Abstract
Background
Atrial tissue fibrosis has previously been identified using delayed-enhancement MRI (DE-MRI) in patients with atrial fibrillation (AF). Although the clinical importance of DE-MRI is well recognized, the visualization of atrial fibrosis and radiofrequency (RF) lesions has still not been achieved in Japan, primarily because of the differences in contrast agents, volume-rendering tools, and technical experience. The objective of this study was to visualize RF lesions by using commercially available tools.
Methods
DE-MRI was performed in 15 patients who had undergone AF ablation (age, 59±4 years, left atrium diameter, 40±2 mm). Specific parameters for MR scanning obtained from previous reports were modified.
Results
Of the 15 images, the images of three patients were uninterpretable owing to low image quality. RF lesions could be visualized in 8 (67%) of the 12 patients.
Conclusions
In the current study, we successfully demonstrated that RF lesions could be visualized in Japanese patients using DE-MRI, although only commercially available tools were used.
Abbreviations: DE-MRI, delayed-enhancement magnetic resonance imaging; AF, atrial fibrillation; RF, radiofrequency
Keywords: Delayed-enhancement MRI, Radiofrequency lesions, Atrial fibrillation, Catheter ablation
1. Introduction
Delayed-enhancement magnetic resonance imaging (DE-MRI) is an established method for characterizing cardiac tissue in various disease processes [1]. Recently, DE-MRI has emerged as an effective method for noninvasively assessing and quantifying the extent of left atrium (LA) structural remodeling [2], which has been reported to be independent of the type of atrial fibrillation (AF) and associated comorbidities. Moreover, selecting appropriate treatment candidates based on the quality and quantity of atrial fibrosis detected using DE-MRI has shown to improve the procedural outcome and prevent unnecessary interventions [3]. Furthermore, a multicenter trial has also demonstrated that atrial fibrosis, as estimated by DE-MRI, is independently associated with the likelihood of recurrent arrhythmias [4]. We recognize the use and importance of DE-MRI for visualizing atrial fibrosis [3]. However, neither atrial fibrosis nor radiofrequency (RF) lesions can be visualized using DE-MRI in Japan, mainly due to the differences in contrast agents, volume-rendering tools and technical experience. Therefore, the objective of the current study was to visualize RF lesions and tissue fibrosis in the left atrium (LA) of Japanese patients who underwent AF ablation using commercially available tools for DE-MRI. In addition, we also investigated the clinical differences between the successful and unsuccessful MRI acquisitions groups.
2. Material and methods
2.1. Patient selection
The study was conducted in consecutive 15 patients who had undergone AF ablation. DE-MRI scans were uninterpretable in 3 patients because the imaging was performed too early after the injection (n=1) and owing to insufficient respiratory gating (n=2). Therefore, the remaining 12 patients were enrolled in this study and were subsequently divided into two groups according to the quality of the acquired MRI (the adequate DE-MRI and the inadequate DE-MRI groups). The study was approved by the local institutional review board (Date: 11.19.2013; Approval number: 20), and written informed consent was obtained from all the patients.
2.2. DE-MRI acquisition
All the patients underwent contrast-enhanced MR imaging using a 1.5-T MR system (Intera Achieva; Philips Medical Systems) equipped with a 32-channel cardiac coil. The scanning technique and parameters for the DE-MRI have been previously reported [5–8]. The DE-MRI of the left atrium (LA) with pulmonary veins (PVs) was acquired using a 3D inversion recovery, respiration navigated, electrocardiogram-gated, T1-FFE sequence in the transverse plane 15 min after the injection of 0.1 mmol/kg gadolinium (Magnevist; Bayer HealthCare). Typical scan parameters were: repetition time (TR)/echo time (TE)=4.7/1.5, voxel size=1.25×1.26×2.60 mm3 (reconstructed to 0.63×0.63×1.30 mm3), flip angle=15, inversion time (TI)=280–330 ms, SENSE with a reduction factor of 2, and 70 reference lines. The TI value was identified from the myocardial TInull using a Look-Locker. The T1 of the LA wall was similar to the myocardial T1 [5]. Data acquisition was limited to 15% of the cardiac cycle. During the MRI, in sinus rhythm (SR) cases, data acquisition was performed during the mid-diastolic phase of the left ventricle. Furthermore, in AF cases, the trigger delay of the cardiac synchronization was set to the shortest value. Saturation bands were placed in the phase-encoding (right–left) line to minimize back-folding from the arms. Fat saturation was applied to suppress any fat signals. The typical scan time for the DE-MRI study was 7–12 min depending on the heart rate (HR) and respiration pattern of each patient; we attempted to maintain the HR at <70 bpm using metoprolol 20 or 40 mg.
2.3. 3D visualization of RF lesions and atrial fibrosis
The method for 3D visualization of the lesions using DE-MRI was as follows: first, source images were transferred to a workstation (AZE Virtual Place; AZE, Tokyo, Japan); second, segmentation was performed with AZE Virtual Place, and the LA was further segmented semi-manually by contouring the endocardial and epicardial borders of the atrium, including the PVs (Fig. 1); third, a voxel-intensity histogram analysis of the LA wall measured the intensities (>1SD) of the Des, and the degree of intensity was categorized into a color-coded scale (green: 1SD–2SD: yellow: 2SD–3SD; red: >4SD) [2]; and finally, 3D reconstruction of the LA and PVs with the DEs was achieved automatically.
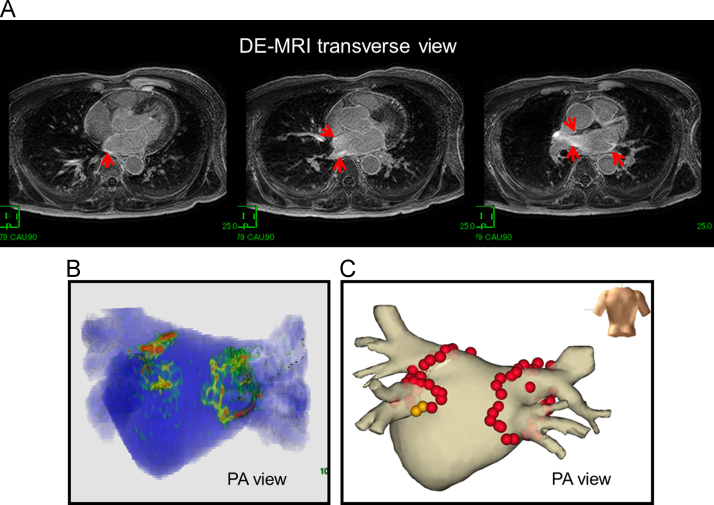
Fig. 1.
Adequate 3D visualization of an RF lesion in a patient without AF recurrence. (A) The LA wall slice 3 months after AF ablation observed using DE-MRI. The red arrows show delayed enhancement. (B) 3D visualization of RF lesions. (C) AF ablation using the NavX system. The red and brown 3D tags indicate ablation points. The patient has been free of any AF recurrence for 12 months after a single AF ablation, without using any anti-arrhythmic drug.
2.4. Statistics
Data were tested using the Kolmogorov–Smirnov test and are presented as mean±standard deviation for normally distributed variables. The medians and quartiles are given for non-normally distributed variables. Categorical variables are expressed as number and percentage of patients. Continuous variables were analyzed using Student׳s t test. Categorical variables were analyzed using Fisher׳s exact test. A value of p<0.05 was considered statistically significant. All the statistical analyses were performed with SPSS, Release 11.0 software (SPSS, Chicago, IL, USA).
3. Results
3.1. Patient characteristics
The patient characteristics are displayed in Table 1. The primary AF type was paroxysmal AF in most patients (9/12 [75%]). Moreover, the mean left atrial diameter and the mean left atrial appendage (LAA) flow were 40±2 mm and 63±25 cm/s, respectively.
Table 1.
Clinical characteristics of the patient population.
| Age (years) | 59±4 |
| Male (n-%) | 10(83) |
| Type of AF (n-%) | |
| Paroxysmal | 9(75) |
| Persistent | 2(17) |
| Long-lasting | 1(8) |
| AF history (months) | 51(24; 81) |
| Antiarrhythmic drugs (n) | 1(0; 1) |
| Arterial hypertension (n,%) | 5(42) |
| Diabetes mellitus (n,%) | 3(25) |
| Lone AF, (n-%) | 4(33) |
| LAA flow (cm/s) | 63±25 |
| Left atrial diameter (mm) | 40±2 |
| LV ejection fraction (%) | 56±1 |
AF=atrial fibrillation, LV=left ventricle, LAA=left atrial appendage.
3.2. Cardiac rhythm, heart rate, and usage of metoprolol during MRI
Data on cardiac rhythm, HR, and metoprolol use during MRIs are displayed in Table 2. Most of the patients were in SR during the MRI. The mean HR was controlled to around 70 bpm by using metoprolol.
Table 2.
Cardiac rhythm, heart rate and usage of metoprolol during MRI.
| Cardiac rhythm during MRI | |
|---|---|
| Sinus rhythm (n-%) | 11(92%) |
| Atrial fibrillation (n-%) | 1(8%) |
| Usage of metoprolol (n-%) | 12(100%) |
| Dose of metoprolol | |
| 20 mg (n-%) | 5(42%) |
| 40 mg (n-%) | 7(58%) |
| Heart rate (bpm) | 72±8 |
MRI=magnetic resonance image.
3.3. Successful 3D visualization of the RF lesion: adequate DE-MRI group vs. inadequate DE-MRI group
RF lesions were successfully visualized in 3D in 8 of the 12 patients (67%). The quality of the MRI was inadequate in the remaining 4 patients who had undergone MRI immediately after AF ablation (median of 3 days (2; 11) in the inadequate DE-MRI group vs. 44 days (32; 300) in the adequate DE-MRI group, p=0.004) (Table 3). Only one patient had AF during the MRI, while the remaining 11 patients were in SR. Furthermore, the HR was lower in the adequate DE-MRI group than in the inadequate DE-MRI group (68±5 bpm vs. 79±9 bpm, respectively, p=0.021). The other clinical parameters did not differ between the two groups (Table 3). Figs. 1 and 2 show representative cases from the adequate and inadequate MRI groups, respectively; both cases were free from AF. However, two cases (patients 1 and 2) experienced AF recurrence during the MRI (Fig. 3). Of note, lesion gaps were documented at the bottom of the right inferior pulmonary vein (RIPV) and near the top of the right superior pulmonary vein (RSPV) in patient 1 (Fig. 3A, B, and C) and at the roof of the left superior pulmonary vein (LSPV) in patient 2 (Fig. 3D and E).
Table 3.
Clinical parameters grouped by the quality of the DE-MRI.
| Adequate MRI group | Inadequate MRI group | Pvalue | |
|---|---|---|---|
| (n=8) | (n=4) | ||
| Cardiac rhythm during MRI | 1.00 | ||
| Sinus rhythm (n, %) | 7(92%) | 4(100%) | |
| Atrial fibrillation (n,%) | 1(8%) | 0(0%) | |
| Use of metoprolol (n, %) | 8(100%) | 4(100%) | N.A. |
| Dose of metoprolol | 0.22 | ||
| 20 mg (n, %) | 2(25%) | 3(75%) | |
| 40 mg (n, %) | 6(75%) | 1(25%) | |
| Heart rate (bpm) | 68±5 | 79±9 | 0.021 |
| Time to MRI (days) | 44(32; 300) | 3(2; 11) | 0.004 |
| After AF ablation | |||
| AF history (months) | 51(27; 72) | 73(12; 201) | 0.067 |
| Lone AF, (n, %) | 3(38%) | 1(25%) | 0.81 |
| LAA flow (cm/s) | 53±21 | 82±25 | 0.059 |
| Left atrial diameter (mm) | 42±8 | 37±5 | 0.35 |
| LV ejection fraction (%) | 57±9 | 54±9 | 0.61 |
MRI=magnetic resonance image, AF=atrial fibrillation, LAA=left atrial appendage, LV=left ventricle.
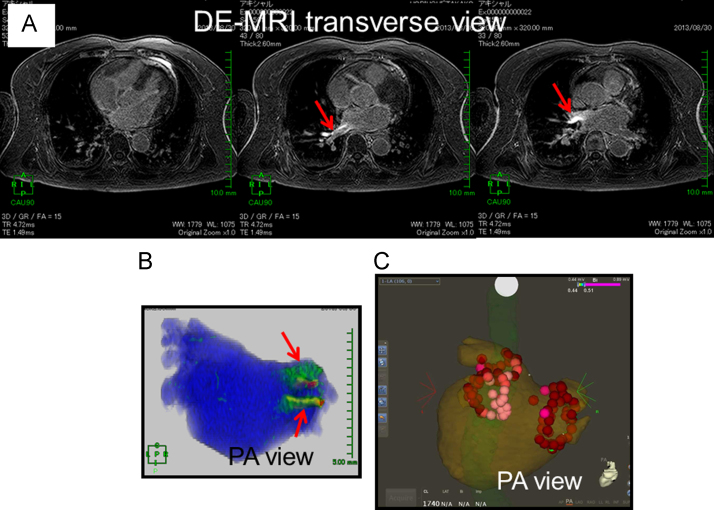
Fig. 2.
Inadequate 3D visualization of an RF lesion in a patient without an AF recurrence. (A) The LA wall slice observed using DE-MRI 2 days after the AF ablation; any delayed enhancement (DE) caused by RF application was not visible. Of note, red arrows indicate artifacts, which may have been caused by the laser application to the right hemi-diaphragm for respiratory navigation. (B) 3D visualization of the RF lesions. The red arrows indicate artifacts, not DE. (C) AF ablation using the CARTO3 system. The red and pink 3D tags indicate the ablation points. The patient has been free from any AF recurrence for 6 months after a single AF ablation, without using any anti-arrhythmic drug.
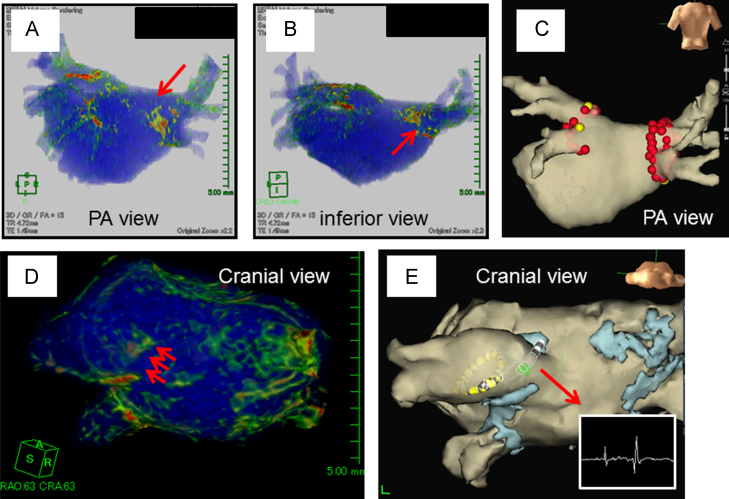
Fig. 3.
Adequate 3D visualization of an RF lesion in a patient with an AF recurrence. (A) PA (postero-anterior) view. The red arrow indicates a gap near the RSPV. (B) Inferior view. The red arrow indicates a gap at the bottom of the RIPV. (C) AF ablation using the NavX system in the first session. A, B, C are all images from patient 1. Complete PVI was accomplished without any RF applications to the LA posterior wall near the LPV. No DEs are visible in the same region in A. AF recurred 3 months following ablation in the patient; inadequate lesion formation at the RPV may have caused the AF recurrence. (D) Cranial view. The red arrow indicates a gap near the roof of the LSPV. (E) AF ablation using the NavX system in the second session. D and E are images from patient 2. The sharp potential was recorded at the gap of DEs. PV was re-isolated using a single RF application at this site. RSPV=right superior pulmonary vein, RIPV=right inferior pulmonary vein, LSPV=left superior pulmonary vein, PVI=pulmonary vein isolation, LPV=left pulmonary vein, RPV=right pulmonary vein.
4. Discussion
4.1. Main findings
The visualization of the RF lesions and atrial fibrosis using DE-MRI has been thoroughly investigated by a group from the University of Utah [2–4, 9–11]. Unfortunately, the complete visualization of DE requires specialized software. Thus, the precise methods for DE visualization clinically have still not been fully established. The study presented here demonstrates that RF lesions caused by AF ablation can be visualized using commercially available tools. Moreover, in order to achieve adequate visualization, the following are important: (1) A low HR (<70 bpm) during the MRI (achieved using metoprolol in this study), and (2) the MRI should be conducted at least 30 days after the AF ablation procedure.
4.2. When should we conduct DE-MRI to visualize RF lesions after AF ablation?
Peters et al. [5] reported that there was a significant relationship between DE thickness and the inverse of the number of days since the ablation and showed that DE thickness dramatically decreases with time, as the duration since AF ablation increases, reflecting a decrease in inflammation and maturation of scar tissue. This result is consistent with studies that have reported a decline in DE among patients examined early after and subsequent to myocardial infarctions. These results suggest that RF-induced lesions show transient inflammatory changes and slow the development of scar tissue, and that DE-MRI cannot adequately visualize RF lesions 30 days after AF ablation. In our cases, DE-MRI could not be used to visualize RF lesions in 4 (33%) of the 12 patients who had undergone DE-MRI 2, 2, 3, and 14 days after AF ablation, which is consistent with the results of a previous report [5]. Because inflammation and scar formation are stabilized, we recommend DE-MRI should be used to assess RF lesions at least 60 days after AF ablation.
4.3. Is the control of HR essential for achieving an adequate DE-MRI?
This study demonstrated that a lower HR during MRI was important for improving the quality of DE-MRI. As for the DE-MRI acquisition, the University of Utah group reported that ECG gating can be used to acquire a small subset of phase encoding views during the diastolic phase of the left atrial cardiac cycle [2,11]. The time interval between the R-peak of the ECG and the start of the data acquisition was defined by examining the cine images of the left atrium in order to determine the period of minimal left atrial motion. They concluded that a typical value is an interval of 60% of the mean RR interval for patients in SR. Obviously, a regular HR and a long RR interval during each cardiac cycle can reduce the cardiac motion, which results in achieving a better DE-MRI. Nonetheless, we do not consider the observed differences in HR (~10 bpm) between the adequate DE-MRI group and the inadequate DE-MRI group in the current study (68±5 vs. 79±9 bpm) to be critical because the precise anatomy of the PVs and the left atrium could still be depicted in both groups (Figs. 1A and 2A). We conclude that the impact of HR is likely less significant for the visualization of DE compared to that of the time from the AF ablation to the MRI acquisition date (at least >30 days).
4.4. RF lesions assessed by DE-MRI and AF recurrence
McGann et al. reported that a gap in RF lesions at the PV antrum analyzed using DE-MRI correlated with incomplete electrical isolation [11]. In our study, during a follow-up of 5.3±2.5 months, AF recurred in 2 (17%) of the 12 patients. Moreover, DE-MRI showed a gap in the RF lesions in both patients (Fig. 3). An inadequate RF lesion formation could cause PV-LA reconduction. In contrast, a complete RF lesion formation without a gap was clearly demonstrated in the remaining 10 patients without AF recurrences. These findings are complimentary to those of the University of Utah group, and the quality of the visualization appears to be equivalent to that obtained in their work [11].
4.5. What is different from the University of Utah protocol?
The DE-MRI acquisition was conducted according to the University of Utah protocol. However, the differences in the protocol are as follows: (1) The TE of the scan (2.3 ms) was “not” chosen to ensure that fat and water are out of phase. (2) The ECG gating was used to acquire a subset of phase-encoding views during the mid-diastolic phase of the “left ventricle (LV)” cardiac cycle, whereas the diastolic phase of the “LA” was used previously.
The University of Utah group suggested that the TE of the scan be chosen so that fat and water are “out of phase”, and that the signal intensity of partial volume fat-tissue voxels are reduced, allowing for an improved delineation of the LA wall boundary (“paradoxical suppression”) [7,8]. However, in this study, we chose not to follow this part of the protocol to avoid any unexpected suppression of the LA anatomy. Furthermore, with our method, we noted dramatically less motion artifact of the LA when ECG gating was used during the mid-diastolic phase of the “left ventricle” cardiac cycle than that during the diastolic phase of the LA [12]. We believe that our protocol actually allowed for the identification of a precise LA wall boundary without using the “paradoxical suppression” technique. Our typical mean RR interval value was 70% for patients in SR. In the 2 cases of AF that occurred during the MRI, the LA motion differed during each cardiac cycle throughout AF even after using ECG gating. Thus, the trigger delay of the cardiac synchronization was set to the shortest value. Therefore, the impact of the ECG gating was likely to be less in patients with AF than in those with SR.
4.6. Study limitations
Our study has three major limitations. First, the sample size was relatively small. Second, the relationship between the gap assessed by DE-MRI and the PV-LA reconduction could not be demonstrated because no follow-up AF procedures were performed; this relationship should be investigated in a further study. Third, while most of the patients were in SR during MRI acquisition, we were unable to determine image quality of MRI in patients with AF during MRI acquisition. Finally, we may have overestimated the size of the RF lesion, because we had not previously assessed the original atrial fibrosis using DE-MRI before AF catheter ablation.
5. Conclusion
To adequately visualize RF lesions, an MRI acquisition should be performed at least 30 days after AF ablation in patients preferably with a low HR in SR. By improving some of the clinical and procedural parameters during MRI, we could visualize RF lesions using DE-MRI, although we used only commercially available tools.
Conflict of interest
None.
Financial support
None.
Acknowledgments
We would like to thank Mr. John Martin for his assistance with language.
References
- 1.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–2738. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 2.Oakes R.S., Badger T.J., Kholmovski E.G. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGann C., Akoum N., Patel A. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrouche N.F., Wilber D., Hindricks G. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. J Am Med Assoc. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 5.Peters D.C., Wylie J.V., Hauser T.H. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 6.Daccarett M., Badger T.J., Akoum N. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuppahally S.S., Akoum N., Badger T.J. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–884. doi: 10.1016/j.ahj.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akoum N., Fernandez G., Wilson B. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1104–1109. doi: 10.1111/jce.12199. (PubMed PMID: 23844972.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmar B.R., Jarrett T.R., Burgon N.S. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J Cardiovasc Electrophysiol. 2014;25:457–463. doi: 10.1111/jce.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi K., Akkaya M., Akoum N. Cardiac MRI assessment of atrial fibrosis in atrial fibrillation: implications for diagnosis and therapy. Heart. 2014;100:590–596. doi: 10.1136/heartjnl-2013-303884. [DOI] [PubMed] [Google Scholar]
- 11.McGann C.J., Kholmovski E.G., Oakes R.S. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 12.Pang J., Sharif B., Arsanjani R. Accelerated whole-heart coronary MRA using motion-corrected sensitivity encoding with three-dimensional projection reconstruction. Magn Reson Med. 2014 doi: 10.1002/mrm.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]