Abstract
Interaction of programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) plays a critical role in regulating the delicate balance between protective immunity and tolerance. Human neuroimmune cells express very low or undetectable levels of PD-1/PD-L1 in normal physiological condition.We seek to examine if exposure of these cells to drug of abuse such as methamphetamine (METH) alters the profile of PD-1/PD-L1 levels, thereby dampens the innate immune response of the host cells. Thus, we assessed the changes in the levels of PD-1/PD-L1 in primary human macrophages, brain endothelial cells (hBECs), astrocytes, microglia, and neurons after exposure to METH. We observed that stimulation of these neuroimmune cells by METH responded differentially to PD-1/PD-L1 expression. Stimulation of macrophage culture with 50 μM of METH exhibited immediate gradual upregulation of PD-L1, while increase in PD-1 took 2-4 hours later than PD-L1. The response of hBECs to PD-1/PD-L1 induction occurred at 24 hours, while increase of PD-1/PD-L1 levels in neurons and microglia was immediate following METH exposure. We found that astrocytes expressed moderate levels of endogenous PD-1/PD-L1, which was diminished by METH exposure. Our findings show a differential expression of PD-1/PD-L1 in neuroimmune cells in response to METH stimulation, suggesting that PD-1/PD-L1 interplay in these cell types could orchestrate the intercellular interactive communication for neuronal death or protection in the brain environment.
Keywords: Methamphetamine, programmed cell death 1, neuroinflammation, neurodegeneration, neuroimmune cells
Introduction
Methamphetamine (METH) is the most addictive psycho stimulant illicit drug [1]. METH ranks the top illicit drug widely used in the world as an euphoric and peaceful feeling stimulant, besides cannabis. A 64.4 million METH users account for 48.3 billion dollars, while a 164 million users of cannabis account for 141.8 dollars, in world black market value [2]. The Hawaiian Islands and the mid-western states top the highest users of METH among teenagers and young adults in the US [3]. METH initially causes the release of dopamine, serotonin and norepinephrine followed by a gradual depletion of dopamine, causing highly addictive effect among METH users [4,5]. The chronic use of METH causes a long-term structural damage to regions of the brain that regulate mood and cognitive function [6]. The possible mechanisms of neurotoxicity with METH dependence are primarily attributed to deleterious metabolic effects of the dopaminergic [7-9], serotonergic [10], noradrenergic [11], and glutamatergic systems [12,13].
Recent studies have indicated the involvement of neuroimmune factors, such as cytokines, chemokines, and cellular adhesion molecules in perpetuating METH-induced neuronal degeneration and neuropsychiatric complications [14-17]. It is likely that immune-mediated tolerance and cell apoptotsis may play a role in METH-induced neuroinflammation and neurodegeneration. Although METH abuse is implicated in immune dysregulation, the relationship between METH exposure and the ability of the host to elicit protective immunity like T-cell immune reactions is not known. T cells express co-stimulatory and co-inhibitory accessory receptors, wherein a delivery of additional co-stimulatory signals by antigen presenting cells (APC) is crucial for regulatory function of T cellsduring viral infection or substance abuse. In other word, T cell activation requires a T cell receptor mediated signaling, in which a positive or a negative co-stimulation by APC such as macrophage decide the impact and duration of the T cell activation [18].
In this complex regulation of T cell response in neuroimmune environment and peripheral tolerance, programmed cell death 1 (PD-1) receptor and its ligand PD-L1 or PD-L2 have emerged as critical inhibitory signaling pathways. PD-L1 (A B7-homologue-1/B7-H1) is a new member of the B7-CD28 family, which is the main ligand of PD-1 receptor. The interaction of PD-L1/PD-L2 with PD-1 is known to induce a co-inhibitory signal in activated T-cells, thereby promotes T-cell apoptosis, energy exhaustion and immune-mediated tissue damage [19]. Thus, blocking the interactions of PD-L1 and PD-1 has emerged to be highly beneficial in the treatment of cancers [20-22] and viral infections [23,24]. In the brain, blocking the engagement PD-L1 and PD-1 plays a critical role in preventing neuroinflammation, such as in chronic active multiple sclerosis [25,26]. Thus, expression of PD-L1 and PD-1 has been shown in brain cell types and also in various neuropathological conditions [27-30].
In general neuroimmune cells are known to express very low or undetectable levels of PD-1/PD-L1 in normal physiological conditions. The present study seeks to investigate whether exposure of neuroimmune cells to METH as in real life scenario of drug abusers can induce the expression of PD-L1 and PD-1 levels. To achieve this goal, we examined the METH-induced alterations of PD-1/PD-L1 levels in primary human macrophages, brain endothelial cells (hBECs), astrocytes, microglia, and neurons in culture. Uncovering this knowledge gap will be of great significance since the regulation of PD-1/PD-L1 in neuroimmune cells during drug abuse is not known to date. This study has the potential to unravel the mechanisms of brain cell death via an interactive regulation of PD-L1/PD-1 under METH exposure. Further, the role of the infiltrated macrophage in regulating PD-L1/PD-1 as co-stimulatory or co-inhibitory to brain cells will be novel finding in drug abuse.
Materials and methods
Antibodies
Antibodies to PD-L1, PD-1, CD68, von Willebrand factor, and GFAP were obtained from Abcam (Cambridge, MA). MAP2 antibody and all secondary Alexa Fluor antibodies were from Invitrogen.
Monocytes
Monocytes isolation was carried out at tissue core facility in the Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, by counter current centrifugal elutriation of leukopheresis packs from HIV-1, HIV-2, and hepatitis B-seronegative blood donors. Monocytes were cultured in 12 well plates (300,000 cells/well) in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated pooled human serum, 1% glutamine, 50 μg/ml gentamicin, 10 μg/ml ciprofloxacin (Sigma), and 1000 U/ml highly purified recombinant human macrophage colony-stimulating factor. Purity of macrophage is assessed by CD68 antibody.
Human brain endothelial cells
Primary human brain endothelial cells (hBECs) were obtained from Dr. Yuri Persidsky’s Laboratory, Temple University, PA, and hBECs were cultured as described previously [31]. Briefly, all cell culture plates and glass cover slips were pre-coated with collagen (0.09 mg/mL), aspirated the excess collagen and dried in sterile hood for 4 hours. Dried glass cover slips and plates were further pre-coated by fibronectin. Cells were plated on 12-well glass cover slips (40,000 cells/well) for immunohistochemistry analysis. DMEM/F-12 media containing 10 mM Hepes, 13 mM sodium bicarbonate (pH 7), 10% fetal bovine serum, penicillin and streptomycin (100 μg/ml each, Invitrogen) were used for cell culture. Cell culture media was changed every 3rd day until tight monolayers were formed in about 6-7 days. Purity of hBECs is assessed by antibody to von Willebrand factor or GLUT1.
Source of astrocytes, microglia and neurons
Cortical neurons, astrocytes and Microglia were obtained from the neural tissue core facility in the Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center. The core facility routinely isolates these cells from elective abortus specimens of human fetal brain tissues. All tissues were obtained in full compliance with the ethical guidelines of both the National Institutes of Health (NIH) and the University of Nebraska. Briefly, dissociated tissues were incubated with 0.25% trypsin for 30 min, neutralized with 10% fetal bovine serum, and further dissociated by trituration.
Astrocytes culture
Astrocytes were cultured in DMEM/F-12 media containing HEPES (10 mM), sodium bicarbonate (13 mM, pH 7), 10% fetal bovine serum, penicillin and streptomycin (100 µg/ml each, invitrogen) as described [32]. Purity of astrocytes was assessed by GFAP antibody, which normally showed 100% enrichment of astrocytes. 40,000 cells/well were plated on 12-well plates containing glass cover slips for immunocytochemistry. Cell culture media was changed every 3rd day until cells were confluent in about 4-5 days. Purity of astrocytes was assessed by GFAP antibody, resulting in 100% purity of astrocytes.
Microglia culture
Microglia were isolated and purified as previously described [33]. Briefly, brain tissue was dissociated with 0.25% trypsin for 30 min at 37°C. The resulting single-cell suspension was cultured in DMEM (Sigma Chemical Co.) supplemented with 10% fetal bovine serum and 1,000 U of MCSF per ml. After 14 days in culture, the nonadherent microglia cells were collected and purified by preferential adhesion. These procedures resulted in >98%-pure microglial cell populations. Microglia were cultivated at density of 40,000 cells/well in 12-well glass cover slips. Purity of microglia was assessed by Iba-1 antibody, resulting in 100% purity of microglia.
Neuron culture
Neurons were cultured on poly-D-lysine pre-coated cover slips and multi well plates (BD Labware, Bedford, MA) in Neurobasal™ Medium containing 0.5 mM glutamine, 50 µg/ml each of penicillin and streptomycin in combination with GIBCO™B-27 supplements with antioxidants as described previously [34]. Purity of neurons was assessed by MAP-2 antibody (Invitrogen), which normally showed 100% enrichment of neurons. 80,000 cells/well were plated on 12-well plates containing glass cover slips for immunocytochemistry. Cell culture media was changed every 3rd day until cells were confluent in about 10-12 days.
METH treatment
The non-toxic working concentrations of METH were determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) cell viability assays in response to METH dose-dependent studies. Briefly, cells were cultured in 96-well microtiter plates up to 90-100% confluency. Cells were treated for 48 hrs in the presence or absence of various concentrations of METH (5-500 μM) followed by addition of 100 μl MTT (5 mg/ml MTT in 10% FBS in 1X PBS) and 45 min incubation at 37°C. Following incubation, 100 μl DMSO was added just after aspirating the MTT solution and the plates were further incubated at room temperature for 15 min. Absorbance of the purple formazan was detected by a microtiter plate reader at 490 nm wavelength. We determined that the non-toxic highest concentration of METH for macrophage, endothelial cells, astrocytes, and microglia was 50 μM of METH, while that of neurons was 25 μM for 48 hrs exposure time point. Thus, all cell types cultured on glass cover slips in 12-well plates were exposed to 50 μM working concentration of METH, while neurons were exposed to 25 μM at the indicated time points for immunofluorescence fluorescent staining and microscopy analysis.
Immunofluorescence staining and microscopy analysis
All cell types exposed to given METH concentrations for up to 48 hrs were washed with PBS, fixed in acetone-methanol (1:1 v/v) fixative. Cellular antigens were blocked with 3% bovine serum albumin at room temperature for 1 hr in the presence of 0.4% Triton X-100. This was followed by incubation with respective primary antibodies like rabbit anti-PD-L1 (5 µg/ml) and mouse anti-PD-1 (1/50 dilution) overnight at 4°C. Post-incubation cells were washed with PBS followed by incubation for 1 hr with secondary antibodies, anti-rabbit-IgG alexa fluor Fluor 594 for PD-L1 and anti-mouse-IgG alexa fluor Fluor 488 for PD-1. Cover slips were then mounted onto glass slides with immunomount containing DAPI (Invitrogen), and fluorescence microphotographs were captured by fluorescent microscopy (Eclipse TE2000-U, Nikon microscope, Melville, NY) using NIS elements (Nikon, Melville, NY) software. Fluorescence intensity from four independent experiments was quantified by Sigma Scan Pro program, Image Analysis version 5.0.0, 1987-1999 SPSS Inc.
Statistical analysis
All immunoreactive fluorescence intensities were quantified by SigmaScan Pro program, Image Analysis version 5.0.0, 1987-1999 SPSS Inc. The non-specific background fluorescence intensity from untreated controls was subtracted from all experimental conditions. Quantification of specific fluorescence intensity was then carried as the difference between untreated controls and METH treated conditions. Comparisons between controls and experiments were performed by one-way ANOVA with Dunn’s post-hoc tests. Differences were considered significant at p-value <0.05, where, *P≤0.05, **P≤0.01, ***P≤0.001 and ****P≤0.0001 compared with controls. Statistical analysis were performed using GraphPad Prism version 6.00 for Mac OS X, GraphPad Software, La Jolla California USA, www.graphpad.com.
Results
METH exposure increases PD-1/PD-L1 in macrophages
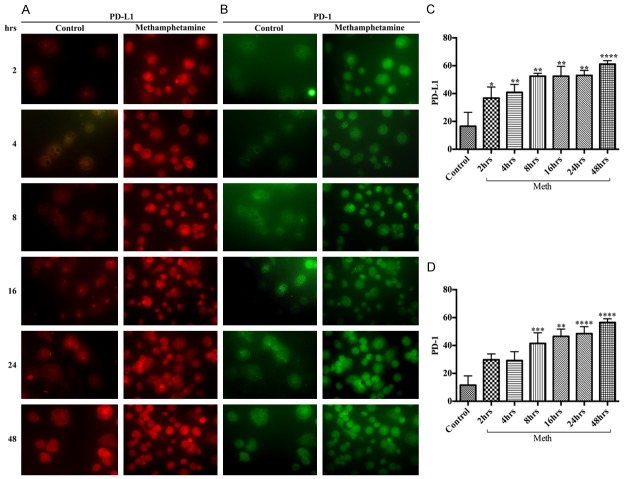
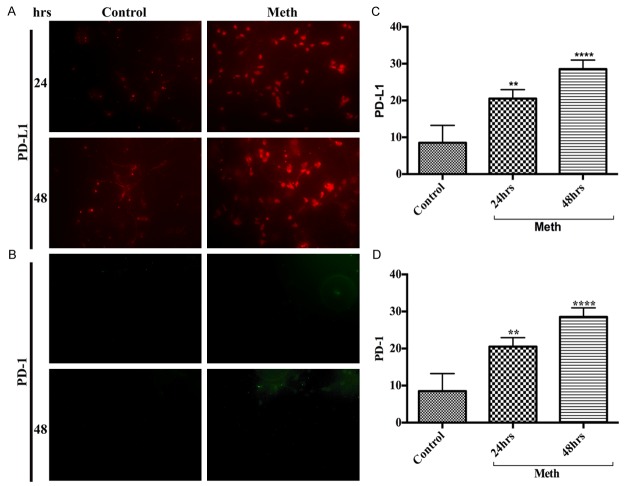
Macrophages are the major innate immune cells for antigen presentation that infiltrate into the brain through the BBB endothelium at the inflammatory sites. The up-regulation of PD-1/PD-L1 in infiltrated macrophage during drug abuse is expected to promote the initiation of neuroiflammation. Exposure of macrophage culture to 50 μM of METH showed a differential time-dependent increase in the expression between PD-1 and PD-L1. Wherein, METH exposure significantly increased PD-L1 levels starting at 2 hours (Figure 1A, 1C), while increase in PD-1 levels occurred only after 4 hours (Figure 1B, 1D), but both PD-1 and PD-L1 reached the maximum level of expression at 48 hours (Figure 1A-D). The levels of PD-1 and PD-L1 in the respective untreated controls were found to be very low, suggesting that induction of PD-L1 and PD-1 were mediated by METH exposure in human macrophages. Since the induction of PD-L1 precedes PD-1 in METH exposure, the data also suggests the order of ligand-receptor interaction in the programmed cell death-1 signaling pathway.
Figure 1.
METH exposure up-regulated PD-L1 and PD-1 levels in human macrophages. Representative immunocytochemical staining of (A) PD-L1 and (B) PD-1, in human macrophages with/without METH at different time points; Ranks-Kruskal-Wallis test for quantitative densitometric analysis of (C) PD-L1 and (D) PD-1 levels. *P≤0.05, **P≤0.01, ***P≤0.001 and ****P≤0.0001 are the statistical significance compared with controls.
Delayed induction of PD-1/PD-L1 in hBECs
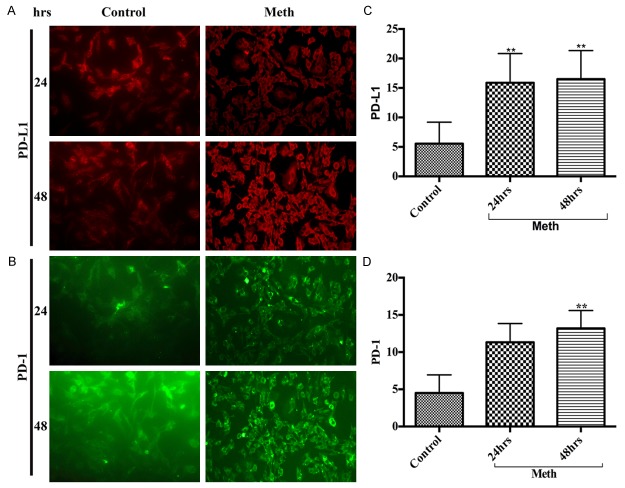
Adhesion of macrophages or T cells to the brain endothelial cells (BBB) is the primary step for recruiting immune cells into the brain. Our rationale is that changes in PD-1/PD-L1 expression in hBECs in response to METH stimulus may disrupt BBB and facilitate the migration of macrophages or T cells into the brain. We found an extremely low basal level of PD-1/PD-L1 in hBECs, but exposure of hBECs to 50 μM of METH significantly increased the levels of PD-L1 (Figure 2A, 2C) and PD-1 (Figure 2B, 2D) compared with controls. However, the up-regulation of PD-1/PD-L1 levels in hBECs started at 24 hours exposure, much later than that of macrophage. The maximum expression of PD-1/PD-L1 in hBECs was achieved at 48 hours of METH exposure. The role of PD-1/PD-L1 in hBECs for accelerating immune cells migration across the BBB and into the brain remains to be investigated.
Figure 2.
A delayed increase of PD-L1 and PD-1 in hBECs. Representative immunocytochemical staining of (A) PD-L1 and (B) PD-1, in hBECs with/without METH at 24 hrs and 48 hrs; Ranks-Kruskal-Wallis test for quantitative densitometric analysis of (C) PD-L1 and (D) PD-1 levels. **P≤0.01 is the statistical significance compared with controls.
METH exposure decreases PD-1/PD-L1 levels in astrocytes
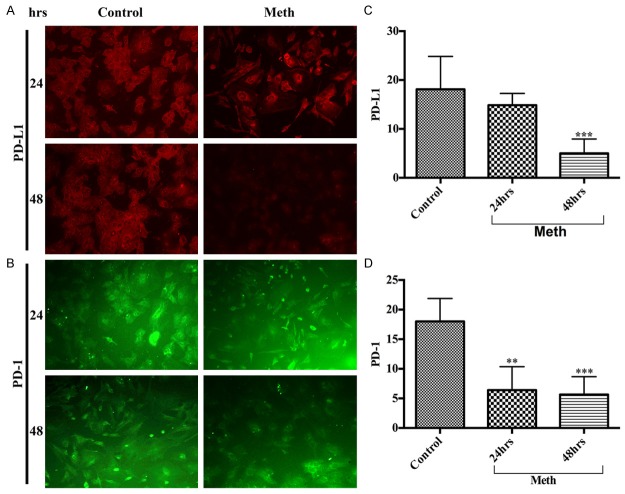
The astrocyte end-feet encircle the cerebral vessel endothelium, which form the perivascular unit of the BBB. We have shown that perivascular inflammation initiated neuronal degeneration in animal model of chronic METH administration. Here, we aim to address whether activation of PD-1/PD-L1 in astrocytes play a role in the development of perivascular inflammation. Unlike any other brain cell types, astrocytes exhibited high basal level expression of PD-L1 (Figure 3A, 3C) and PD-1 (Figure 3B, 3D) in controls compared with METH-treated. Interestingly, the endogenous levels of PD-1/PD-L1 in astrocytes decreased time-dependently in response to METH exposure, while the levels of PD-1/PD-L1 remained stable in untreated controls. These findings suggest an inherent protective role of PD-1/PD-L1 in astrocytes, perhaps affording towards neuroprotection. We will validate this argument in future studies with cell viability assays in astrocyte-neuron co-culture and in our animal model of chronic METH administration for better insight and understanding of the mechanism.
Figure 3.
METH down-regulated PD-L1 and PD-1 levels in human astrocytes. Representative immunocytochemical staining of (A) PD-L1 and (B) PD-1, in human astrocytes with/without METH at 24 hrs and 48 hrs; Ranks-Kruskal-Wallis test for quantitative densitometric analysis of (C) PD-L1 and (D) PD-1 levels. **P≤0.01 and ***P≤0.001 are the statistical significance compared with controls.
METH increases the levels of PD-1/PD-L1 in microglia
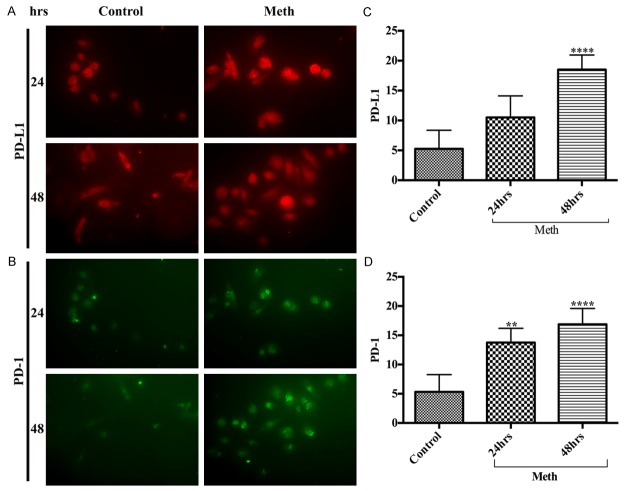
Microglia are the immune surveillance and major antigen presenting cells in the brain. The innate and adaptive immunity nature of microglia serves as the first line of host defense in the brain. Nevertheless, microglia are also the scavenging cells of the brain and a source of neuroinflammation during uncontrolled microgliosis. Microglia maintains a minimal basal level of PD-1/PD-L1, but this homeostasis appears to change drastically under inflammatory conditions. Since METH induces gliosis, we examined the alterations of PD-1/PD-L1 levels in human microglia following exposure to 50 μM METH at various time points. Our data revealed that METH exposure significantly increased the levels of PD-L1 (Figure 4A, 4C) and PD-1 (Figure 4B, 4D) in microglia time-dependently compared with basal controls. Our future goal is to examine the underlying molecular mechanism(s) of PD-L1-PD-1 interaction in METH exposure and to evaluate whether activation of PD-1/PD-L1 in microglia is an immune response to neuroprotection or neurodegeneration.
Figure 4.
METH exposure elevated the expression of PD-L1 and PD-1 in human microglia. Representative immunocytochemical staining of (A) PD-L1 and (B) PD-1, in human microglia with/without METH at 24 hrs and 48 hrs; Ranks-Kruskal-Wallis test for quantitative densitometric analysis of (C) PD-L1 and (D) PD-1 levels. **P≤0.01 and ****P≤0.0001 are the statistical significance compared with controls.
METH impacts a rapid turnover of PD-1/PD-L1 in neurons
We have demonstrated that neurons are highly sensitive to METH-induced inflammatory response both in-vivo and in-vitro findings [13,35]. We attributed the mechanisms of METH-induced neuronal degeneration to oxidative damage and metabolic energy deficiency. Here, we examined the role of PD-1/PD-L1 activation as a possible cause of neuronal loss in METH exposure. The basal levels of PD-1/PD-L1 in neuronal culture exhibited none to very low expression in untreated cells. But when neuronal culture was exposed to 25 μM of METH, the expression of PD-L1 (Figure 5A, 5C) and PD-1 (Figure 5B, 5D) markedly increased and reached the maximum increments at 24 hours in a time-dependent manner. Our data suggests the involvement of PD-1/PD-L1 activation in METH-induced neurodegenerative process, which requires further verification in future.
Figure 5.
METH up-regulated the expression of PD-L1 and PD-1 in human neurons. Representative immunocytochemical staining of (A) PD-L1 and (B) PD-1, in human neurons with/without METH at 24 hrs and 48 hrs; Ranks-Kruskal-Wallis test for quantitative densitometric analysis of (C) PD-L1 and (D) PD-1 levels. **P≤0.01 and ****P≤0.0001 are the statistical significance compared with controls.
Discussion
The programmed cell death-1 (PD-1) receptor and its ligand PD-L1 are known to express very poorly in human brain cells under normal physiological conditions [36], however, activated neuroimmune cells such as T cells, B cells, macrophages, dendritic cells, astrocytes and microglia [16,17], and non-immune cells such as endothelial and epithelial cells [18,19] appear to increase expression profile of PD-L1/PD-1 levels. For the first time, here we reported the differential induction of PD-L1/PD-1 levels in neuroimmune and non-neuroimmune cells by nontoxic concentrations of METH. The physiological relevance and significance of our present findings implicate the changes in PD-L1/PD-1 regulation in neuroimmune response in drug abuse.
Our findings revealed that neuroimmune cells, macrophages and microglia exhibited extremely low basal levels of PD-1/PD-L1, which were significantly elevated time-dependently by METH exposure. Induction of PD-L1 appeared to occur earlier than that of PD-1, suggesting that prior synthesis of the ligand PD-L1 might be essential for the activation of PD-1 signaling pathway. Our observation of PD-1/PD-L1 up-regulation in macrophage and microglia by METH implicated the dampening of the defensive innate immunity response of these cells in the peripheral as well as in the central nervous system. Elevation of PD-1 and PD-L1 expression in microglia by METH is expected to cause microgliosis and enhance neuroinflammation. It has been reported that activation of PD-1/PD-L1 by inflammatory cytokines as well as in animal model of mice lacking the ligands PD-L1 or PD-L2 led to neuroinflammation [36] and worsened the outcome of cerebral infarct volume and stroke [37].
We also observed that the basal levels of PD-1/PD-L1 in non-neuroimmune cells such as human brain endothelial cells (hBECs) were extremely low, but it was induced by METH treatment at a very slow process (see Figure 2A-D). Such extremely low expression of PD-1/PD-L1 in hBECs has been shown [26], and PD-L1/PD-L2 seemed to express well in human hBECs depending on pathological conditions, but mouse brain endothelial cells did not express PD-L1/PD-L2 [38]. Our future goal will be to examine whether induction of PD-1/PD-L1 by METH in hBECs plays a role in BBB impairment for acceleration of immune cells adhesion and migration across the BBB, and into the brain. This brings to the highlight of recent important discovery of PD-L1/PD-L2 expression in post-mortem human brain tissue sections [26]. Here, Pittet et al. (2011) observed that PD-L2 that is highly expressed in the BBB of control brain tissue was significantly diminished in brain tissue from multiple sclerosis (MS), while PD-L1 was not detectable at the BBB endothelium either in control or MS tissue sections. Our findings indicated a delayed induction of PD-L1 by METH, as such it will be of great interest if there is a switch mechanism of diminishing the endogenously expressed PD-L2 at the BBB with an upregulation of PD-L1 in chronic METH administration.
Interestingly, we observed that astrocyte was the only brain cell type showing a good basal level of PD-1/PD-L1, which seemed to decrease time-dependently in response to METH exposure. Conversely, METH treatment increased the level of PD-1/PD-L1 time-dependently in neuronal culture, suggesting METH-induced neuronal degeneration. The function of the endogenous PD-1/PD-L1 in astrocyte is unknown. We wonder whether it acts as neuroimmune surveillance in the brain environment as a neuroprotective mechanism, which needs to be verified in astrocyte-neuron interactive studies in the presence or absence of METH. We suggest that upregulation of PD-1/PD-L1 in macrophage and in microglia by METH, and to some extent in hBEC and in neuron may indicate the role of PD-1/PD-L1 in weakening of the defense response of these neuroimmune cells. As such, it may promote the progression of inflammatory neuronal degeneration. However, the present studies are limited to the screening of PD-1/PD-L1 expression in neuroimmune cells following exposure to METH. Our future studies will focus on the comprehensive understanding of the underline signaling pathway(s) in co-culture system of neuron-microglia, neuron-astrocyte, and microglia-astrocyte. We will then validate the co-culture findings in an in vivo study, which will provide a better insight in cumulative influence of different neuroimmune cells over PD-L1 and PD-1 interaction.
Acknowledgements
This work was supported by NIH/NIAAA grants 1R21AA022734-01A1 to JH. Primary human endothelial cells were kindly provided by Dr. Yuri Persidsky, School of Medicine, Temple University, PA.
Disclosure of conflict of interest
None.
Abbreviations
- APC
Antigen presenting cells
- METH
methamphetamine
- BBB
blood brain barrier
- hBEC
human brain endothelial cells
References
- 1.Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ. Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology. 2009;34:2430–2441. doi: 10.1038/npp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNODOC. United Nations Office on Drugs and Crime. Vienna: 2011. World drug report. [Google Scholar]
- 3.Grant KM, Kelley SS, Agrawal S, Meza JL, Meyer JR, Romberger DJ. Methamphetamine use in rural Midwesterners. Am J Addict. 2007;16:79–84. doi: 10.1080/10550490601184159. [DOI] [PubMed] [Google Scholar]
- 4.Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- 5.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 6.Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friend DM, Keefe KA. A role for D1 dopamine receptors in striatal methamphetamine-induced neurotoxicity. Neurosci Lett. 2013;555:243–247. doi: 10.1016/j.neulet.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ares-Santos S, Granado N, Moratalla R. The role of dopamine receptors in the neurotoxicity of methamphetamine. J Intern Med. 2013;273:437–453. doi: 10.1111/joim.12049. [DOI] [PubMed] [Google Scholar]
- 10.Payer DE, Nurmi EL, Wilson SA, McCracken JT, London ED. Effects of methamphetamine abuse and serotonin transporter gene variants on aggression and emotion-processing neurocircuitry. Transl Psychiatry. 2012;2:e80. doi: 10.1038/tp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yui K, Goto K, Ikemoto S. The role of noradrenergic and dopaminergic hyperactivity in the development of spontaneous recurrence of methamphetamine psychosis and susceptibility to episode recurrence. Ann N Y Acad Sci. 2004;1025:296–306. doi: 10.1196/annals.1316.037. [DOI] [PubMed] [Google Scholar]
- 12.Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL. Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry. 2014;76:47–56. doi: 10.1016/j.biopsych.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. Methamphetamine inhibits the glucose uptake by human neurons and astrocytes: stabilization by acetyl-L-carnitine. PLoS One. 2011;6:e19258. doi: 10.1371/journal.pone.0019258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potula R, Persidsky Y. Adding fuel to the fire: methamphetamine enhances HIV infection. Am J Pathol. 2008;172:1467–1470. doi: 10.2353/ajpath.2008.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A. 2005;68:2133–2145. doi: 10.1080/15287390500177156. [DOI] [PubMed] [Google Scholar]
- 17.House RV, Thomas PT, Bhargava HN. Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacol Immunotoxicol. 1994;16:1–21. doi: 10.3109/08923979409029897. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirza N, Duque MA, Dominguez AL, Schrum AG, Dong H, Lustgarten J. B7-H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals. J Immunol. 2010;184:5466–5474. doi: 10.4049/jimmunol.0903561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014;7:1–17. doi: 10.1016/j.hemonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 22.Flemming A. Cancer: PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov. 2012;11:601. doi: 10.1038/nrd3806. [DOI] [PubMed] [Google Scholar]
- 23.Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, Orlandini A, Missale G, Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS One. 2013;8:e77780. doi: 10.1371/journal.pone.0077780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittet CL, Newcombe J, Prat A, Arbour N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J Neuroinflammation. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo JC, Anders RA, Sun Y, Chen L, Wang Y, Alegre ML, Fu YX. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berghoff AS, Ricken G, Widhalm G, Rajky O, Hainfellner JA, Birner P, Raderer M, Preusser M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL) Clin Neuropathol. 2014;33:42–49. doi: 10.5414/np300698. [DOI] [PubMed] [Google Scholar]
- 29.Ortler S, Leder C, Mittelbronn M, Zozulya AL, Knolle PA, Chen L, Kroner A, Wiendl H. B7-H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: implications for the lesion pathogenesis of multiple sclerosis. Eur J Immunol. 2008;38:1734–1744. doi: 10.1002/eji.200738071. [DOI] [PubMed] [Google Scholar]
- 30.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 32.Floreani NA, Rump TJ, Abdul Muneer PM, Alikunju S, Morsey BM, Brodie MR, Persidsky Y, Haorah J. Alcohol-induced interactive phosphorylation of Src and toll-like receptor regulates the secretion of inflammatory mediators by human astrocytes. J Neuroimmune Pharmacol. 2010;5:533–545. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao CC, Gekker G, Hu S, Peterson PK. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 34.Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol Neurodegener. 2011;6:23. doi: 10.1186/1750-1326-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittet CL, Newcombe J, Antel JP, Arbour N. The majority of infiltrating CD8 T lymphocytes in multiple sclerosis lesions is insensitive to enhanced PD-L1 levels on CNS cells. Glia. 2011;59:841–856. doi: 10.1002/glia.21158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]