Abstract
Background
Para-Hisian pacing (PHP) helps differentiate retrograde conduction over an accessory pathway (AP) from retrograde conduction over the atrioventricular (AV) node. This study examined a potential limitation of this technique, focusing on the measurement of the ventriculoatrial (V–A) interval from the coronary sinus (CS) during PHP.
Methods
Our subjects were 9 patients undergoing electrophysiological studies before successful catheter ablation of a posteroseptal AP. During PHP, retrograde conduction occurred over an AP when the pacing stimulus to atrium (S–A) interval recorded near the AP remained unchanged whether the His bundle (HB) was captured or not (pattern 1), or when a loss of HB capture was associated with an increase in the S–A interval and no change in the V–A interval near the AP (pattern 2).
Results
Patterns 1 and 2 were observed in 5 (56%) and 2 (22%) patients, respectively. However, in the remaining 2 patients (22%), loss of HB capture during PHP was associated with an increase in the S–A interval (as in pattern 2), whereas the V–A interval near the AP could not be measured because no ventricular electrogram was visible on the CS recording (pattern 3); therefore, the presence of AP could not be confirmed by PHP. In patterns 2 and 3, the atrial activation sequence remained unchanged whether the HB was captured or not.
Conclusions
PHP may not be able to discriminate between a retrograde septal AP and AV nodal conduction in patients whose proximal CS recording shows no visible ventricular electrogram.
Keywords: Para-Hisian pacing, Atrioventricular node, Accessory pathway
1. Introduction
Para-Hisian pacing (PHP) is useful for making the distinction between retrograde conduction over an accessory pathway (AP) versus over the atrioventricular (AV) node through the measurement of differences in the timing and sequence of retrograde atrial activation depending on whether the His bundle (HB) is captured or not [1–3]. Retrograde activation during PHP is measured as the interval between the pacing stimulus and (a) the earliest atrial electrogram (S–A interval) and (b) the ventriculoatrial (V–A) interval [1–3]. While some existing case reports have described the pitfalls of this technique [4–10], its potential inability to discriminate between retrograde AV nodal and septal AP conduction has not been discussed. The purpose of this study was to clarify this point, with a focus on the limitation of the measurement of the V–A interval from the proximal coronary sinus (CS).
2. Materials and methods
2.1. Study population
We studied 3 women and 6 men with a mean age of 50.1±26.1 years who underwent electrophysiological studies including PHP and successful ablation of a septal AP (Table 1). Two patients (nos. 3 and 6) presented with a decremental AP and one patient (no. 3) presented with a slowly conducting AP. The study complied with the guidelines of the declaration of Helsinki and was approved by the institutional review board of Gunma University Hospital (No. 729, approval date: Jan 17, 2010). All patients provided written informed consent to participate in this study.
Table 1.
Selected baseline characteristics.
| Patient no. | Age | Sex | Delta |
During sinus rhythm, ms |
During tachycardia, ms |
Properties of AP |
Successful ablation site | Retrograde conduction over AV nodea | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AH | HVa | AH | HA | Decrementality | Responses to ATP injection | ||||||
| 1 | 49 | M | + | 75 | 30 | 348 | 145 | − | − | On the mitral annulus | − |
| 2 | 52 | M | + | 60 | 37 | 179 | 128 | − | ND | On the mitral annulus | − |
| 3 | 79 | M | + | 86 | 46 | ND | ND | − | ND | Inside the CS | − |
| 4 | 16 | M | − | 93 | 46 | 168 | 148 | − | – | On the mitral annulus | − |
| 5 | 77 | M | + | 95 | 39 | ND | ND | − | – | Inside the CS | + |
| 6 | 17 | F | − | 78 | 52 | 254 | 72 | + | ND | On the mitral annulus | − |
| 7 | 76 | F | − | 105 | 37 | 232 | 183 | − | – | On the mitral annulus | − |
| 8 | 22 | M | − | 67 | 61 | 230 | 230 | − | ND | Inside the CS | + |
| 9 | 63 | F | − | 90 | 34 | 113 | 293 | − | ND | Inside the CS ostium | − |
AP=accessory pathway; CS=coronary sinus; F=female; M=male; ND=not determined; +=present; −=absent.
Estimated after successful ablation.
2.2. Electrophysiological study
The patients underwent baseline electrophysiological studies in the fasting, non-sedated state after discontinuation of all antiarrhythmic drugs for ≥5 half-lives. A 6F 2-mm spaced, pentapolar, deflectable-tip electrode catheter was placed in the HB region, and multipolar, deflectable-tip electrode catheters were placed in the high right atrium, right ventricle, and CS. Programmed electrical stimulation was performed with a SEC-3102TM cardiac stimulator (Nihon Kohden, Tokyo, Japan). Retrograde AP conduction was confirmed by preexcitation of the atrium during tachycardia with a ventricular paced event during HB refractoriness.
2.3. Para-Hisian pacing
PHP was performed while gradually changing the pacing output to obtain recordings during right ventricular pacing near the HB versus during HB capture, with the mapping catheter maintained in a stable position. The response to PHP was determined by changes in the following variables between HB capture and non-capture: (1) retrograde atrial activation sequence; (2) the S–A interval recorded at multiple sites, including near the earliest atrial activation site; and (3) the V–A interval near the earliest atrial activation site. These variables were examined before ablation. An identical retrograde atrial activation sequence indicated that retrograde conduction was occurring over the same system during HB capture and non-capture (AP or AV node). Retrograde conduction was classified as occurring strictly over an AP if (1) S–A intervals recorded at any site remained essentially identical (pattern 1; the AP/AP pattern in the original report by Hirao et al. [1]), or if (2) local V–A intervals remained unchanged at all sites despite a lengthening of the S–A interval associated with HB non-capture (pattern 2) [1,2]. Thus, the loss of HB capture without a change in retrograde atrial activation and an increase in the S–A interval indicated retrograde conduction over an AP or over the AV node [1,2]. If the V–A interval recorded near the site of earliest atrial activation remained unchanged, retrograde conduction was occurring over an AP [1,2].
3. Results
We observed 3 patterns of response to PHP. Pattern 1, observed in 56% of patients (nos. 4, 6, 7, 8, and 9), was characterized by S–A and V–A intervals in the septal region that were identical whether HB was captured or not, along with an unchanged atrial activation sequence (Fig. 1). In these 5 patients, a local ventricular electrogram was visible on the CS recording near the AP. Pattern 2 (AP/APL in the study by Hirao et al. [1]), observed in patient nos. 1 and 5 (22% of the study sample), was characterized by a loss of HB capture associated with an increase in the S–A interval without change in the atrial activation sequence or the V–A interval near the AP due to an increase in the S–V interval (Fig. 2). Pattern 3, observed in patient nos. 2 and 3 (22% of the study sample), was characterized by a loss of HB capture associated with an increase in the S–A interval, as in pattern 2. However, in pattern 3 patients, neither the S–V nor the V–A interval near the AP could be measured, as the ventricular electrogram was invisible on the CS recording even when ventricular sensitivity was enhanced to a maximum level of 0.1 mV/cm or the CS catheter was adjusted to obtain the local ventricular electrogram (Fig. 3). These observations are summarized in Tables 1 and 2. A mixed pattern expressing retrograde conduction over an AP and over the AV node was not observed in any patients. Following the AP ablation procedure, VA conduction persisted in patient nos. 2, 4, 5, and 8. In these 4 patients, ventricular burst and premature stimulation revealed decremental retrograde conduction, consistent with the AV node.
Fig. 1.
Pattern 1 of PHP (patient no. 4). The first pacing stimulus (S) captures the ventricular myocardium without direct capture of the His bundle, producing a wide first QRS complex and the earliest atrial activation at CS 7–8. The second pacing stimulus (S) directly captures the HB, producing a relatively narrow QRS complex. The S–A interval (bold bidirectional arrows) remains fixed and the atrial activation sequence remains unchanged. Note the visible far-field ventricular electrogram (V) in the proximal CS near the posteroseptal AP and the absence of change in the local S–V (dotted bidirectional arrows) and V–A intervals (thin bidirectional arrows). All intervals are in milliseconds. I, II, and V1=surface ECG; HRA=high right atrium; HBEp to HBEd=proximal to distal His bundle; CSp to CSd=proximal to distal coronary sinus; RVA=right ventricular apex.
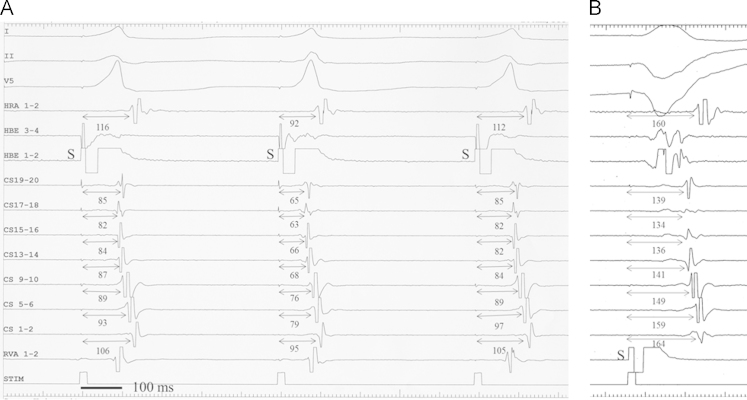
Fig. 2.
Pattern 2 of PHP (patient no. 5). The pacing stimulus (S) in A did not capture the HB, and produced a wide QRS complex. Direct capture of the HB in B produced (a) a relatively narrow QRS complex, (b) a shortening of the S–A interval (bold bidirectional arrows), and (c) the advancement of ventricular activation (V) near the AP and a shortening of the S–V interval (bidirectional dotted arrows). The V–A interval (thin bidirectional arrows) remained fixed at 52 ms at CS 5–6. Other abbreviations as in Fig. 1.
Fig. 3.
Pattern 3 of PHP (Panel A) and ventriculoatrial conduction over the AV node (Panel B) in patient no. 2. (A) HB capture is lost with the first and third QRS complex and is present with the second QRS complex in association with a decrease in the S–A interval (bidirectional arrows). The retrograde atrial activation sequence is identical throughout. Since no ventricular electrogram was visible at the site of earliest atrial activation (CS 17–18), the V–A interval near the AP could not be measured. (B) Note that S–A intervals (bidirectional arrows) indicating retrograde conduction time over the AV node are obviously longer than the corresponding S–A intervals in Panel A. Other abbreviations as in Fig. 1.
Table 2.
Summary of para-Hisian pacing measurements.
| Patient no. |
HB capture |
HB non-capture |
Pattern | ||||
|---|---|---|---|---|---|---|---|
| S–A | S–V | V–A | S–A | S–V | V–A | ||
| 1 | 137 | 69 | 68 | 153 | 80 | 73 | 2 |
| 2 | 63 | ND | ND | 82 | ND | ND | 3 |
| 3 | 196 | ND | ND | 213 | ND | ND | 3 |
| 4 | 105 | 71 | 34 | 103 | 71 | 32 | 1 |
| 5 | 137 | 82 | 55 | 164 | 112 | 52 | 2 |
| 6 | 104 | 74 | 30 | 108 | 74 | 34 | 1 |
| 7 | 113 | 80 | 33 | 115 | 79 | 36 | 1 |
| 8 | 182 | 141 | 41 | 180 | 122 | 58 | 1 |
| 9 | 186 | 127 | 59 | 186 | 131 | 55 | 1 |
S–A=the interval between the pacing stimulus and the earliest atrial electrogram; S–V=the interval between the pacing stimulus and the ventricular electrogram at the proximal CS; V–A=the interval between the ventricular electrogram and the earliest atrial electrogram at the proximal CS.
4. Discussion
Retrograde conduction over a posteroseptal AP was accurately identified in 7 of the 9 patients (78%), whereas in 2 patients (22%), retrograde AP conduction was not identifiable because of the absence of a visible ventricular electrogram near the AP. CS recordings provide important information needed to locate a left-sided AP [11] and measure the V–A interval near the AP during PHP [1], since in a majority of patients, atrial and ventricular electrograms can be recorded simultaneously [12]. Since the CS is immediately adjacent to the atrial myocardium, although it is separated from the ventricular myocardium by a layer of fat [13–16], the amplitude of the ventricular electrograms recorded from the CS is usually <50% of the atrial electrogram amplitude, and their morphological characteristics are those of far-field signals, such as a lower amplitude with a narrower electrode spacing and a low signal frequency [12]. Furthermore, as in 2 of our patients, the far-field ventricular electrograms may not be visible from the CS, especially in the left posteroseptal region, probably because it is too distant from the mitral annulus [13–15].
Shortening of the S–A interval associated with capture of the HB, as observed in patterns 2 and 3, is due to conduction over the His–Purkinje system and earlier activation of the ventricular myocardium near the AP [1–3]. This observation is more likely in patients with a left lateral or anterolateral AP located far from the site of PHP [1], although it does occur in a minority of patients with a posteroseptal AP [1]. In the present study, a shortening of the S–A interval associated with capture of the HB was observed in 44% of our patients, suggesting that this phenomenon is not rare, even in patients with a posteroseptal AP. We hypothesize that when present in a patient with a posteroseptal AP, it is the result of a variation in the distribution of a septal branch toward the posterior septum [17,18]. In these patients, capture of the HB results in earlier activation of the ventricular posteroseptal myocardium near the AP, which shortens the S–A interval (Fig. 4). Theoretically, this phenomenon may be less likely to occur in patients with a “right-sided” posteroseopal AP than a “left-sided” posteroseptal AP because (1) the PHP site is closer to the right-sided posteroseptum than the left-sided posteroseptum, and (2) at least the proximal portion of the right bundle branch had no branching structure. In the present study, APs were successfully ablated on the mitral annulus or inside the CS in 4 patients in whom pattern 2 or 3 was observed, consistent with a “left-sided” AP.
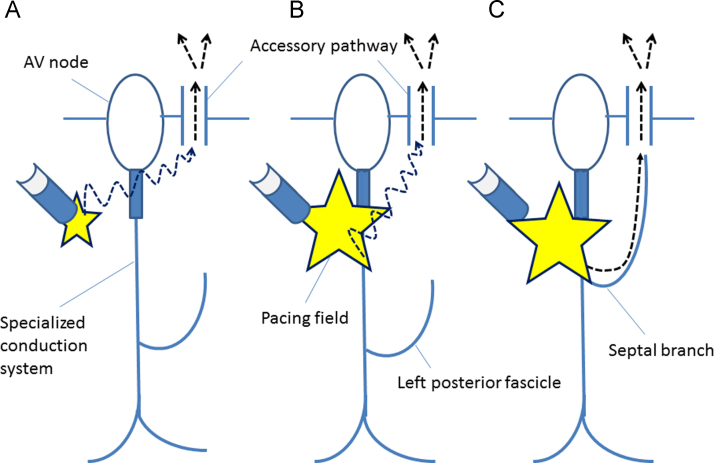
Fig. 4.
Schematic representation of the conduction pathway (dotted arrows) to the atrium over the posteroseptal AP during PHP without HB capture (A) and with direct HB capture (B and C) based on hypothetical variations in the distributions of the left posterior fascicle. (A) The S–A interval during PHP without HB capture corresponds to the conduction time across the working myocardium between the pacing site and the posteroseptal atrium near the AP (dotted arrow). (B) During PHP with direct HB capture and an S–A interval similar to that observed during PHP without HB capture, the wavefront conducting across the working myocardium (dotted arrow) reaches the posteroseptal atrium earlier than the wavefront propagating along the left posterior fascicle. (C) During PHP with direct HB capture and a shorter S–A interval than that observed during PHP without HB capture, the wavefront conducting through the septal branch distributed toward the posteroseptal region reaches the posteroseptal atrium earlier than the wavefront propagating across the working myocardium.
Furthermore, the difference in the mean S–A interval between HB capture and non-capture in the 4 patients with conduction pattern 2 or 3 was only 20±4 ms (range, 16–27 ms), and was shorter than the HV interval after ablation in 3 patients (75%). By contrast, in patients with strictly AV nodal retrograde conduction, whereby ventricular capture without HB capture propagates to the atrium via the ventricular myocardium and right bundle branch, the difference in the S–A interval between capture and non-capture should theoretically be at least equal to (a) the interval between the pacing site and entry of the wavefront into the right bundle branch, plus (b) the interval between the entry into the right bundle branch and the HB, which is nearly the same as the HV interval [1,2]. The value of this difference, however, has not been reported previously. Thus, we hypothesize that a smaller difference in the S–A interval between capture and non-capture of the HB excludes the AV node as the retrograde pathway. Further studies are needed to confirm this hypothesis.
In summary, this study illustrates a plausible pitfall of PHP: in >20% of patients presenting with a posteroseptal AP, we could not determine whether retrograde conduction occurred via the AV node or via the AP because of the absence of a ventricular electrogram on the CS recording, which precluded the measurement of the V–A interval near the septal AP during PHP. In these patients, the AP versus the AV node should not be discriminated solely on the basis of a shortening of the S–A interval when the HB is captured. This pitfall is not a real limitation of PHP. Instead, PHP should be performed using another deflectable catheter or ablation catheter to obtain the local ventricular electrogram near the AP from the intraventricular cavity, making it easier to distinguish VA conduction through the AP from that occurring through the AV node. In addition, by using ventricular premature stimulation during tachycardia, retrograde conduction through the septal AP and AV node can be easily differentiated.
Conflict of interest
The authors have no conflict of interest to disclose.
Acknowledgments
Data collection: T.Ii., Y.K., T.N., T.Ir., M.O; data analysis and interpretation: T.Ii., Y.K., T.N., M.K.; manuscript composition: T. Ii., Y.K.; manuscript approval: T.Ii., Y.K., T.N., T.Ir., M.K.
References
- 1.Hirao K., Otomo K., Wang X. Para-Hisian pacing. A new method for differentiating retrograde conduction over an accessory AV pathway from conduction over the AV node. Circulation. 1996;94:1027–1035. doi: 10.1161/01.cir.94.5.1027. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa H., Jackman W.M. Para-Hisian pacing: useful clinical technique to differentiate retrograde conduction between accessory atrioventricular pathways and atrioventricular nodal pathways. Heart Rhythm. 2005;2:667–672. doi: 10.1016/j.hrthm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Hirao K., Yamamoto N., Toshida N. Diagnostic significance of the morphological change in the atrial electrogram during para-Hisian pacing. Jpn Circ J. 2000;64:928–932. doi: 10.1253/jcj.64.928. [DOI] [PubMed] [Google Scholar]
- 4.Adachi M., Igawa O., Miake J. QRS complex widening due to loss of left bundle branch capture: pitfall of para-Hisian pacing. J Interv Card Electrophysiol. 2009;25:213–216. doi: 10.1007/s10840-008-9345-8. [DOI] [PubMed] [Google Scholar]
- 5.van Opstal J.M., Crijns H.J. Paradoxical increase of stimulus to atrium interval despite His-bundle capture during para-Hisian pacing. Europace. 2009;11:1702–1704. doi: 10.1093/europace/eup232. [DOI] [PubMed] [Google Scholar]
- 6.Sauer W.H., Lowery C.M. A potential para-Hisian pacing pitfall. J Cardiovasc Electrophysiol. 2009;20:448. doi: 10.1111/j.1540-8167.2008.01347.x. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian A., Chauhan V.S. Paradoxical extranodal response during para-Hisian pacing: what is the mechanism? Pacing Clin Electrophysiol. 2009;32:1582–1583. doi: 10.1111/j.1540-8159.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 8.Selvaraj R.J., Yerram S., Ramasamy C. An unusual response to para-Hisian pacing: What is the explanation? Heart Rhythm. 2013;10:1586–1588. doi: 10.1016/j.hrthm.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Ho K.L., Nair K., Chauhan V.S. Paradoxical response during para-Hisian pacing. What is the mechanism? Heart Rhythm. 2012;9:1732–1733. doi: 10.1016/j.hrthm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Yue A.M., Foster W.M. Para-Hisian pacing: is an accessory pathway present? Heart Rhythm. 2012;9:624–625. doi: 10.1016/j.hrthm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama M., Kaneko Y., Taniguchi Y. Coronary sinus recordings of double potentials associated with retrograde conduction through left atrioventricular accessory pathways. J Cardiovasc Electrophysiol. 2004;15:1371–1376. doi: 10.1046/j.1540-8167.2004.04422.x. [DOI] [PubMed] [Google Scholar]
- 12.Giudici M., Winston S., Kappler J. Mapping the coronary sinus and great cardiac vein. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):414–419. doi: 10.1046/j.1460-9592.2002.00414.x. [DOI] [PubMed] [Google Scholar]
- 13.Shinbane J.S., Lesh M.D., Stevenson W.G. Anatomic and electrophysiologic relation between the coronary sinus and mitral annulus: implications for ablation of left-sided accessory pathways. Am Heart J. 1998;135:93–98. doi: 10.1016/s0002-8703(98)70348-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamanouchi Y., Igawa O., Hisatome I. Activation mapping from the coronary sinus may be limited by anatomic variations. Pacing Clin Electrophysiol. 1998;21(11 Pt 2):2522–2526. doi: 10.1111/j.1540-8159.1998.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 15.El-Maasarany S., Ferrett C.G., Firth A. The coronary sinus conduit function: anatomical study (relationship to adjacent structures) Europace. 2005;7:475–481. doi: 10.1016/j.eupc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Sealy W.C., Mikat E.M. Anatomical problems with identification and interruption of posterior septal Kent bundles. Ann Thorac Surg. 1983;36:584–595. doi: 10.1016/s0003-4975(10)60690-x. [DOI] [PubMed] [Google Scholar]
- 17.Demoulin J.C., Kulbertus H.E. Histopathological examination of concept of left hemiblock. Br Heart J. 1972;34:807–814. doi: 10.1136/hrt.34.8.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi Y., Konishi N., Enoki N. A morphological study of the left bundle branch in the normal human heart. Acta Pathol Jpn. 1988;38:417–424. doi: 10.1111/j.1440-1827.1988.tb02315.x. [DOI] [PubMed] [Google Scholar]