarkable LV dyssynchrony develops similar to left bundle branch block and right ventricular pacing.
The morphology of the QRS waves in WPW syndrome changes according to the degree of fused conduction both via the AP and via the compact A-V node. The mechanism of conduction observed in this case might be proven by a comparison between the QRS wave morphology observed in this case and that during pacing from the RA near the AP or near the normal A-V conduction system. If the QRS wave morphology in this case is similar to that when pacing from the RA near the AP, and not similar to the wave via the normal A-V conduction system when pacing from the RA near the normal A-V conduction system, the QRS is likely being conducted through the AP. One limitation of this study was that we did not investigate QRS wave morphology with this pacing method before the RFCA. If we had, we might have been able to determine the mechanism of conduction of the QRS wave and LV dyssynchrony.
In previous studies, several months were needed to achieve an improvement in LV function [7,12], and the reason for the improvement was mechanical remodeling of the LV myocardium. In the present case, LV function was more improved 6 months after RFCA than it was the day after RFCA. Mechanical remodeling of the LV myocardium may have normalized LV function during the 6 months after RFCA.
There is a concern about the possibility that some patients with WPW syndrome who have LV dyssynchrony and atypical delta waves, as in the present case, may be diagnosed with DCM. In these WPW syndrome patients, although medical therapy might not improve LV dysfunction, RFCA of the AP might improve the LV dysfunction [12]. Before making a diagnosis of DCM accompanied by LV dyssynchrony, we should exclude WPW syndrome with atypical delta waves. Furthermore, atypical delta waves in a 12-lead ECG should be evaluated without depending on an ECG auto-analysis.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Hiejima K., Satake S. Concealed Wolff–Parkinson–White syndrome. Jpn Circ J. 1978;42:299–311. doi: 10.1253/jcj.42.299. [DOI] [PubMed] [Google Scholar]
- 2.Katoh T., Ohara T., Kim E.M. Non-invasive diagnosis of concealed Wolff–Parkinson–White syndrome by detection of concealed anterograde pre-excitation. Jpn Circ J. 2001;65:367–370. doi: 10.1253/jcj.65.367. [DOI] [PubMed] [Google Scholar]
- 3.Rostock T., Sydow K., Steven D. A new algorithm for concealed accessory pathway localization using T-wave-subtracted retrograde P-wave polarity during orthodromic atrioventricular reentrant tachycardia. J Interv Card Electrophysiol. 2008;22:55–63. doi: 10.1007/s10840-008-9253-y. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K., Suzuki F., Hiejima K. Quantitative analysis of concealed conduction into accessory atrioventricular pathways in Wolff–Parkinson–White syndrome. Pacing Clin Electrophysiol. 1997;20:1342–1353. doi: 10.1111/j.1540-8159.1997.tb06789.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomaske M., Janousek J., Razek V. Adverse effects of Wolff–Parkinson–White syndrome with right septal or posteroseptal accessory pathways on cardiac function. Europace. 2008;10:181–189. doi: 10.1093/europace/eun005. [DOI] [PubMed] [Google Scholar]
- 6.Fazio G., Mongiovi M., Sutera L. Segmental dyskinesia in Wolff–Parkinson–White syndrome: a possible cause of dilatative cardiomyopathy. Int J Cardiol. 2008;123:e31–e34. doi: 10.1016/j.ijcard.2006.11.109. [DOI] [PubMed] [Google Scholar]
- 7.Kwon B.S., Bae E.J., Kim G.B. Septal dyskinesia and global left ventricular dysfunction in pediatric Wolff–Parkinson–White syndrome with septal accessory pathway. J Cardiovasc Electrophysiol. 2010;21:290–295. doi: 10.1111/j.1540-8167.2009.01612.x. [DOI] [PubMed] [Google Scholar]
- 8.Emmel M., Balaji S., Sreeram N. Ventricular preexcitation associated with dilated cardiomyopathy: a causal relationship? Cardiol Young. 2004;14:594–599. doi: 10.1017/S1047951104006031. [DOI] [PubMed] [Google Scholar]
- 9.Udink ten Cate F.E., Wiesner N., Trieschmann U. Dyssynchronous ventricular activation in asymptomatic Wolff–Parkinson–White syndrome: a risk factor for development of dilated cardiomyopathy. Indian Pacing Electrophysiol J. 2010;10:248–256. [PMC free article] [PubMed] [Google Scholar]
- 10.Udink ten Cate F.E., Kruessell M.A., Wagner K. Dilated cardiomyopathy in children with ventricular preexcitation: the location of the accessory pathway is predictive of this association. J Electrocardiol. 2010;43:146–154. doi: 10.1016/j.jelectrocard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Ticzon A.R., Damato A.N., Caracta A.R. Interventricular septal motion during preexcitation and normal conduction in Wolff–Parkinson–White syndrome: echocardiographic and electrophysiologic correlation. Am J Cardiol. 1976;37:840–847. doi: 10.1016/0002-9149(76)90107-7. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaku T., Hirooka K., Taniguchi T. Successful catheter ablation to accessory atrioventricular pathway as cardiac resynchronization therapy in a patient with dilated cardiomyopathy. Europace. 2009;11:121–123. doi: 10.1093/europace/eun318. [DOI] [PubMed] [Google Scholar]