Abstract
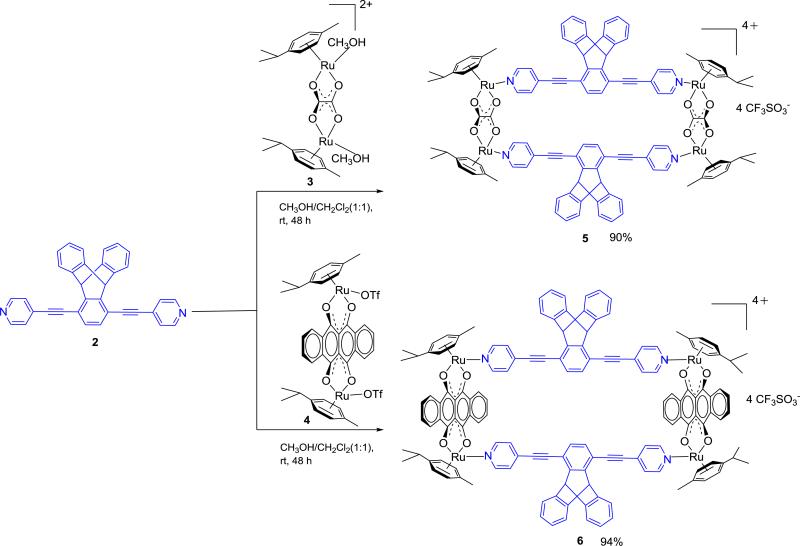
A suite of two new tetraruthenium metallarectangles 5 and 6 have been obtained from [2 + 2] self-assemblies between dipyridylethynyltriptycene 2 and one of the two dinuclear arene ruthenium clips, [Ru2 (μ-η4-OO∩OO) (η6-p-cymene)2][OTf]2 ; (OO∩OO = oxalate 3; 6,11-dihydroxy-5,12-naphthacenedionato (dotq) 4; OTf = triflate). These molecular rectangles are fully characterized by 1H NMR spectroscopy, electrospray mass spectrometry. A single crystal of 6 was suitable for X-ray diffraction structural characterization. These new metallarectangles showed fluorescence behavior in solution, have been examined for emission quenching effects with various aromatic compounds, and show high quenching selectivity and sensitivity towards nitroaromatics, particularly picric acid and trinitrotoluene. Excited-state charge transfer from the rectangles to nitro aromatic substrates can be used to develop selective fluorescent sensors for nitro aromatics.
Keywords: Fluorescent, Ruthenium, Self-assembly, Nitro aromatics, Metalla-rectangle
1. Introduction
The design and study of discrete supramolecular structures through abiological self-assembly is an active field of research fuelled by the single-step formation of complicated structural motifs and incorporation of constituent building blocks with the desired functionalities.1 Of the various self-assembly protocols, metal-ligand coordination driven self-assembly is especially appealing and effective.2 Its highly directional and predictable nature provides limitless possibilities to design novel molecular materials. With the tremendous progress that has been made in the preparation and characterization of metallosupramolecules, attention is now being focused on fully exploring and developing their sensor and host-guest properties.3,4
Nitroaromatics, especially trinitrotoluene (TNT), dinitrotoluene (DNT), and picric acid (PA), are the primary constituents of many unexploded land mines worldwide.5 Nitroaromatics used in organic synthesis, drug analysis, rocket fuel, fireworks, and matches release contaminants into the environment that are toxic to living organisms.6 It is of great interest to develop efficient fluorescent sensors that rapidly detect nitroaromatic compounds in an effort to prevent terrorism and reduce environmental problems associated with explosive residues. Recently, optical detection techniques based on fluorimetric assays for sensing nitroaromatics have attracted a great deal of attention.7 The pioneer work of Swager et al. in developing poly(phenylene ethynylene)s polymers has been extensively employed for sensing nitroaromatics.8 The response characteristics of these materials depend on the strength of the chemical (or physical) interaction between the analyte and photoactive polymer, and the permeability of analyte in the polymer. To increase the permeability of fluorescent poly(phenylene ethynylene)s polymer films, sterically demanding pentiptycene and triptycene moieties were incorporated, allowing nitroaromatics to quench polymer fluorescence more rapidly and efficiently. Additionally, the incorporation of rigid three dimensional pentiptycene and triptycene molecules into the polymeric backbone prevents low quantum yields due to π-stacking, excimer formation, and self-quenching.8b,c and e Pentiptycene and triptycene containing fluorescent-conjugated organic polymers have been employed extensively as sensing materials. The potential of pentiptycene and triptycene containing conjugated metal-organic discrete assemblies of finite shapes and sizes as sensors for nitroaromatics has not yet been explored.
Herein, we report the synthesis of two new [2 + 2] metal-organic discrete assemblies 5 and 6 by coordination driven self-assembly. The assemblies include oxalate and 6,11-dihydroxy-5,12-naphthacenedionato-based O,O-bridging ligands between the Ru centers of acceptors 3 and 4, respectively, with new dipyridylethynyltriptycene donor 2 used as a bridging donor unit. Ethynyl spacer and triptycene functionalities were integrated into donor 2 to endow supramolecules 5 and 6 with photoluminescent properties that are exploited for detection of electron-poor guests like nitro-aromatics. The triptycene functionality also prevents the self-quenching of initial fluorescence intensity in solution that occurs due to intermolecular stacking.
2. Experimental Section
2.1. Materials and physical measurements
1,4-Diethynyl-9,10-dihydro-9,10-[1’,2’]benzeno-anthracene 1, was synthesized following the reported procedure.9 Arene-ruthenium acceptor clips 310 and 411 were synthesized under a dry nitrogen atmosphere by standard Schlenk technique following the reported procedures. Solvents were dried and distilled according to the standard literature procedures.12 1H and 13C NMR spectra were recorded on a Bruker 300 MHz spectrometer. The chemical shifts (δ) in 1H NMR are reported relative to residual solvent signals. ESI-MS spectra for compound 2 were recorded on Triple Quadrupole LC-Mass spectrometry (Finnigan TSQ Quantum Ultra EMR). ESI-MS spectra for self-assemblies 5 and 6 were recorded on a Micromass QuattroII triple-quadrupole mass spectrometer using electrospray ionization with a Mass Lynx operating system. Absorption spectra were recorded by a CARY 100 Conc UV/Vis spectrophotometer. Elemental analyses were performed using an Elemental GmbH Vario EL-3 instrument. Fluorescence titration studies were carried out on a HORIBA FluoroMax-4 fluorometer.
2.2. Fluorescence Quenching Titration Study
A 2 mL stock solution (5.0 × 10−6 M) of macrocycles 5 and 6 were placed in a 1 cm wide quartz cell. Quenchers (1,3,5-trinitrobenzene, picric acid, 2,4-dinitrophenol, nitrobenzene, benzoquinone and benzoic acid) stock solution (1.0 × 10−4 M) was added in an incremental fashion. The whole titration experiment was carried out at 298 K, and each titration measurement was repeated at least three times to obtain concordant values. For all measurements, macrocycle 5 was excited at λex = 283 nm, and the corresponding emission intensity was monitored from 300 nm. Both excitation and emission slit widths were 3 nm. No change in emission spectra shape was noted except for significant gradual quenching of the initial fluorescence intensity of 5 and 6 upon titration with electron-deficient nitroaromatics. Analysis of normalized fluorescence emission intensity (I0/I) as a function of increasing quenchers concentration ([Q]) was well described by the Stern-Volmer equation: I0/I = 1 + KSV[G]. Stern-Volmer constants were calculated from the slope of Stern-Volmer plots.
2.3. Synthesis of compounds
2.3.1. Synthesis of compound 1,4-bis(pyridin-4-ylethynyl)-9,10-dihydro-9,10-[1,2]benzene anthracene 2
The flask containing Pd(PPh3)2Cl2 (0.057 g, 0.08 mmol) and copper(I) iodide (0.025 g, 0.13 mmol) was evacuated and then filled with nitrogen. In sequence, deoxygenated triethylamine (10 mL), a deoxygenated solution of 4-iodopyridine (0.389 g, 1.9 mmol) in anhydrous THF (5 mL), and a deoxygenated solution of compound 1 (0.250 g, 0.83 mmol) in triethylamine (5 mL) were added to the flask via cannula. After 12 h at 50 °C, the reaction mixture was poured into a flask containing dichloromethane (20 mL) and saturated NH4Cl (20 mL) solution. The organic layer was separated, washed with water, dried over Na2SO4, and concentrated. Residue was purified by column chromatography (neutral alumina, methanol/ dichloromethane, 2/98, v/v) to afford 2 as a pale yellow solid. Yield 85%. M. p. 179-181 °C. 1H NMR (300 MHz, CDCl3): δ = 8.71 (d, J = 6.9 Hz, 4H), 7.52 (d, J = 6.8 Hz, 4H), 7.47- 7.49 (m, 4H), 7.20 (s, 2H), 7.05-7.08 (m, 4H), 5.95 (s, 2H). 13C NMR (75 MHz, CDCl3): δ = 150.0, 147.7, 144.1, 131.1, 128.2, 125.7, 125.6, 124.1, 118.2, 91.4, 91.1, 52.1. MS (ESI): m/z calcd = 456.16, found = 457.17. Anal. Calcd for C34H20N2: C, 89.45; H, 4.42; N, 6.14. Found: C, 89.07; H, 4.67; N, 5.99.
2.3.2. Synthesis of metallarectangle 5
A CH2Cl2 (1 mL) solution of donor 2 (0.017 g, 0.04 mmol) was added dropwise to a CH3OH solution (1 mL) of ruthenium acceptor 3 (0.034 g, 0.04 mmol). The mixture was then stirred for 48 h at room temperature. The reaction mixture was filtered and solvent was removed under reduced pressure. The resulting solid was washed with diethyl ether and then dried to furnish 5 as a yellow powder. Yield 90%. 1H NMR (300 MHz, CD3NO2): δ = 8.13 (d, J = 5.8 Hz, 8H, Hi), 7.66-7.69 (m, 16H, Hg and Hh ), 7.18-7.21 (m, 8H, Hf ), 6.11 (s, 4H, He), 5.94 (d, J = 6.4 Hz, 8H, Hcym), 5.78 (d, J = 6.4 Hz, 8H, Hcym), 5.54 (s, 4H, Hd), 2.85-2.96 (m, 4H, Hc), 2.27 (s, 12H, Hb), 1.42 (d, J = 7.0 Hz, 24H, Ha) ppm; 13C NMR (75 MHz, CD3NO2): δ = 185.7, 154.1, 149.4, 145.5, 135.9, 129.2, 127.1, 125.4, 118.9, 105.6, 102.9, 100.5, 96.1, 91.2, 84.9, 83.3, 52.9, 32.7, 22.6, 18.5; MS (ESI) for 5 (C116H96F12N4O20Ru4S4): 726.44 [5–3OTf]3+. Anal. Calcd for C116H96F12N4O20Ru4S4: C, 53.05; H, 3.68; N, 2.13; Found: C, 50.34; H, 3.59; N, 2.30.
2.3.3. Synthesis of metallarectangle 6
A CH2Cl2 (1 mL) solution of donor 2 (0.017 g, 0.04 mmol) was added dropwise to a CH3OH solution (1 mL) of ruthenium acceptor 4 (0.042 g, 0.01 mmol). The mixture was then stirred for 48 h at room temperature. The reaction mixture was filtered and then solvent was removed under reduced pressure. The resulting solid was washed with diethyl ether and then dried to furnish 6 as a green powder. Yield 94%. 1H NMR (300 MHz, CD3NO2): δ = 8.82-8.85 (m, 8H, Hk), 8.71 (d, J = 6.8 Hz, 8H, Hj), 7.99-8.02 (m, 8H, Hi), 7.53 (d, J = 6.8 Hz, 8H, Hh), 7.19-7.22 (m, 8H, Hg), 6.57-6.60 (m, 8H, Hf), 6.54 (s, 4H, He), 6.02 (d, J = 6.2 Hz, 8H, Hcym), 5.79-5.82 (m, 12H, Hcym and Hd), 3.10-3.15 (m, 4H, Hc), 2.34 (s, 12H, Hb), 1.42 (d, J = 6.8 Hz, 24H, Ha) ppm; 13 C NMR (75 MHz, CD3NO2): δ = 170.8, 153.2, 149.4, 145.3, 135.2, 134.3, 128.9, 128.5, 126.8, 125.2, 118.8, 108.6, 105.3, 101.0, 95.2, 91.2, 85.3, 84.0, 52.8, 32.1, 22.7, 18.1; MS (ESI) for 6 (C148H112F12N4O20Ru4S4): 859.93 [6–3OTf]3+.
2.4. Single-crystal X-ray Crystallography
A single crystal of rectangle 6 was mounted onto a loop, and the data were collected at 100 K on an ADSC Quantum 210 CCD diffractometer with synchrotron radiation (λ = 0.80000 Å) at Macromolecular Crystallography Beamline 6B1, Pohang Accelerator Laboratory (PAL), Pohang, Korea. The raw data were processed and scaled using the program HKL2000. The structure was solved by direct methods, and refinements were carried out with full-matrix least-squares on F2 with appropriate software implemented in the SHELXTL program package.
X-ray data for 6: C83H80F6N2O13Ru2S2, formula weight = 1693.75, Monoclinic, wavelength = 0.80000 Å, T = 100(2) K, space group = P21/n, unit cell dimensions a = 17.581(4) Å, α = 90°, b = 25.642(5) Å, β = 113.73(3)°, c = 19.481(4) Å, γ = 90°, V = 8040(3) Å3, Z = 4, ρcalcd = 1.399 g cm−3, absorption coefficient = 0.681 mm-1, F(000) = 3480, crystal size = 0.35 × 0.20 × 0.15 mm3, theta range for data collection = 1.49 to 25.07°, index ranges = −20≤h≤20, -28≤k≤28, −22≤l≤22, reflections collected = 40281, independent reflections = 10910 [R(int) = 0.0208], completeness to theta = 25.00°, 77.1 %, absorption correction = semi-empirical from equivalents, max. and min. transmission = 0.9047 and 0.7965, refinement method = full-matrix least-squares on F2, data / restraints / parameters = 10910 / 170 / 976, goodness-of-fit on F2= 1.682, final R indices [I>2sigma(I)] R1 = 0.1287, wR2 = 0.3693, R indices (all data) R1 = 0.1421, wR2 = 0.3829, largest diff. peak and hole = 1.146 and -0.701 e.Å-3.
3. Results and Discussion
Ligand 2 was synthesized in 85% yield by coupling with 4-iodopyridine and 1,4-diethynyl-9,10-dihydro-9,10-[1’,2’]benzenoanthracene 1 in the presence of Pd(0)/CuI catalyst (Scheme 1).
Scheme 1.
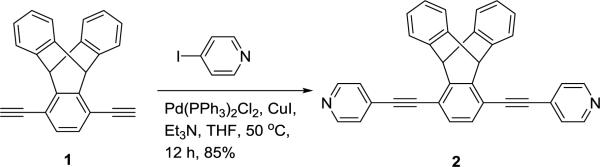
Synthesis of dipyridylethynyltriptycene donor 2.
Solutions containing equimolar amounts of [Ru2(μ-η4-C2O2)(MeOH)2(η6-p-cymene)2][O3SCF3]2 (3) or [Ru2(μ-η4-C18H8O4)(η6-p-cymene)2][O3SCF3]2 (4) and dipyridylethynyltriptycene donor 2 in CH2Cl2/CH3OH were stirred for 48 h at room temperature. This resulted in the quantitative self-assembly of 5 and 6, respectively (Scheme 2). Analytically pure rectangles were isolated as crystalline solids upon addition of diethyl ether to concentrated reaction mixtures. All rectangles were fully characterized by 1H, 13C NMR, electrospray mass spectrometry (ESI-MS), and UV-Vis absorption and emission. A single crystal of complex 6 was used to determine its solid-state structure via X-ray crystallography.
Scheme 2.
Synthesis of metallarectangles 5 and 6.
In 1H NMR spectra of 5 and 6, the signals corresponding to the Hα nuclei on the pyridine rings undergo upfield shifts (△δ = 0.1-0.6 ppm) relative to compound 2, indicating metal pyridine coordination. Likewise, the aromatic proton signals of the triptycene functional group of rectangles 5 and 6 showed significant upfield shifts due to increased electron density upon coordination. The aromatic proton resonances of p-cymene ligands were observed as two doublets at δ = 6.02 and 5.79–5.82 ppm, while the signals for naphthacenedione protons were observed as two multiplets at δ = 8.82–8.85 and 7.99–8.02 ppm for 6. The appearance of prominent peaks in the ESI-MS spectra of the multiply charged ions for 5 at m/z = 726.44 [5–3OTf]3+ and for 6 at 859.93 [6–3OTf]3+ indicate the formation of [2+2] self-assembled products. The observed peaks are isotopically resolved and agreed well with their theoretically predicted patterns (Figure S5 and S6, Supporting Information).
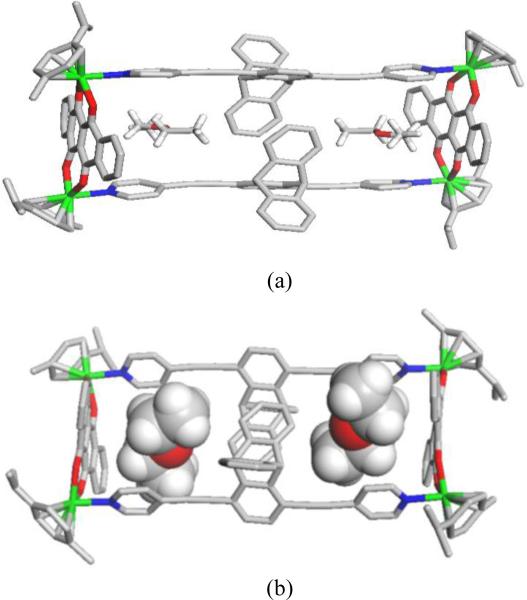
3.1. Crystal structure of 6
The molecular structure of 6 was further confirmed by X-ray crystallography (Figure 1 and Table 1). Single crystals of rectangle 6 were obtained by slow evaporation of Et2O into a concentrated solution of the complex in methanol. X-Ray diffraction analysis revealed that complex 6 had a tetranuclear rectangular architecture. The molecular structure of 6 consisted of two dipyridylethynyltriptycene units linked by two [Ru2(μ-η4-C18H8O4)(η6-p-cymene)2]2+ building blocks to form a M4L2 rectangle. Each Ru center was coordinated to one p-cymene moiety in a η6 fashion with a dotq bridging ligand and one pyridyl group of the dipyridylethynyltriptycene linker. This resulted in molecular rectangle 6, which contained two diethyl ether molecules inside the rectangular molecular cavity with overall dimensions of 8.35 Å × 20.65 Å.
Figure 1.
(a) Molecular structure of 6 showing two encapsulated diethyl ether molecules. (b) Side view. Color code: green, Ru; blue, N; red, O; grey, oxygen. H atoms are not shown for clarity.
Table 1.
Selected bond lengths [Å] and angles [°] for 6
| Bonds | Length [A] | Bond | Length [A] |
|---|---|---|---|
| Ru(1)-N(1) and Ru(1)#-N(1)# | 2.109(8) | Ru(1)-O(1) | 2.109(8) |
| Ru(1)-O(2) | 2.039(6) | Ru(2)-N(2) and Ru(2)#-N(2)# | 2.115(10) |
| Ru(2)-O(3) | 2.018(7) | Ru(2)-O(4) | 2.026(6) |
| Bonds | Angle [°] | Bonds | Angle [°] |
|---|---|---|---|
| O(1)-Ru(1)-O(2) | 85.8(2) | O(1)-Ru(1)-N(1) | 82.5(3) |
| O(2)-Ru(1)-N(1) | 82.3(3) | O(3)-Ru(2)-O(4) | 84.5(2) |
| O(3)-Ru(2)-N(2) | 85.1(3) | O(4)-Ru(2)-N(2) | 82.1(3) |
3.2. Detection of Nitroaromatics by Fluorescence Quenching
Fluorescence quenching-based detection of chemical explosives has been employed extensively due to the high sensitivity, easy visualization, and short response time required for detecting chemical explosives.13 The electron-deficient character of nitroaromatic explosives makes them efficient fluorescence quenchers through photo-induced electron transfer (PET) processes. The strategy used to design new fluorescent metallarectangles 5 and 6 involved the following unique features: (a) triptycene and ethynyl functionalities were introduced to create fluorescent and π-electron rich assemblies; (b) open spaces in the metallarectangles were suitable to accommodate small electron-deficient nitroaromatics; (c) triptycene molecules and bulky capped p-cymene moieties on each ruthenium metal center help to avoid intermolecular stacking and reduce self-quenching of initial fluorescence intensity in solution.
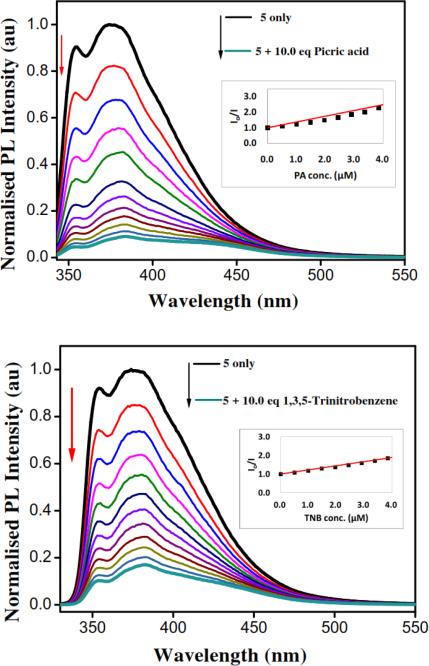
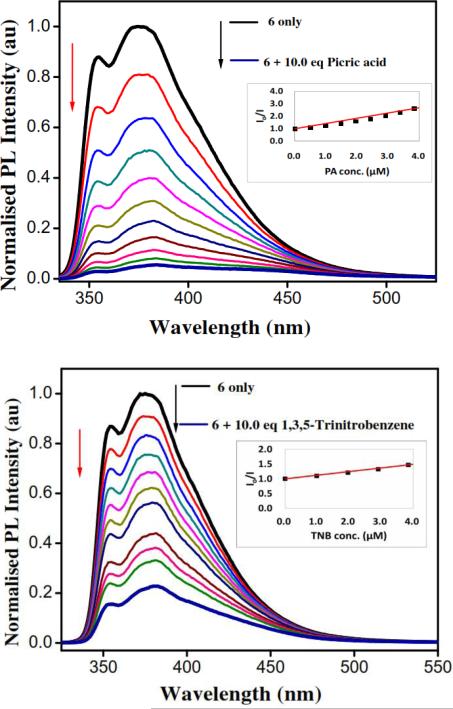
The electronic absorption spectra of metallarectangles in methanol solutions (1.0 × 10−5 M) exhibit intense bands at λabs = 282 and 350 nm for 5 and λabs = 280, 351, 572, and 614 for 6 (Supporting information, Figure S7). High-energy bands observed at 330 nm in the spectra of 2 were also present in each of the rectangles. This suggests that the π→π* transitions expected for an extended aromatic system of dipyridyl ligand were preserved upon self-assembly. The photoexcitation of 5 in methanol solution (1.0 × 10−6 M) at 283 nm resulted in emission around 375 nm, whereas 6 showed emission bands at 379 nm. To demonstrate the ability of metallarectangles 5 and 6 at sensing electron-deficient nitroaromatic explosives, 5 and 6 were titrated with picric acid and 1,3,5-trinitrobenzene in methanolic solution. Titration of a 1.0 × 10−6 M methanolic solution of 5 (Figure 2) and 6 (Figure 3) with a 1.0 × 10−4 M methanolic solution of picric acid and 1,3,5-trinitrobenzene showed gradual quenching of the initial fluorescence intensity.
Figure 2.
Quenching of initial fluorescence intensity of 6 (1.0 × 10−6 M) upon gradual addition of picric acid and 1,3,5-trinitrobenzene in methanol (1.0 × 10−4 M ) at room temperature and corresponding Stern-Volmer plots.
Figure 3.
Quenching of initial fluorescence intensity of 5 (1.0 × 10−6M) upon gradual addition of picric acid and 1,3,5-trinitrobenzene in methanol (1.0 × 10−4 M) at room temperature and corresponding Stern-Volmer plots.
Emission of metallarectangles 5 and 6 was above 350 nm whereas picric acid absorbs irradiation below 300 nm. This evidence ruled out the possibility of energy transfer from excited rectangles to explosives quenched due to a lack of overlap between the emission of supramolecular rectangles and absorption of picric acid. Thus, the observed fluorescence quenching is due to photoinduced electron transfer from the excited state of metallarectangles to the ground state of nitroaromatics. The main reason for facile electron transfer is the presence of conjugated labile π-electrons in the fluorophore and the strong oxidant tendency of 1,3,5-trinitobenzene and picric acid.
3.2.1. Analysis of normalized fluorescence intensity (I0/I) as a function of picric acid and 1,3,5-trinitrobenzene concentration
Stern-Volmer constants KSV = 3.7 × 105 M−1 and 2.2 × 105 M−1 were obtained from the slope of a linear Stern-Volmer plots for picric acid and 1,3,5-trinitrobenzene with metallarectangle 5, respectively (Figure 2). The R2 values 0.882 and 0.977 were obtained from the Stern-Volmer plots for picric acid and 1,3,5-trinitrobenzene with metallarectangle 5, respectively. Stern-Volmer constants KSV= 1.2 × 105 M−1 and 4.2 × 105 M−1 were obtained from the slope of a linear Stern-Volmer plot for picric acid and 1,3,5-trinitrobenzene with metallarectangle 6, respectively (Figure 3). The R2 values 0.922 and 0.983 were obtained from the Stern-Volmer plots for picric acid and 1,3,5-trinitrobenzene with metallarectangle 6, respectively.
The investigation was extended to nitro-aromatics known as electron-poor molecules, such as nitrobenzene and 2,4-dinitrophenol (Figure S8, S9, S12 and S13, Supporting Information). The results indicate that the increased electron-withdrawing nature of nitro-aromatics may induce enhanced π–π interactions with π-electron rich molecular rectangles 5 and 6. The selectivity of 5 and 6 for nitroaromatic compounds over other electron-deficient aromatic systems such as benzoic acid (BA) and benzoquinone (BQ) was also investigated. No significant quenching was noticed with polar aromatics such as benzoic acid (Figure S11 and S15, Supporting Information). The weak quenching response towards carboxylic acid also ruled out the possibility of simple acid–base reactions of analytes with molecular rectangles.
The fluorescence quenching results suggest that metallarectangles 5 and 6 only have quenching responses for nitroaromatic analytes in comparison to other tested aromatic compounds due to their low reduction potential value compared to nitroaromatics. This result support proposed quenching mechanism, in which electron-deficient nitroaromatics act as fluorescence quenchers via photoinduced electron transfer from the electron-rich fluorophores.
4. Conclusions
In conclusion, we herein report the synthesis and characterization of metallarectangles 5 and 6 prepared via directional self-assembly of a dinuclear Ru-acceptor and a new dipyridyl donor. The incorporation of ethynyl and triptycene functionalities creates assemblies fluorescent and π-electron rich. These new, electron-rich, and photoluminescent metallarectangles have been examined for emission quenching effects with various aromatic compounds. The metallarectangles show high quenching selectivity and sensitivity towards nitroaromatics, particularly picric acid and 1,3,5-trinitrobenzene. Conjugated organic polyethynyl compounds have been used as potential sensors for chemical explosives. Our unique self-assembled organometallic rectangles 5 and 6 can be used as molecular sensors to detect picric acid and 1,3,5-trinitrobenzene.
Supplementary Material
Triptycene and ethynyl functionalities containing new ligand has been synthesized.
Fluorescent and π-electron rich metallarectangles 5 and 6 were synthesized.
Presence of triptycene prevents self-quenching by avoiding intermolecular stacking.
Metallarectangles 5 and 6 show high emission quenching selectivity for nitroaromatics.
Acknowledgments
The Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2013R1A1A2006859) supported this work. The Priority Research Centers program (2009-0093818) through the NRF is also appreciated. X-ray diffraction experiments using synchrotron radiation were performed at Pohang Accelerator Laboratory in Korea. P.J.S. thanks the USA National Institutes of Health (Grant GM-057052) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary material
CCDC 986299 contains the supplementary crystallographic data for complex 6. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Supplementary data associated with 1H, 13C NMR and ESI-MS spectral of ligand 2, complexes (5 and 6). Fluorescence quenching spectra of complexes with different quenchers.
References
- 1.a Steed JW, Turner DR, Wallace KJ. Core Concepts in Supramolecular Chemistry and Nanochemistry. John Wiley & Sons; West Sussex, U.K.: 2007. [Google Scholar]; b Steed JW, Atwood JL. Supramolecular Chemistry. John Wiley & Sons; West Sussex, U.K.: 2000. [Google Scholar]
- 2.a Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; b Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; c Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001;509 [Google Scholar]; d Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; e Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; f Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; g Severin K. Chem. Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; h Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]; i Lee SJ, Lin W. Acc. Chem. Res. 2008;41:521. doi: 10.1021/ar700216n. [DOI] [PubMed] [Google Scholar]; j Rauchfuss TB, Severin K. In: In Organic Nanostructures. Atwood JL, Steed JW, editors. Wiley-VCH: Weinheim; Germany: 2008. p. 179. [Google Scholar]; k Han Y-F, Jia W-G, Yu W-B, Jin G-X. Chem. Soc. Rev. 2009;38:3419. doi: 10.1039/b901649j. [DOI] [PubMed] [Google Scholar]; l Therrien B. Eur. J. Inorg. Chem. 2009:2445. [Google Scholar]; m De S, Mahata K, Schmittel M. Chem. Soc. Rev. 2010;39:1555. doi: 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]; n Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Jin P, Dalgarno SJ, Atwood JL. Coord. Chem. Rev. 2010;254:1760. [Google Scholar]; p Caulder DL, Raymond KN. Acc. Chem. Res. 1999;32:975. [Google Scholar]; q Northrop BH, Zheng YR, Chi K-W, Stang PJ. Acc. Chem. Res. 2009;42:1554. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]; r Chakrabarty R, Mukherjee PS, Stang PJ. Chem. Rev. 2011;111:6810. doi: 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]; s Cook TR, Zheng YR, Stang PJ. Chem. Rev. 2013;113:734. doi: 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]; t Mishra A, Kang SC, Chi K-W. Eur. J. Inorg. Chem. 2013:5222. [Google Scholar]; u Vajpayee V, Bivaud S, Goeb S, Croue V, Allain M, Popp BV, Garci A, Therrien B, Salle M. Organometallics. 2014;33:1651. [Google Scholar]
- 3.a Carr JD, Lambert L, Hibbs DE, Hursthouse MB, Abdul Malik KM, Tucker JHR. Chem. Commun. 1997:1649. [Google Scholar]; b Lehaire M-L, Scopelliti R, Piotrowski H, Severin K. Angew. Chem., Int. Ed. 2002;41:1419. doi: 10.1002/1521-3773(20020415)41:8<1419::aid-anie1419>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]; c Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org. Lett. 2004;6:651. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]; d Grote Z, Scopelliti R, Severin K. J. Am. Chem. Soc. 2004;126:16959. doi: 10.1021/ja044874n. [DOI] [PubMed] [Google Scholar]; e Palacios MA, Nishiyabu R, Marquez M, Anzenbacher P. J. Am. Chem. Soc. 2007;129:7538. doi: 10.1021/ja0704784. [DOI] [PubMed] [Google Scholar]; f Ghosh S, Mukherjee PS. Organometallics. 2008;27:316. [Google Scholar]; g Xu M, Wu S, Zeng F, Yu C. Langmuir. 2009;26:4529. doi: 10.1021/la9033244. [DOI] [PubMed] [Google Scholar]; h Ghosh S, Chakrabarty R, Mukherjee PS. Inorg. Chem. 2009;48:549. doi: 10.1021/ic801381p. [DOI] [PubMed] [Google Scholar]; i Rochat S, Severin K. Org. Biomol. Chem. 2009;7:1147. doi: 10.1039/b820592b. [DOI] [PubMed] [Google Scholar]; j Anslyn EV. J. Am. Chem. Soc. 2010;132:15833. doi: 10.1021/ja108349y. [DOI] [PubMed] [Google Scholar]; k Gao J, Rochat S, Qian X, Severin K. Chem. Eur. J. 2010;16:5013. doi: 10.1002/chem.200903119. [DOI] [PubMed] [Google Scholar]; l Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Chem. Rev. 2011;111:6603. doi: 10.1021/cr100242s. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Dsouza RN, Pischel U, Nau WM. Chem. Rev. 2011;111:7941. doi: 10.1021/cr200213s. [DOI] [PubMed] [Google Scholar]; n Shanmugaraju S, Joshi SA, Mukherjee PS. Inorg. Chem. 2011;50:11736. doi: 10.1021/ic201745y. [DOI] [PubMed] [Google Scholar]; o Shanmugaraju S, Bar AK, Joshi SA, Patil YP, Mukherjee PS. Organometallics. 2011;30:1951. [Google Scholar]; p Vajpayee V, Song YH, Lee MH, Kim H, Wang M, Stang PJ, Chi K-W. Chem.-Eur. J. 2011;17:7837. doi: 10.1002/chem.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Mishra A, Ravikumar S, Song Y, Prabhu NS, Hong SH, Kim H, Cheon S, Noh J, Chi K-W. Dalton Trans. 2014;43:6032. doi: 10.1039/c3dt53186d. [DOI] [PubMed] [Google Scholar]
- 4.a Zheng YR, Zhao Z, Kim H, Wang M, Ghosh K, Pollock JB, Chi K-W, Stang PJ. Inorg. Chem. 2010;49:10238. doi: 10.1021/ic1018373. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang M, Vajpayee V, Shanmugaraju S, Zheng YR, Zhao Z, Hyunuk K, Mukherjee PS, Chi K-W, Stang PJ. Inorg. Chem. 2011;50:1506. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Maureen RA. Chem. Eng. News. 1997;10:14. [Google Scholar]; b Yang JS, Swager TM. J. Am. Chem. Soc. 1998;120:11864. [Google Scholar]; c Jenkins TF, Leggett DC, Miyares PH, Walsh ME, Ranney TA, Cragin JH, George V. Talanta. 2001;54:501. doi: 10.1016/s0039-9140(00)00547-6. [DOI] [PubMed] [Google Scholar]
- 6.a Etnier E. Regul. Toxicol. Pharmacol. 1989;9:147. doi: 10.1016/0273-2300(89)90032-9. [DOI] [PubMed] [Google Scholar]; b Honeycutt M, Jarvis A, McFairland V. Ecotoxicol. Environ. Saf. 1996;35:282. doi: 10.1006/eesa.1996.0112. [DOI] [PubMed] [Google Scholar]; c Lachance B, Robidoux P, Hawari J, Ampleman G, Thiboutot S, Sunahara G. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 1999;444:25. doi: 10.1016/s1383-5718(99)00073-x. [DOI] [PubMed] [Google Scholar]; d Robidoux PY, Sunahara GI, Savard K, Dodard S, Martel M, Gong P, Hawari J. Environ. Toxicol. Chem. 2004;23:1026. doi: 10.1897/03-308. [DOI] [PubMed] [Google Scholar]; e Ownby D, Belden J, Lotufo G, Lydy M. Chemosphere. 2005;58:1153. doi: 10.1016/j.chemosphere.2004.09.059. [DOI] [PubMed] [Google Scholar]; f Ownby D, Belden J, Lotufo G, Lydy M. Chemosphere. 2005;58:1161. doi: 10.1016/j.chemosphere.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Máñez R, Sancenoń F, Hecht M, Biyical M, Rurack K. Anal. Bioanal. Chem. 2011;399:55–74. doi: 10.1007/s00216-010-4198-2. [DOI] [PubMed] [Google Scholar]
- 8.a Zhou Q, Swager TM. J. Am. Chem. Soc. 1995;117:7017. [Google Scholar]; b Yang J-S, Swager TM. J. Am. Chem. Soc. 1998;120:5321. [Google Scholar]; c Yang J-S, Swager TM. J. Am. Chem. Soc. 1998;120:11864. [Google Scholar]; d McQuade DT, Pullen AE, Swager TM. Chem. Rev. 2000;100:2537. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]; e Williams VE, Swager TM. Macromolecules. 2000;33:4069. [Google Scholar]; f Yamaguchi S, Swager TM. J. Am. Chem. Soc. 2001;123:12087. doi: 10.1021/ja016692o. [DOI] [PubMed] [Google Scholar]; g Cumming CJ, Aker C, Fisher M, Fox M, la Grone MJ, Reust D, Rockley MG, Swager TM, Towers E, Williams V. IEEE Trans. Geosci. Rem. Sens. 2001;39:1119. [Google Scholar]; h Zahn S, Swager TM. Angew. Chem., Int. Ed. 2002;41:4226. doi: 10.1002/1521-3773(20021115)41:22<4225::AID-ANIE4225>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; i Amara JP, Swager TM. Macromolecules. 2005;38:9091. [Google Scholar]; j Zhao D, Swager TM. Macromolecules. 2005;38:9377. [Google Scholar]; k Thomas SW, III, Amara JP, Bjork RE, Swager TM. Chem. Commun. 2005:4572. doi: 10.1039/b508408c. [DOI] [PubMed] [Google Scholar]; l Toal SJ, Trogler WC, Mater J. Chem. 2006;16:2871. [Google Scholar]; m Narayanan A, Varnavsky OP, Swager TM, Goodson T., III J. Phys. Chem. 2008;C(112):881. [Google Scholar]
- 9.Zhu Z, Swager TM. Org. lett. 2001;3:3471. doi: 10.1021/ol0164886. [DOI] [PubMed] [Google Scholar]
- 10.Yan H, Süss-Fink G, Neels A, Stoeckli-Evans H. J. Chem. Soc., Dalton Trans. 1997:4345. [Google Scholar]
- 11.Barry NPE, Furrer J, Therrien B. Helv. Chim. Acta. 2010;93:1313. [Google Scholar]
- 12.Perrin DD, Armarego WLF, editors. Purification of Laboratory Chemicals. 2nd edition Pergamon Press; Oxford, UK: 1988. [Google Scholar]
- 13.a Rouhi AM. Chem. Eng. News. 1997;75:14. [Google Scholar]; b Czarnik AW. Nature. 1998;394:417. [Google Scholar]; c Desvergne JP, Czarnik AW, editors. Chemosensors of Ion and Molecule Recognition. Kluwer Academic Publishers; Boston: 1997. [Google Scholar]; d de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, ice TER. Chem. Rev. 1997;97:1515. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]; e McQuade DT, Pullen AE, Swager TM. Chem. Rev. 2000;100:2537. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]; f Content S, Trogler WC, Sailor MJ. Chem. Eur. J. 2000;6:2205. doi: 10.1002/1521-3765(20000616)6:12<2205::aid-chem2205>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; g Dinolfo PH, Hupp JT. Chem. Mater. 2001;13:3113. [Google Scholar]; h Albert KJ, Myrick ML, Brown SB, James DL, Milanovich FP, Walt DR. Environ. Sci. Technol. 2001;35:3193. doi: 10.1021/es010829t. [DOI] [PubMed] [Google Scholar]; i Lee SJ, Lin W. J. Am. Chem. Soc. 2002;124:4554. doi: 10.1021/ja0256257. [DOI] [PubMed] [Google Scholar]; j Splan KE, Keefe MH, Aaron M, Massari KA, Hupp JT. Inorg. Chem. 2002;41:619. doi: 10.1021/ic010992p. [DOI] [PubMed] [Google Scholar]; k Yinon J. Anal. Chem. 2003;75:99A. [Google Scholar]; l Splan KE, Massari AM, Morris GA, Sun SS, Reina E, Nguyen ST, Hupp JT. Eur. J. Inorg. Chem. 2003:2348. [Google Scholar]; m Jiang H, Lin W. J. Am. Chem. Soc. 2003;125:8084. doi: 10.1021/ja034402t. [DOI] [PubMed] [Google Scholar]; n Jiang H, Lin W. J. Am. Chem. Soc. 2004;126:7426. doi: 10.1021/ja049145m. [DOI] [PubMed] [Google Scholar]; o Rose A, Zhu Z, Madigan CF, Swager TM, Bulovic V. V. Nature. 2005;434:876. doi: 10.1038/nature03438. [DOI] [PubMed] [Google Scholar]; p Balakrishnan K, Datar A, Zhang W, Yang X, Naddo T, Huang J, Zuo J, Yen M, Moore JS, Zang L. J. Am. Chem. Soc. 2006;128:6576. doi: 10.1021/ja0618550. [DOI] [PubMed] [Google Scholar]; q Naddo T, Che Y, Zhang W, Balakrishnan K, Yang X, Yen M, Zhao J, Moore JS, Zang L. J. Am. Chem. Soc. 2007;129:6978. doi: 10.1021/ja070747q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.