Abstract
The effects of short-term as well as long-term antioxidant supplementation on exercise performance in the heat or the cold are unknown. Based on our recent studies we hypothesized that short-term supplementation with alpha-ketoglutaric acid (α-KG) and 5-hydroxymethylfurfural (5-HMF) would decrease oxidative stress but without significant impairment of maximal exercise performance in the heat or the cold. During a 5-week period young and welltrained participants performed 5 incremental treadmill tests to exhaustion under different temperature conditions (normal: 20°C, cold: +7°C, heat: +33°C) and with different nutritional supplements (placebo or α-KG and 5-HMF) prior to the tests applying a randomized cross over design. The first test was performed under normal temperature, the second and fourth under cold and the third and fifth test under heat conditions. Reactive oxygen metabolites and the biological antioxidant activity in serum were determined (Free Carpe Diem, Diacron International) before the first and after each exercise test. We demonstrated that reactive oxygen metabolites and maximal exercise performance remained unchanged in the cold as well in the heat with and without short-term antioxidant supplementation. Thus, short bouts of intense exercise in the heat or the cold seem not to produce significant oxidative stress in well-trained subjects and therefore pre-treatment with antioxidants may not have beneficial effects. However, future studies will focus on potentially favorable effects in sedentary or diseased subjects and/or on effects of more prolonged antioxidant supplementation when performing endurance exercise for a long duration under extreme temperature conditions.
Keywords: Antioxidants, oxidative stress, biomarkers, heat, cold, exercise performance
Introduction
High intensity exercise produces free radicals including reactive oxygen species (ROS) associated with high rates of mitochondrial oxygen consumption but also related to various other mechanisms [1]. In addition, reactive nitrogen species (RNS) are generated by the contracting skeletal muscle cell [2]. Moreover, exercising under extreme environmental conditions, i.e. hypoxia, cold or heat, may all enhance the generation of free radicals [3-6]. There are several lines of evidence that high levels of free radicals might impair exercise performance by favoring muscle fatigue [7,8] and/or the reduction of force production [9], e.g. by oxidizing regulatory proteins of sarcoplasmic reticulum calcium release channels and/or changes of myofilament structure and function [10].
Based on these findings, it might be suspected that supplementation with antioxidants when exercising under extreme environmental conditions should scavenge free radicals and therefore help maintaining exercise performance. However, research findings are controversial. Whereas some studies did not find any beneficial effects of supplementation with antioxidants on muscle strength, fatigue, and performance [11] others for example, demonstrated delayed muscle fatigue after supplementation with N-acetylcysteine (NAC) [12,13]. Controversies might partly be explained by the use of different antioxidants and/or differences regarding the time and duration of supplementation. Recently, we found that short-term supplementation with alpha-ketoglutaric acid (α-KG) and 5-hydroxymethylfurfural (5-HMF) did not prevent the hypoxia induced decrease of exercise performance despite attenuation of oxidative stress [14]. However, subsequently we demonstrated that aerobic exercise performance was less impaired in acute normobaric hypoxia after 3 weeks with supplementation of α-KG and 5-HMF when compared with a broad-based antioxidants supplement or placebo [15]. Thus, long-term α-KG and 5-HMF supplementation may have effectively prevented oxidative stress formation and may have increased NO bioavailability which in turn preserved exercise performance in hypoxia [16,17]. Also other studies showed that the combined oral intake of α-KG compounded with 5-HMF improved exercise capacity and reduced oxidative stress, e.g. after lung surgery [18]. In addition, 5-HMF has been shown to elicit protective properties against hypoxic injury [19] and on the vascular endothelium as demonstrated in human umbilical vein endothelial cells [17,20]. The effects of short-term as well as long-term antioxidant supplementation on exercise performance in the heat or the cold are unknown. Based on our recent studies we hypothesized that short-term supplementation with α-KG and 5-HMF would decrease oxidative stress but without significant impairment of maximal exercise performance in the heat or the cold.
Material and methods
Participants
Seven male sport students (age: 22.7 ± 2.6 years, height: 178.1 ± 3.9 cm, weight: 71.4 ± 6.9 kg) were informed about the study aims and gave written informed consent for participation. The participants were healthy and regularly active with a mean training volume of 8.9 ± 7.4 training hours per week and a maximal oxygen uptake (VO2max) of 56.5 ± 8.7 ml/min/kg. The study was carried out in conformity with the ethical standard laid down in the 1975 declaration of Helsinki and was approved by the institutional review board and by the ethical Committee of the University of Innsbruck, Austria.
Study procedure and exercise performance
The study protocol for each participant consisted of 5 incremental treadmill tests to exhaustion each separated by a washout period of one week. Tests were performed under different temperature conditions with and without antioxidative supplementation prior to the tests applying a randomized crossover design. During the 5-week period participants were encouraged not to change their eating or training habits. Additionally, 24 hours before each test session participants were asked not to consume alcohol or nicotine, nor to perform a heavy training session.
All exercise tests were performed in a climate chamber (pole of cold, Austria) under 3 different climate conditions adapted from the study of Quindry et al. [23]. The conditions were room temperature (20°C, 40% humidity), cold (+7°C, 40% humidity) and heat (+33°C, 40% humidity). The order of the climate condition was as follows: the room temperature setting was the first test to be completed. During the second and fourth week, tests were completed under cold conditions and during week 3 and 5 under heat conditions.
Treadmill spiroergometry (Oxycon mobile, Viasys Healthcare GmbH Jaeger, Germany) test to exhaustion was performed as described in detail elsewhere [23]. Shortly, the test started at 5 km/h walking speed with an incline of 5% for 2 min followed by 2 min at 5 km/h walking speed with an incline of 10% and 1 min at 6 km/h walking speed with an incline of 10%, then the incline was increased by 2% per minute up to 20%, then participants started running and running speed was increased by 1 km/h per min up to exhaustion. VO2peak was defined as the VO2 averaged during the last 30 s before exhaustion. Before and after each exercise test participants rated their comfort [24], skin wetness [25], motivation and perceived exertion [26]. Body temperature (measurement at the ear by Thermoscan IRT 6022: Braun, Germany) was measured before and after the tests. Capillary blood samples were collected from fingertip at baseline and after each exercise test for the determination of lactate (Biosen C-Line, EKF, Magdeburg, Germany), oxidative stress and total antioxidant level (detailed description of theses parameters are provided below). For the oxidative stress and defense parameters blood was centrifuged at 3000 g for 5 min, and values were determined immediately from serum (Free Carpe Diem, DIACRON international).
Supplementation
Participants started with the supplementation of the substances (either placebo or α-KG plus 5-HMF; Cyl® Airnergy drink; both in the form of a liquid) approximately 48 h before exercise testing in the heat and the cold. Subjects ingested their supplements over 2 days prior to exercise testing, twice daily, once in the morning and once in the evening. The total antioxidant dose during these 2 days was 60 mg of 5-HMF and 4.8 g α-KG. The placebo was identical-looking and tasting. The order of supplementation under each condition was random.
Measurement of oxidative stress and total antioxidant capacity
Oxidative stress
Hydroperoxides were measured by the plasma levels of diacron reactive oxygen metabolites (d-ROMs) by using the Free Carpe Diem device (FREE® Carpe Diem; Diacron International, Italy). d-ROM levels are detected based on the ability of transition metals to catalyze, in the presence of peroxides, the formation of free radicals that are then trapped by an alchilamine. The alchilamine reacts to form a colored radical that can be detected by a spectrophotometer at 505 nm. The results are expressed in arbitrary units, namely Carratelli units (U.CARR). A single U.CARR corresponds to 0.08 ng/100 mL of H2O2 [27].
Total antioxidant capacity
The biological antioxidant activity of plasma (BAP) was determined using the Free Carpe Diem device (FREE® Carpe Diem; Diacron International, Italy). When trivalent FeCl3 is dissolved in a colorless solution containing a chelation acid derivative, it turns red as a result of the action of the Fe+3 ions. However, it is decolorized by the reduction of Fe+3 to Fe+2 ions caused by the antioxidant activity of plasma added to the reaction solution. The antioxidant potential of plasma is evaluated spectrophotometrically by measuring the degree of decolorization. The normal value for BAP in healthy subjects is > 2200 μmol/L [27].
Statistical analysis
All statistical analyses were performed using the SPSS program version 16.0 (SPSS Inc., Chicago, IL). The paired t-test or Wilcoxon test was used to compare categorical variables or continuous variables between baseline and supplementation trials. ANOVA or Kruskal-Wallis test, with post hoc analysis (Bonferroni corrected), were used to compare differences among trials. Bivariate correlation analyses were performed by Pearson or Spearman as appropriate. Data are presented as means ± SD or median (interquartile range, IQR). A p-value < 0.05 was considered statistically significance.
Results
Exercise performance in the heat and cold with and without antioxidant supplement
Peak exercise time, VO2peak, peak heart rates and blood lactate concentrations determined at the end of incremental exercise tests at room temperature, and with and without antioxidant supplement in the cold and the heat are shown in Table 1. None of the variables differed significantly between conditions. The ratings of perceived exertion (RPE) during submaximal and maximal exercise are depicted in Figure 1. Although not statistically different, RPE tended to increase at comparable exercise intensities from cold to warmer conditions.
Table 1.
Peak exercise performance and exercise responses at various conditions
| Condition | Room temperature (°C) | Core temperature (°C) | Exercise time (minutes) | VO2peak (ml/min/kg) | HRpeak (bpm) | Lactate (mmol/L) |
|---|---|---|---|---|---|---|
| Room temperature | +20 | 37.03 ± 0.35 | 12.60 ± 0.68 | 56.47 ± 8.73 | 191.00 ± 6.66 | 14.47 ± 1.99 |
| Cold with placebo | +7 | 37.23 ± 0.57 | 12.51 ± 0.97 | 54.71 ± 9.06 | 188.86 ± 7.90 | 16.03 ± 3.65 |
| Cold with supplement | +7 | 37.21 ± 0.52 | 12.51 ± 0.77 | 54.26 ± 5.57 | 189.14 ± 7.62 | 15.98 ± 2.21 |
| Heat with placebo | +33 | 37.34 ± 0.43 | 12.34 ± 1.17 | 56.54 ± 8.87 | 189.86 ± 8.97 | 14.33 ± 3.41 |
| Heat with supplement | +33 | 37.51 ± 0.48 | 12.47 ± 0.82 | 59.83 ± 7.23 | 191.43 ± 8.10 | 14.35 ± 2.70 |
Values are expressed as mean ± SD. There are no significant differences between conditions.
Figure 1.
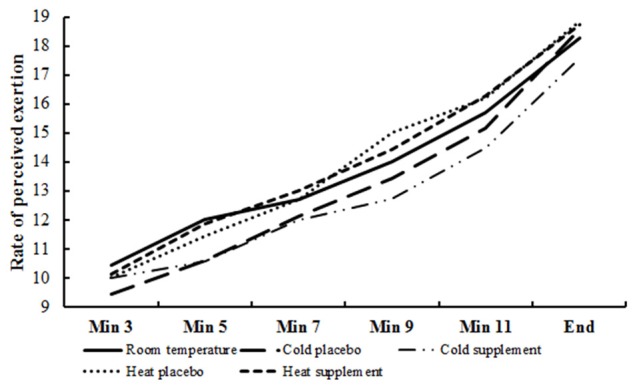
Ratings of perceived exertion during incremental exercise testing under various conditions.
Oxidative stress and antioxidant biomarkers at baseline and after maximal exercise in the heat and the cold with and without supplementation of α-KG and 5-HMF
Whereas the amount of blood oxidative stress (dROMs) after maximal exercise in various conditions did not differ significantly from baseline (Figure 2), the amount of antioxidant biomarkers (BAP) were elevated in both the cold with placebo (P = 0.040) and the cold with supplementation of α-KG and 5-HMF (P = 0.008) compared to baseline (Figure 3).
Figure 2.
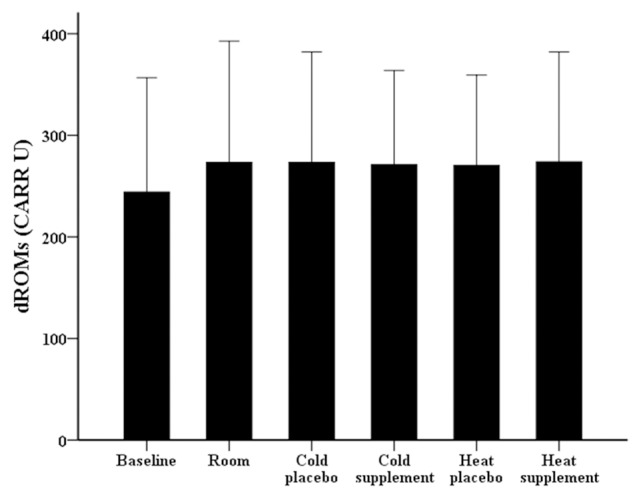
The amount of oxidative stress (dROMs) at baseline (rest) and after maximal exercise under various conditions. There are no significant differences between conditions.
Figure 3.
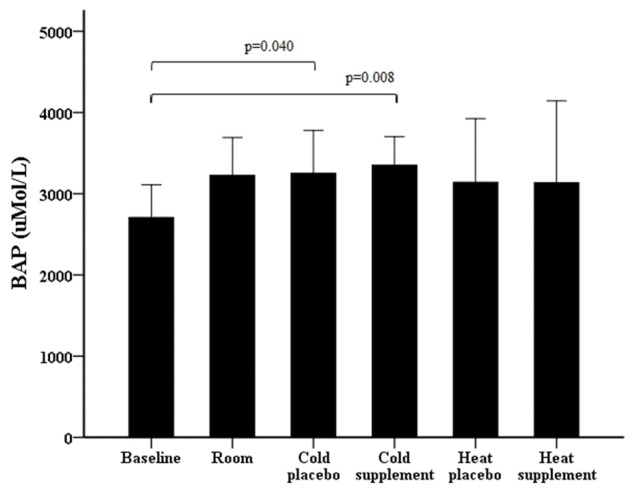
The amount of antioxidants (BAP) at baseline (rest) and after maximal exercise under various conditions.
Discussion
The main findings of the present study are that short-term supplementation with α-KG and 5-HMF did not change peak exercise performance and related physiological responses and did not affect oxidative stress during exercise neither in the cold nor the heat. However, the amounts of antioxidant biomarkers (BAP) were elevated after maximal exercise in the cold with placebo and with supplementation of α-KG and 5-HMF. RPE during submaximal exercise tended to increase with increasing temperature independently of antioxidant supplementation.
Unexpectedly, there were no changes in maximal performance and related physiological responses neither in the heat nor the cold even without antioxidant supplementation compared to normal ambient temperature conditions. Thus, no performance reductions occurred that could have been compensated for by short-term antioxidant supplementation. Some studies reported a performance decline at extreme temperature conditions [22,28,29] but others did not [22,30,31]. With respect to cold environment, studies indicate that only under extreme conditions (i.e. approximately below -10°C) performance impairment would occur [28,29]. The very cold exposure is thought to induce bronchus constriction due to ventilating heavily cold air, to reduce blood flow in superficial parts of the muscle, to reduce muscle contraction velocity, to alter motor unit recruitment and to induce oxidative stress, and thus reducing exercise performance [3,28,29,32]. The degree of cold exposure in the present investigation was probably too low to induce such detrimental effects. With respect to hot exposure, time seems to be critical. Pirnay et al. showed that performance of an incremental exercise test was only affected after thermal exposure leading to a significantly increased body temperature (body temperature was already increased to 38.6°C when the test started and was 39.2°C after exhaustion), but this was not the case when starting the exercise immediately at the beginning of heat exposure (body temperature was 36.9°C when the test started and was 37.4°C after exhaustion) [22]. Heat stress is thought to induce oxidative stress and redistribute blood flow away from the working muscles to the skin, thereby reducing performance [6,22]. In the present study, exercise tests started immediately after entering the climate chamber and body temperature did not significantly increase. Thus detrimental effects of a raised body temperature should not have developed. Additionally, in the present study participants were really well trained (VO2max: 56.5 ± 8.7 ml/min/kg). Farney et al. showed that in trained men strenuous exercise did not increase blood oxidative stress [33]. This may explain why we did not see any significant increases in d-ROMs and related performance changes.
We found that the amount of antioxidant biomarkers (BAP) were elevated in cold with placebo and with supplement but this was not the case in the heat. Our test series was conducted in October when outside temperatures reached already low values (approximately 0°C) during some days. Therefore it could be speculated that some adaptation to cold may have occurred, which was shown to enhance antioxidant defense after exercise [34].
Our data indicate that RPE during submaximal exercise slightly increases with temperature elevation independent of antioxidant supplementation. These observations are in accordance with Maw et al. who demonstrated that RPE was significantly lower in the cold compared to exercise performed in the heat [35]. Supplementation did not show any effect on RPE as was also reported by Keong et al. [36].
Some limitations of this study should be mentioned. Firstly the small sample size could have led to a type II error which however, is limited by the use of a cross over design. Secondly, the timing of blood sampling may have influenced present outcomes. Accordingly, Michailidis et al. noted that sampling time is crucial when investigating aerobic exercise-induced oxidative stress [37].
Conclusion
The present findings demonstrate that reactive oxygen metabolites and maximal exercise performance remained unchanged in the cold as well in the heat with and without short-term antioxidant supplementation. Interestingly, the amounts of antioxidant biomarkers were elevated after maximal exercise in the cold with placebo and with supplementation of α-KG and 5-HMF. Since short bouts of intense exercise in the heat or the cold seem not to produce significant oxidative stress in well-trained subjects pre-treatment with antioxidants may not have beneficial effects. However, future studies will focus on potentially favorable effects in sedentary or diseased subjects and/or on effects of more prolonged antioxidant supplementation when performing endurance exercise for a long duration under extreme temperature conditions.
Acknowledgements
We are especially grateful to the University of Innsbruck for continuous support of our research work.
Disclosure of conflict of interest
None.
References
- 1.Aruoma OI. Free radicals and antioxidant strategies in sports. J Nutr Biochem. 1994;5:370–381. [Google Scholar]
- 2.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 3.Martarelli D, Cocchioni M, Scuri S, Spataro A, Pompei P. Cold exposure increases exercise-induced oxidative stress. J Sports Med Phys Fitness. 2011;51:299–304. [PubMed] [Google Scholar]
- 4.McAnulty SR, McAnulty L, Pascoe DD, Gropper SS, Keith RE, Morrow JD, Gladden LB. Hyperthermia increases exercise-induced oxidative stress. Int J Sports Med. 2005;26:188–192. doi: 10.1055/s-2004-820990. [DOI] [PubMed] [Google Scholar]
- 5.Miller LE, McGinnis GR, Kliszczewicz B, Slivka D, Hailes W, Cuddy J, Dumke C, Ruby B, Quindry JC. Blood oxidative-stress markers during a high-altitude trek. Int J Sport Nutr Exerc Metab. 2013;23:65–72. doi: 10.1123/ijsnem.23.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Rhoads RP, Baumgard LH, Suagee JK, Sanders SR. Nutritional interventions to alleviate the negative consequences of heat stress. Adv Nutr. 2013;4:267–276. doi: 10.3945/an.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 10.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24:249–266. doi: 10.1139/h99-021. [DOI] [PubMed] [Google Scholar]
- 12.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of n-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med. 1997;156:1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 13.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 14.Gatterer H, Greilberger J, Philippe M, Faulhaber M, Djukic R, Burtscher M. Short-term supplementation with alpha-ketoglutaric acid and 5-hydroxymethylfurfural does not prevent the hypoxia induced decrease of exercise performance despite attenuation of oxidative stress. Int J Sports Med. 2013;34:1–7. doi: 10.1055/s-0032-1312584. [DOI] [PubMed] [Google Scholar]
- 15.Mariacher C, Gatterer H, Greilberger J, Djukic R, Greilberger M, Philippe M, Burtscher M. Effects of antioxidant supplementation on exercise performance in acute normobaric hypoxia. Int J Sport Nutr Exerc Metab. 2014;24:227–235. doi: 10.1123/ijsnem.2013-0118. [DOI] [PubMed] [Google Scholar]
- 16.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 17.Cao G, Cai H, Cai B, Tu S. Effect of 5-hydroxymethylfurfural derived from processed cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013;140:273–279. doi: 10.1016/j.foodchem.2012.11.143. [DOI] [PubMed] [Google Scholar]
- 18.Matzi V, Lindenmann J, Muench A, Greilberger J, Juan H, Wintersteiger R, Maier A, Smolle-Juettner FM. The impact of preoperative micronutrient supplementation in lung surgery. A prospective randomized trial of oral supplementation of combined alpha-ketoglutaric acid and 5-hydroxymethylfurfural. Eur J Cardiothorac Surg. 2007;32:776–782. doi: 10.1016/j.ejcts.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Li MM, Wu LY, Zhao T, Wu KW, Xiong L, Zhu LL, Fan M. The protective role of 5-hydroxymethyl-2-furfural (5-hmf) against acute hypobaric hypoxia. Cell Stress Chaperones. 2011;16:529–537. doi: 10.1007/s12192-011-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HK, Choi YW, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim YT, Yoon S. 5-hydroxymethylfurfural from black garlic extract prevents tnfalpha-induced monocytic cell adhesion to huvecs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and nf-kappab activation. Phytother Res. 2011;25:965–974. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- 21.Oksa J, Kaikkonen H, Sorvisto P, Vaappo M, Martikkala V, Rintamäki H. Changes in maximal cardiorespiratory capacity and submaximal strain while exercising in cold. J Therm Biol. 2004;29:815–818. [Google Scholar]
- 22.Pirnay F, Deroanne R, Petit JM. Maximal oxygen consumption in a hot environment. J Appl Physiol. 1970;28:642–645. doi: 10.1152/jappl.1970.28.5.642. [DOI] [PubMed] [Google Scholar]
- 23.Quindry J, Miller L, McGinnis G, Kliszczewiscz B, Slivka D, Dumke C, Cuddy J, Ruby B. Environmental temperature and exercise-induced blood oxidative stress. Int J Sport Nutr Exerc Metab. 2013;23:128–136. doi: 10.1123/ijsnem.23.2.128. [DOI] [PubMed] [Google Scholar]
- 24.Holmér I. Human wear trials for cold weather protective clothing systems. In: Williams JT, editor. Textiles for cold weather apparel. Cambridge, UK: Woodhead Publishing Ltd.; 2009. pp. 256–273. [Google Scholar]
- 25.Yoo S, Barker RL. Comfort properties of heat resistant protective workwear in varying conditions of physical activity and environment. Part II: Perceived comfort response to garments and its relationship to fabric properties. Textile Research Journal. 2005;75:531–539. [Google Scholar]
- 26.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 27.Takuro M, Katsunori T, Mariko M, Naoya Y, Tomoshi T, Keitaro M, Go H, Hiroo I, Ryoichiro D, Ryusuke M, Takeshi N. Video-assisted thoracic surgery attenuates perioperative oxidative stress response in lung cancer patients: A preliminary study. Acta Med Nagasaki. 2014;59:19–25. [Google Scholar]
- 28.Oksa J, Kaikkonen H, Sorvisto P, Vaappo M, Martikkala V, Rintamaki H. Changes in maximal cardiorespiratory capacity and submaximal strain while exercising in cold. J Therm Biol. 2004;29:815–818. [Google Scholar]
- 29.Sandsund M, Saursaunet V, Wiggen Ø, Renberg J, Færevik H, van Beekvelt MC. Effect of ambient temperature on endurance performance while wearing cross-country skiing clothing. Eur J Appl Physiol. 2012;112:3939–3947. doi: 10.1007/s00421-012-2373-1. [DOI] [PubMed] [Google Scholar]
- 30.Renberg J, Sandsund M, Wiggen Ø, Reinertsen RE. Effect of ambient temperature on female endurance performance. J Therm Biol. 2014;45:9–14. doi: 10.1016/j.jtherbio.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Sandsund M, Sue-Chu M, Helgerud J, Reinertsen RE, Bjermer L. Effect of cold exposure (-15 degrees C) and salbutamol treatment on physical performance in elite nonasthmatic cross-country skiers. Eur J Appl Physiol Occup Physiol. 1998;77:297–304. doi: 10.1007/s004210050337. [DOI] [PubMed] [Google Scholar]
- 32.Oksa J. Neuromuscular performance limitations in cold. Int J Circumpolar Health. 2002;61:154–162. doi: 10.3402/ijch.v61i2.17448. [DOI] [PubMed] [Google Scholar]
- 33.Farney TM, McCarthy CG, Canale RE, Schilling BK, Whitehead PN, Bloomer RJ. Absence of blood oxidative stress in trained men after strenuous exercise. Med Sci Sports Exerc. 2012;44:1855–1863. doi: 10.1249/MSS.0b013e3182592575. [DOI] [PubMed] [Google Scholar]
- 34.Hong JH, Kim KJ, Suzuki K, Lee IS. Effect of cold acclimation on antioxidant status in cold acclimated skaters. J Physiol Anthropol. 2008;27:255–262. doi: 10.2114/jpa2.27.255. [DOI] [PubMed] [Google Scholar]
- 35.Maw GJ, Boutcher SH, Taylor NA. Ratings of perceived exertion and affect in hot and cool environments. Eur J Appl Physiol Occup Physiol. 1993;67:174–179. doi: 10.1007/BF00376663. [DOI] [PubMed] [Google Scholar]
- 36.Keong CC, Singh HJ, Singh R. Effects of palm vitamin e supplementation on exerciseinduced oxidative stress and endurance performance in the heat. J Sports Sci Med. 2006;5:629–639. [PMC free article] [PubMed] [Google Scholar]
- 37.Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med Sci Sports Exerc. 2007;39:1107–1113. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]


