Abstract
Purpose
Accumulation of collagen crosslinks (advanced glycation end products [AGEs]) produced by non-enzymatic glycation deteriorates bone's mechanical properties and fracture resistance. Although a single AGE, pentosidine, is commonly used as a representative marker, it is unclear whether it quantitatively reflects total fluorescent AGEs in bone. The goal of this study was to establish the relationship between pentosidine and total AGEs in cancellous and cortical bone.
Methods
Pentosidine and total AGEs were quantified in 170 human bone samples. Total fluorescent AGEs were measured in 28 additional cancellous and cortical bone specimens of the same apparent volume that were incubated in control or in vitro glycation solutions. Correlations between pentosidine and total AGEs and differences between cortical and cancellous groups were determined.
Results
Pentosidine was correlated with total AGEs in cancellous bone (r=0.53, p<0.0001) and weakly correlated in cortical bone (r=0.23, p<0.05). There was more pentosidine (p<0.01) and total AGEs (p<0.001) in cancellous than in cortical bone. The in vitro glycation sub-study showed that cancellous bone accumulated more AGEs than cortical bone (p<0.05).
Conclusion
The relationship between pentosidine and total AGEs and their magnitude of accumulation differed in cancellous and cortical bone of the same apparent volume, and were dependent on the surface-to-volume ratios of each sample. It is important to consider the bone types as two separate entities, and it is crucial to quantify total AGEs in addition to pentosidine to allow for more comprehensive analysis of the effects of non-enzymatic glycation in bone.
Keywords: Non-enzymatic glycation, pentosidine, advanced glycation end-products, bone, crosslinks
Introduction
Bone is subjected to a variety of molecular level modifications with aging that alters its extracellular matrix [1]. In particular, type I collagen, which comprises 90% of bone's organic matrix, undergoes numerous biochemical modifications including non-enzymatic glycation (NEG) [2, 3]. NEG in bone induces a cascade of biochemical processes, collectively known as the Maillard reaction, which involves a spontaneous interaction between an extracellular sugar-derived aldehyde group and, for example, the ε-amino group of collagen-bound hydroxylysine or lysine. The resulting glucosyl-lysine rearranges to form an Amadori product or Schiff base adduct, both of which undergo further reactions with other amino groups [3-5]. These processes produce a family of molecules known as advanced glycation end products (AGEs) that form as crosslinks within and across collagen fibers, and many of them are naturally fluorescent [3, 5, 6].
AGEs have been quantified by two different methods. The first method involves measurement of AGEs using a fluorometric assay in which AGEs are quantified based on bulk fluorescence and normalized to collagen content. The fluorometric method has been validated, showing significantly higher AGE content in in vitro glycated bone compared to non-glycated controls [7]. In contrast, the second method characterizes AGEs by a single crosslink, pentosidine. This method involves a high performance liquid chromatography technique that separates bone components based on their ionic properties using gradient elution, and ultimately, leads to isolation and determination of pentosidine concentration [8, 9]. Among the numerous non-enzymatic crosslinks in bone (e.g. pentosidine, glucosepane, methylimidazolium, glyoxalimidazolium, carboxymethyllysine, carboxyethyllysine) [10, 11], pentosidine is currently the most commonly measured AGE in bone [2, 3, 10].
The age-related degradation of bone's mechanical properties has been associated with the accumulation of pentosidine. Recent studies have shown that pentosidine explains up to 23% of the variation in bone fracture toughness [12] or as low as only 9% of the variance in trabecular ductility [13]. However, it is not known whether pentosidine quantitatively reflects the content of other AGEs that may also contribute to the overall effect of non-enzymatic glycation in bone. Given that pentosidine is present in small amounts in bone [5, 14], it is likely that other AGEs contribute to changes in bone fracture resistance. Thus, it is important to determine whether pentosidine measurement provides an overall assessment of the effects of NEG on bone [5, 13]. The accumulation of AGEs in bone is the net result of rate of formation by NEG and rate of removal by bone remodeling [3, 15]. Thus, the intrinsic differences in bone remodeling rates between cancellous and cortical bone would affect the AGEs removal rate, suggesting that crosslink presence may differ between cancellous and cortical bone due to their notably different turnover rates. These bone types also have different surface-to-volume ratios. It is possible that larger surface areas that provide increased contact between free floating sugars and amino acid residues on collagen may lead to amplified crosslink formation. These factors combined may alter the molecular profile of AGEs, and measures of pentosidine may not necessarily similarly represent total AGEs in both bone types.
To address the above concerns, cortical and cancellous bone specimens, obtained from adult human tibiae and femurs of varying ages were measured for pentosidine and total fluorescent AGE content. Additionally, an in vitro glycation sub-study was conducted on paired cancellous and cortical bone specimens of same apparent volume to validate ex vivo results and determine whether differences in bone surface area alter the accretion of AGEs. Our goal was to establish the extent to which pentosidine can be used as a surrogate marker to represent total fluorescent AGEs in both cancellous and cortical bone, and to elucidate a possible mechanism for the differential accumulation of AGEs in the two bone types.
Methods
Specimen Collection
A total of 170 bone specimens (79 cancellous, 91 cortical) were obtained from the proximal end of human tibiae (64 cancellous, 82 cortical) and femurs (15 cancellous, 9 cortical) from male and female donors (age range: 18 to 97, male n=94, female n=76). A subset of cancellous (n=31) and cortical (n=31) specimens were paired in order for direct comparison of AGEs between cancellous and cortical bone. All donors were tested negative for known bone metabolic diseases, and none of the donors were diagnosed with osteoarthritis (National Disease Research Interchange and International Institute for the Advancement of Medicine). These specimens were stored in saline at -80°C until used for quantification of pentosidine and/or total fluorescent AGEs.
In Vitro Glycation
An additional 28 human tibial cortical and cancellous bone specimens (14 pairs, age range: 34 to 70, average age: 50.4±13.0) were obtained. Specimens were cut into cubic structures with the same apparent volumes (2×2×2 mm3). These specimens were randomly divided such that 1 pair of cancellous and cortical specimens from each donor were used as controls while a second pair was glycated. A glycation solution was made containing 25 mM ε-amino-n-caproic acid, 5 mM benzamidine, 10 mM N-ethylmaleimide, 30 mM HEPES, and 0.6 M ribose in Hank's buffer. The control solution was made in the same fashion but without any ribose. Bone specimens were placed in centrifuge tubes, submerged in the corresponding solutions, and incubated at 37°C for 7 days as performed in previous studies [7, 16]. The pH for the incubation solutions was monitored daily and maintained between 7.2 and 7.6 using 0.5 M hydrochloric acid or sodium hydroxide to lower or raise the pH, respectively. These specimens were stored in saline at -80°C until quantification of total fluorescent AGEs.
Quantification of Collagen Crosslinks
Bone specimens were first flushed with cold nanopure water until free of blood, and then defatted by three 15 minute washes in 500 μL cold isopropyl ether under constant agitation. The bone samples were lyophilized overnight using a freeze dry system (Labconco), and hydrolyzed according to dry mass in 6N HCl (10 μL/mg bone) for 20 hours at 110°C. Hydrosylates were centrifuged at 13000 rpm at 4°C for 30 minutes to remove any debris. The centrifuged hydrosylates were used for the measurement of pentosidine via ultra-high performance liquid chromatography (UPLC, Waters Corporation), and for quantification of total fluorescent AGE content through a fluorometric assay. All centrifuged hydrosylates were stored at -80°C in complete darkness until use.
Separations via ultra-high performance liquid chromatography for pentosidine were completed according to recently developed protocols [9]. Collagen purity using this methodology has been previously confirmed in our laboratory using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Additionally, intra- and inter-assay variability were both <2% [9]. Each hydrosylate was used to calculate collagen content after hydroxyproline measurement using a hydroxyproline kit (BioRad). A pentosidine stock (International Maillard Reaction Society, Case Western Reserve University) was used as an external standard. Routinely, separations were performed for all standards and each specimen hydrosylate. Separations for hydroxyproline were completed at 60°C with a flow rate of 0.5 mL/min (detector: 471 nm). For fluorescent crosslinks, separations were conducted at 40°C with a flow rate of 0.667 mL/min (pentosidine at 335/385 nm excitation/emission) [9]. The amount of collagen per sample was determined based on hydroxyproline quantity [17], and crosslink content was normalized to the amount of collagen in each specimen.
Total fluorescent AGEs were quantified similarly as in previous studies [7, 9]. Fluorescence was measured for quinine standards (stock: 10 μg/mL quinine per 0.1 N sulfuric acid) and hydrosylates at 360/460 nm excitation/emission using an Infinite 200 microplate reader (Tecan). A chloramine-T solution was added to hydroxyproline standards (stock: 2000 μg/mL L-hydroxyproline per 0.001 N HCl) and sample hydrosylates. The resulting solution was then incubated at room temperature to oxidize hydroxyproline. To quench residual chloramine-T, 3.15 M perchloric acid was added and incubated at room temperature. Finally, a p-dimethylaminobenzaldehyde solution was added and incubated for at 60°C. All standards and specimens were cooled at room temperature in darkness. The absorbance was measured at 570 nm using an Infinite 200 microplate reader (Tecan). Similar to pentosidine measurements, collagen content was calculated based on hydroxyproline quantity [17]. Total fluorescent AGEs were quantified in terms of ng quinine per mg collagen.
Statistical Analyses
Outliers were determined as values that were beyond two standard deviations from the mean of each group. Because data were not normally distributed for in vivo crosslink quantification, non-parametric statistical analyses were used. Spearman correlations were run between pentosidine and total fluorescent AGE content separately in cancellous and cortical groups. Mann Whitney Rank Sum tests were used to determine differences between cancellous and cortical groups in all specimens and also in paired subsets.
For the in vitro glycation sub-study the data were normally distributed and differences between cancellous and cortical specimens in control and glycated groups were determined using paired T-tests. All cancellous specimens were pooled together (control and glycated) as were cortical specimens. Differences between cancellous and cortical specimens were then determined for these pooled groups. All statistical tests were performed with SigmaStat version 2.03 and JMP Pro 9.0.
Results
Age-Related Trends
The cancellous group (n=79) had a mean age of 61.4 ± 20.0 years while the cortical group (n=91) had an average age of 62.1 ± 20.8 years. The age distribution of the two groups was statistically indistinguishable (p=0.66). In cortical bone with both tibiae and femurs pooled together, donor age (Figure 1) was positively correlated with total fluorescent AGEs (r=0.486, p<0.0001), but was not significantly associated with pentosidine (p=0.12). In contrast, neither total fluorescent AGEs (p=0.12) nor pentosidine (p=0.09) demonstrated a statistically significant relationship with donor age in cancellous bone with tibiae and femurs pooled together (Figure 1). However, in femurs alone (Figure 1), there were significant relationships between age and AGEs in cancellous (r=0.799, p<0.001) and cortical (r=0.750, p<0.001) bone. Additionally, there was also an association between age and pentosidine in both cancellous (r=0.280, p<0.001) and cortical (r=0.717, p<0.001) bone. In tibiae alone (Figure 1), there was a significant relationship between age and AGEs only in cortical bone (r=0.460, p<0.001). Also, there was an association between age and pentosidine only in cancellous bone (r=0.385, p<0.01).
Fig. 1.
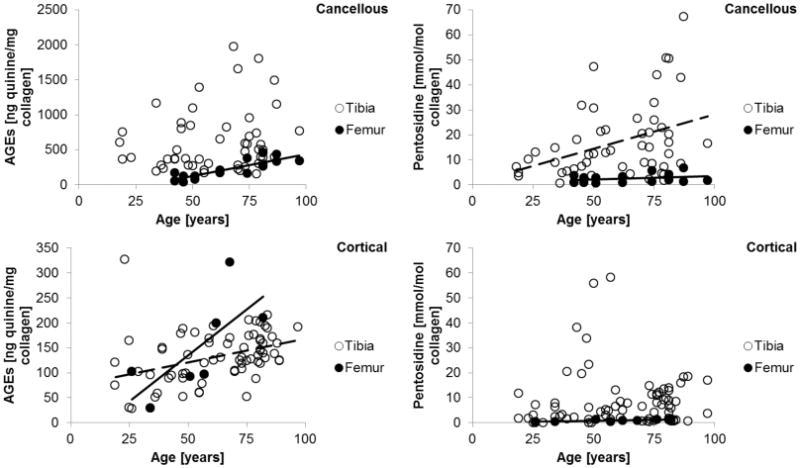
The relationship between advanced glycation end-products and age in (top) cancellous bone and (bottom) cortical bone. Data and representative linear fits for significant correlations are shown as a solid line for femurs and dotted line for tibiae.
Relationship between Pentosidine and Total Fluorescent AGEs
Pentosidine and total fluorescent AGEs were positively correlated in cancellous bone in tibiae (r=0.314, p<0.05) and femurs (r=0.700, p<0.01). Similarly, this association was observed in cortical bone in tibiae (r=0.249, p<0.05), but not in femurs (p=0.422). When tibiae and femurs were pooled together, we found that pentosidine had a significant positive correlation with total fluorescent AGEs in both cancellous (r=0.530, p<0.0001, Figure 2) and cortical (r=0.226, p<0.05, Figure 2) bone.
Fig. 2.

There is a relationship between pentosidine and total fluorescent AGEs in (left) cancellous bone and (right) cortical bone as determined by Spearman correlation.
Differences between Cancellous and Cortical Bone
In tibiae alone, there was 1.9 times more pentosidine (p<0.001) and 4.7 times more total fluorescent AGEs (p<0.001) in cancellous than in cortical bone. However, no differences were observed in pentosidine (p=0.321) or total fluorescent AGEs (p=0.237) in femurs. When combined, there was 1.7 times more pentosidine (p<0.01) and 4.0 times more total fluorescent AGEs (p<0.001) in cancellous than in cortical bone (Figure 3). In the paired subset, there was 2.9 times more AGEs (p<0.001), but no significant difference in pentosidine (p=0.18) between cancellous and cortical bone. In the in vitro glycated sub-study, glycated specimens had 59% and 85% more total AGEs than controls in cortical (p<0.01) and cancellous bone (p<0.01), respectively. Cancellous bone had more total AGEs than cortical bone (Figure 4) in both control (+21%, p=0.08) and glycated (+50%, p<0.05) specimens as well as in both groups pooled together (+43%, p<0.05).
Fig. 3.
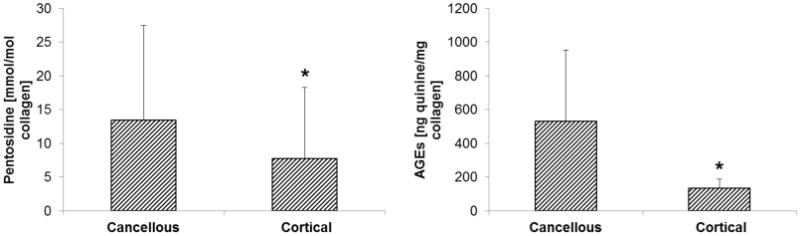
In human cadaveric bone, there was significantly more (left) pentosidine and (right) total fluorescent AGEs in cancellous bone than in cortical bone. Asterisks denote significant differences.
Fig. 4.

In vitro glycation data show that cancellous bone accumulated more total fluorescent AGEs than cortical bone in control, glycated, and pooled groups. Asterisks denote significant differences.
Discussion
Conventionally, pentosidine is used as a surrogate biomarker of AGEs accumulation, but it is not known whether changes in pentosidine concentration quantitatively reflect the content of other possibly more abundant AGEs that are also associated with bone strength [11, 12]. Specifically, it was unknown whether pentosidine alone could be used as a reliable representative of total AGEs to predict bone mechanical properties and fracture risk. Hence, we proposed to establish the association between pentosidine concentration and total fluorescent AGE content. We were able to measure both pentosidine and bulk fluorescent crosslink quantity from the same hydrosylates obtained from a very large selection of human cadaveric cancellous and cortical bone specimens. Furthermore, comparisons between cancellous and cortical bone were made in both paired and unpaired groups showing increased non-enzymatic glycation in cancellous compared to cortical bone. Paired and unpaired groups show the same trends although the level of statistical significance and power varied among the groups. The mechanism by which AGE formation differed between cancellous and cortical bone was unclear, which led us to conduct an in vitro glycation sub-study to investigate AGE accumulation in the two bone types. Overall, we found that there was a significant positive relationship between pentosidine quantities and total fluorescent AGEs in cancellous and cortical bone, and that the increased quantities of these crosslinks in cancellous than in cortical bone may be accounted for by their different surface-to-volume ratios.
We found that total fluorescent AGEs and pentosidine showed strong associations with age in both cancellous and cortical bone in femur specimens only, but the same relationships were not observed in both bone types for tibial specimens. These results are consistent with previous work investigating bone toughness in both femurs and tibiae, which showed that age-related trends were more evident in femur specimens [18]. Similar to Odetti et al's work in which specimens from both femurs and tibiae were pooled together, we also observed age-related trends in pooled specimens in cortical bone only while age-related trends in cancellous bone were not statistically significant [19]. Furthermore, relationships between pentosidine and total fluorescent AGEs as well as differences in cancellous and cortical bone had similar trends in both tibiae and femurs, but the level of statistical significance varied. These data suggest that there may be site-dependent differences in AGE accumulation in cancellous and cortical bone, but further investigation is needed with increased femur sample size to confirm these results. The remainder of our discussion is based on the analyses conducted on pooled data for tibia and femur specimens.
In our study, crosslink content was on the same order of magnitude as previously reported [7, 13, 20]. Previous work in a canine model elegantly demonstrates that the suppression of bone turnover in young healthy canine animals reduces turnover and subsequently increases bone AGE content [21]. Our samples taken from patients >60 years old, perhaps a more clinically relevant population, reveal that cancellous bone, which has biologically faster turnover, also has a higher accumulation of AGEs, suggesting that the rate of AGEs formation may also be altered with increasing age. We also acknowledge that only limited medical histories were available for some donors, and hence there is a possibility that some of the older donors used bisphosphonate drugs, which suppresses turnover. The reduced or imbalanced turnover in this study population would allow for longer exposure of collagen to the extracellular environment and lead to increased AGE production. The varying turnover rates in these donors may be one factor accounting for differences in cortical and cancellous bone (including age-related trends), but further work must be done to clarify and understand these trends.
Furthermore, the elderly, and particularly osteoporotic patients, have a higher prevalence of trabecular rods than plates [22-24]. Recent work in our laboratory showed that rods are more glycated than plates [25] and may be preferentially retained due to an inverse relationship between resorption and extent of glycation [26]. We did find that there were high levels of non-enzymatic glycation in cancellous bone, suggesting that the cancellous specimens in our study did contain older bone tissue.
In particular, the presence of more AGEs in cancellous than in cortical bone may reflect the influence of greater surface-to-volume ratio in cancellous than in cortical bone. Non-enzymatic glycation is a surface-based phenomenon and greater surface area in cancellous than in cortical bone (for the same bone volume) may indeed translate into a higher accumulation of AGEs. To investigate this idea, we performed an in vitro glycation sub-study to induce the non-enzymatic glycation process on specimens that had the same apparent bone volume. The same apparent volume in cancellous and cortical bone specimens translates to having drastically different surface-to-volume ratios where cancellous bone would have increased access for sugars to reach the bone surface and interact with amino acid residues to form AGEs. Our in vitro results indeed support this hypothesis. Moreover, previous findings in our laboratory showed in cancellous bone that levels of total fluorescent AGEs (r=0.54, p<0.01) and pentosidine (r=0.38, p<0.05) were higher in specimens with greater structure model index (SMI) [25]. SMI is a computed measure based on the change in surface area per small increases in volume [27]. Hence, higher SMI represents higher surface-to-volume ratio of the trabecular structures, which would allow increased formation of AGEs, and these results further emphasize the role of bone surface-to-volume ratio in AGEs accumulation.
The above results are in contrast with a previous study by Odetti et al who found that cortical bone was more glycated than cancellous bone [19]. The bone specimens obtained by Odetti et al's study were from patients undergoing surgery due to a fracture or loosening of a prosthetic implant while our specimens were obtained from non-fracture and non-prosthetic donors. Selection of specimens from an injury site introduces a bias as cancellous bone at injury sites undergo active remodeling and results in freshly formed bone that is more immature and consequently less glycated. Furthermore, specimens in our study were carefully paired and/or obtained from anatomically similar locations across selected donors.
We also found that in cancellous bone, pentosidine composed a small portion of the total fluorescent AGE content and explained only 28% of total fluorescent AGEs. The relationship between pentosidine and total fluorescent AGE quantity was even weaker in cortical bone where pentosidine explained only 5% of total fluorescent AGEs. The difference in association between pentosidine and total fluorescent AGEs in cortical compared to cancellous bone may be due to several reasons. First microarchitectural changes and a faster rate of bone loss with aging and disease in cancellous than in cortical bone [28] can affect the relationship between pentosidine and AGEs wherein the older and more glycated rods contain more pentosidine [25] that is more likely to form following Amadori rearrangement at a later stage of the glycation process. Furthermore, as demonstrated by our results, due to a greater access of sugar to bone surface, NEG may proceed faster and result in formation of more AGE crosslinks including pentosidine. More dose- and time- dependent in vitro and in vivo studies are needed to determine the mechanistic basis of the relationship between AGEs and pentosidine in cortical and cancellous bone.
In conclusion, we observed that pentosidine accounts for a small proportion of total fluorescent AGEs accumulation in cancellous and cortical bone. The different relationships between pentosidine and total AGEs observed in cancellous (r2 =0.28, p<0.05) and cortical (r2 =0.05, p<0.05) bone indicates the importance of quantifying total fluorescent AGEs rather than just pentosidine alone to comprehensively analyze the effect of non-enzymatic glycation on bone. Moreover, the increased AGEs in cancellous than in cortical bone may be the result of its increased surface-to-volume ratio, which allows cancellous bone increased access to extracellular sugars for crosslink formation. Because the relationship between pentosidine and total AGEs and their magnitude of accumulation differed in cancellous and cortical bone, it is important to consider each bone tissue type as separate entities when investigating the effects of non-enzymatic glycation on bone.
Acknowledgments
This study was funded by National Institute on Aging grant AG20618 (Vashishth) and National Institute of General Medical Sciences training grant T32 GM067545 (Karim). Human cadaver bones were obtained from the International Institute for the Advancement of Medicine and also from the National Disease Research Interchange through the National Institutes of Health grant 5 U42 RR006042.
Footnotes
Conflicts of Interest: No disclosures.
References
- 1.Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–1310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 3.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5:62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 4.Robins SP, Bailey AJ. Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun. 1972;48:76–84. doi: 10.1016/0006-291x(72)90346-4. [DOI] [PubMed] [Google Scholar]
- 5.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent crosslinks of collagen. Anal Biochem. 1997;253:26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- 9.Sroga GE, Vashishth D. UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:379–385. doi: 10.1016/j.jchromb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vashishth D. Advanced glycation end-products and bone fractures. IBMS BoneKEy. 2009;6:268–278. doi: 10.1138/20090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–832. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–11660. [PubMed] [Google Scholar]
- 15.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 16.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 17.Gross J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. J Exp Med. 1958;107:247–263. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman TL, Yeni YN, Brown CU, Wang Z. Influence of microdamage on fracture toughness of the human femur and tibia. Bone. 1998;23:303–306. doi: 10.1016/s8756-3282(98)00103-3. [DOI] [PubMed] [Google Scholar]
- 19.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–717. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 20.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding M, Hvid I. Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone. 2000;26:291–295. doi: 10.1016/s8756-3282(99)00281-1. [DOI] [PubMed] [Google Scholar]
- 23.Müller R, Gerber SC, Hayes WC. Micro-compression: a novel technique for the nondestructive assessment of local bone failure. Technol Health Care. 1998;6:433–444. [PubMed] [Google Scholar]
- 24.Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 25.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–5703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand T, Rüegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 28.Mundy GR. Pathogenesis of osteoporosis and challenges for drug delivery. Adv Drug Deliv Rev. 2000;42:165–173. doi: 10.1016/s0169-409x(00)00060-0. [DOI] [PubMed] [Google Scholar]


