Abstract
Aim
Non-alcoholic steatohepatitis (NASH) is associated with increased hepatic insulin resistance. Ceramides and other toxic sphingolipids promote inflammation, lipotoxicity and insulin resistance; however, the role of ceramides in the pathogenesis of NASH has not been determined. This study characterizes expression of ceramide-related genes in human livers with NASH and examines the effects of weight loss on NASH and pro-ceramide gene expression in liver.
Methods
Liver biopsies were obtained to assess the histopathological status of non-alcoholic fatty liver disease/NASH prior to and following completion of a 1-year course of implementing either lifestyle changes or a standard enrichment protocol designed to encourage weight loss. Liver biopsy samples were used to measure pro-ceramide gene expression by quantitative reverse transcriptase polymerase chain reaction analysis (qRT–PCR), and serum was used to measure ceramide immunoreactivity.
Results
At baseline, serine palmitoyltransferase (SPTLC)2 (P = 0.02) and ceramide synthase (CER)1 (P = 0.001) mRNA transcripts were less abundantly expressed in livers with NASH relative to normal controls. After weight loss (average 9.3%), SPTLC1 (P = 0.005) and uridine diphosphate glucose ceramide glucosyltransferase (UGCG) (P = 0.001) expression significantly declined while CER1 increased (P = 0.001) among subjects randomized to the lifestyle change subgroup. Reductions in calorie and fat consumption were significantly correlated with changes in ceramide-related gene expression. Finally, both net and relative reductions in serum ceramide levels were significantly greater in the lifestyles compared with the standard enrichment (control) protocol group (both P < 0.005).
Conclusion
NASH is associated with increased insulin resistance and altered ceramide gene expression in liver. Weight loss-mediated reversal of NASH is associated with reduced pro-ceramide gene expression in liver.
Keywords: ceramide, fatty liver, gene expression, lifestyle intervention, non-alcoholic steatohepatitis, weight loss
INTRODUCTION
Non-Alcoholic Fatty Liver disease (NAFLD) is among the commonest causes of chronic liver disease in developed countries.1 NAFLD is associated with obesity, type 2 diabetes and metabolic syndrome, and most likely, insulin resistance mediates its pathogenesis.2 Lipid accumulation exceeding the liver’s capacity for oxidative metabolism, is an early and pivotal event leading to NAFLD.3 The spectrum of NAFLD ranges from simple steatosis (SS), to non-alcoholic steatohepatitis (NASH), which is potentially progressive and can lead to hepatic fibrosis, cirrhosis and liver failure.4 In NASH, fat droplet accumulation is associated with hepatocellular injury and lobular inflammation,5 and occasionally with perivenular or pericellular fibrosis and Mallory–Denk bodies. The mechanisms underlying progression from SS to NASH are not well understood.6 As there are no definitive treatments for NAFLD,7 additional information about factors contributing to disease progression is needed.
Triglycerides are the dominant lipid accumulated in hepatic steatosis. However, other lipids accumulated with NAFLD have not been thoroughly characterized in relation to disease status or progression. Recent studies demonstrated significant alterations in long-chain fatty acyl coenzyme A (CoA), diacylglycerol, free cholesterol, cholesterol ester, ceramide and sphingolipids in NAFLD.8–10 Ceramides and related sphingolipids represent minor components of the accumulated lipids, but their roles as mediators of insulin resistance, cell death and inflammation11 suggest that their contribution to NAFLD progression may be pivotal.
Ceramides are synthesized de novo in response to pro-inflammatory cytokines, oxidative stress and increased free fatty acid levels, all of which exist in obesity and insulin resistance states.12 Ceramide biosynthesis is dependent upon the availability of long-chain saturated fatty acids, which are prevalent in Western diets.12,13 Hepatic ceramide levels are elevated in animal models of obesity with type 2 diabetes.14–17 Bioinformatics strategies for lipidomics have demonstrated strong associations between hepatic ceramide levels and degree of steatosis in an animal model of obesity (ob/ob mice),18 and gene array analysis of human livers revealed positive correlations between expression of pro-ceramide signaling (MAP2K4) and metabolism (uridine diphosphate glucose ceramide glucosyltransferase [UGCG]) genes and hepatic fat content.19 These observations suggest that ceramides and sphingolipids may play important roles in the pathogenesis of NAFLD.
Several studies have shown that ceramides and related sphingolipids interfere with insulin signaling at different levels of the cascade.9,20 Local ceramide accumulation inhibits insulin actions by decreasing phosphorylation and activation of Akt/PKB.21 Glycosphingolipids, in particular the ganglioside GM3, interfere more proximally at the level of insulin receptor phosphorylation.22 Correspondingly, pharmacological inhibition of ceramide and glycosphingolipid synthesis improves insulin sensitivity in cultured cells and in animal models of obesity with type 2 diabetes. Inhibition of de novo ceramide biosynthesis by myriocin improves hepatic and peripheral insulin sensitivity and reduces plasma glucose levels.23 Similarly, inhibition of glycosphingolipid synthesis by AMP-DNM or Genz-123346 improves whole body glucose uptake, glucose tolerance and hemoglobin A1c levels.16 Importantly, inhibiting glycosphingolipid synthesis reduces expression of several genes involved in hepatic lipogenesis, gluconeogenesis, inflammation and hepatic fat accumulation.24,25 Therefore, it is not surprising that this approach has been suggested for treating insulin resistance with NASH.
Despite growing evidence that the ceramides and sphingolipids promote insulin resistance and NAFLD in animal models, pertinent human disease data are lagging. We used quantitative reverse transcriptase polymerase chain reaction (qRT–PCR) assays to measure pro-ceramide gene expression in human liver biopsy samples obtained at baseline and 12 months after enrolling in a study to evaluate the effectiveness of weight loss on liver histopathology and peripheral insulin resistance. The findings suggest that weight loss-mediated reversal of NASH histology and restoration of insulin sensitivity are associated with reduced proceramide gene expression in liver.
METHODS
Participants
In the NASH group (n = 31), subjects were recruited as part of a randomized controlled trial to test the effect of weight loss on NASH (trial registration: www.clinicaltrials.gov, NCT00266019).26 Inclusion criteria were: (i) elevated alanine or aspartate aminotransferase values (ALT >41 or AST >34 U/L); (ii) body mass index (BMI) between 25 and 40 kg/m2; (iii) absence of another form of liver disease; and (iv) evidence of steatohepatitis on liver biopsy (defined as presence of [i] macro-vesicular steatosis, [ii] lobular inflammation and [iii] acinar zone 3 hepatocellular injury or ballooning degeneration).5 Exclusion criteria were: (i) significant alcohol consumption (>1 standard drink/day); (ii) contraindication for liver biopsy; (iii) inability to walk a quarter mile without stopping; (iv) pregnancy; (v) engagement in an active weight loss program or taking weight loss medication; (vi) substance abuse; or (vii) significant psychiatric problems. Written informed consent for enrollment and participation in this study was obtained from all subjects.
For the control group (n = 10), de-identified, histologically-confirmed, tumor-free, liver biopsy specimens from non-obese patients who had undergone resection of their metastatic tumors served as controls. Control liver tissue was obtained distant from the metastatic tumor.
Interventions
Participants with NASH (n = 31) were randomly assigned to a lifestyle intervention (LS; N-21) or standard enrichment protocol (SEP; N-10) group to compare the effects of different weight loss strategies on liver histology.26 The LS intervention was a year-long, standardized program utilizing diet, exercise and behavior modification to achieve 7–10% weight reduction, as reported previously.27,28 SEP participants received basic education about healthful eating, physical activity and weight control.27,29 The Block Food Frequency Questionnaire, which corresponds with dietary records,30 was used to measure changes in macro- and micronutrient intake at baseline and at the end of the study (48 weeks).
Liver biopsies
Liver biopsies were obtained upon entry and completion of the study. The specimens were divided with one portion fixed for histopathological examination, and the other frozen at −80°C for molecular studies. Liver histopathology was interpreted under code using a standardized scoring system that is recommended for NASH-related clinical trials.5
Molecular and biochemical studies
Gene expression was measured in liver tissue by qRT– PCR assays as previously described31 and using gene-specific primers (Supplementary Table S1). mRNA abundance was calculated relative to 18S rRNA, and those ratios were used for statistical analyses.31 Because the frozen liver biopsy samples were quite small (~2 mm in length and 1 mm in diameter), only a limited number of studies were feasible. To make the maximum use of the available tissue, our investigations were limited to qRT–PCR analysis. Serum ceramide levels were measured by enzyme-linked immunosorbent assay as previously described.32
Statistical analyses
Data were analyzed using the Statistical Package for Social Sciences ver. 14.0 for Windows. Intergroup comparisons were made using ANOVA with post-hoc Bonferroni tests. ANCOVA with baseline values serving as covariates, was used to compare the LS and SEP groups with respect to changes in gene expression. χ2-Tests were used for all cross-sectional tests of proportions, and correlations (Pearson’s r) were used to examine the relationships between change in gene expression and changes in dietary parameters. Data depicted in the graphs represent means ± standard errors.
RESULTS
Subjects
Overall, the mean age of the population was 48 years and the mean BMI was 34 kg/m2. Most participants (71%) were men. Twenty-six (84%) were white, four (13%) were Hispanic and one (3%) was American Indian/Alaska Native. Forty-eight percent had type 2 diabetes and 74% had metabolic syndrome.33
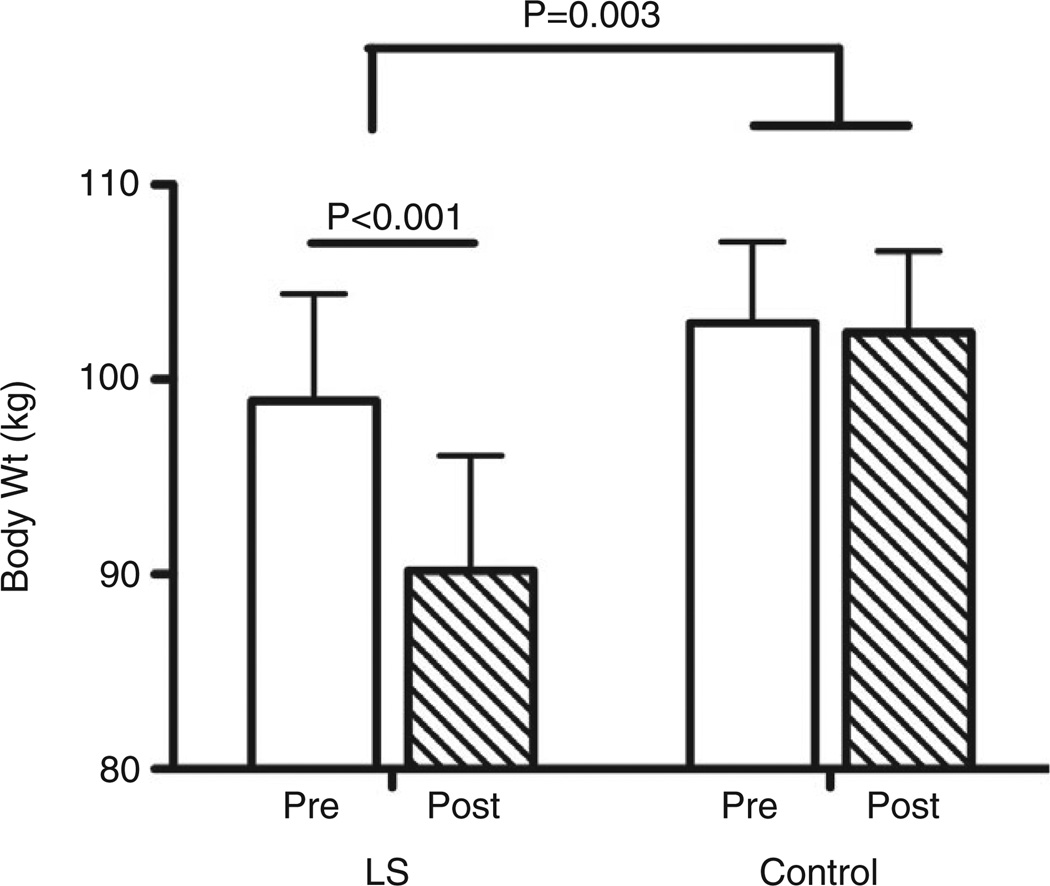
Baseline participant characteristics of subjects randomized to the SEP control or LS group are shown in Supplementary Table S2. The two groups did not differ initially with respect to age, sex or BMI. However, 1 year later, the LS group lost on average 8.7 kg (95% confidence interval [CI] = 5.6–11.7), while the SEP control group lost only 0.5 kg (95% CI = −3.9 to 4.9) (P = 0.005). Moreover, LS group participants achieved and maintained the target 7–10% weight loss, whereas the control group exhibited minimal weight reduction (9.3 ± 7.5% vs 0.2 ± 6.1%, P = 0.003) (Fig. 1).
Figure 1.
Change in bodyweight (kg) by group at 12 months. P = 0.003 for the comparison between lifestyle intervention (LS) vs standard enrichment protocol controls at month 12. Wt, weight.
Expression of ceramide-related genes at baseline
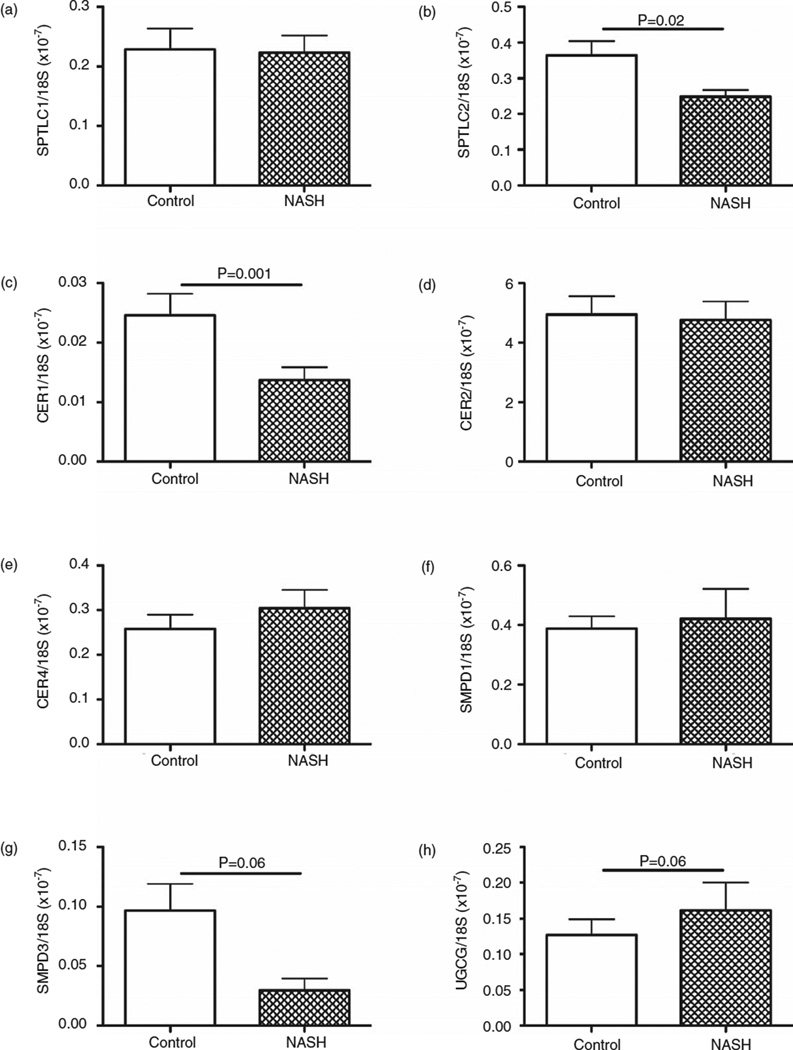
Quantitative RT–PCR analysis was used to measure expression of eight ceramide-related genes, including serine palmitoyltransferase (SPTLC)1 and 2, ceramide synthase (CER)1, 2 and 4, sphingomyelin phosphodiesterase (SMPD)1 and 3, and uridine diphosphate (UDP) glucose ceramide glucosyltransferase (UGCG) (see Supplementary Table S3). Comparisons between baseline NASH (n = 31) and normal control (n = 10) livers revealed significantly lower mean levels of SPTLC2 (P < 0.001), CER1 (P = 0.001) and CER2 (P = 0.04; data not shown) mRNA transcripts in NASH (Fig. 2). There was a trend toward statistically significant difference in the expression of UGCG (P = 0.06) and SMPD1 (P = 0.07). Expression of the other four ceramide-related genes was not significantly different between the groups. Weighted kappa scores showed a high degree of intra-rater agreement for these findings (steatosis grade 0.85, fibrosis stage 0.91, lobular inflammation 0.91, and ballooning degeneration 0.7).34 Of the 31 participants, 28 (90.3%) had adequate liver tissue for gene expression analysis.
Figure 2.
Comparison of baseline ceramide-related gene expression in livers of non-alcoholic steatohepatitis (NASH) (n = 31) vs control (n = 10). Graphs depict mean ± standard error of the mean for (a) SPTLC1, (b) SPTLC2, (c) CER1, (d) CER2, (e) CER4, (f) SMPD-1, (g) SMPD-3, (h) UGCG. mRNA levels were normalized to 18S rRNA. CER, ceramide synthase; SMPD, sphingomyelin phosphodiesterase; SPTLC, serine palmitoyltransferase; UGCG, uridine diphosphate glucose ceramide glucosyltransferase.
Effects of weight loss on ceramide-related gene expression in the liver
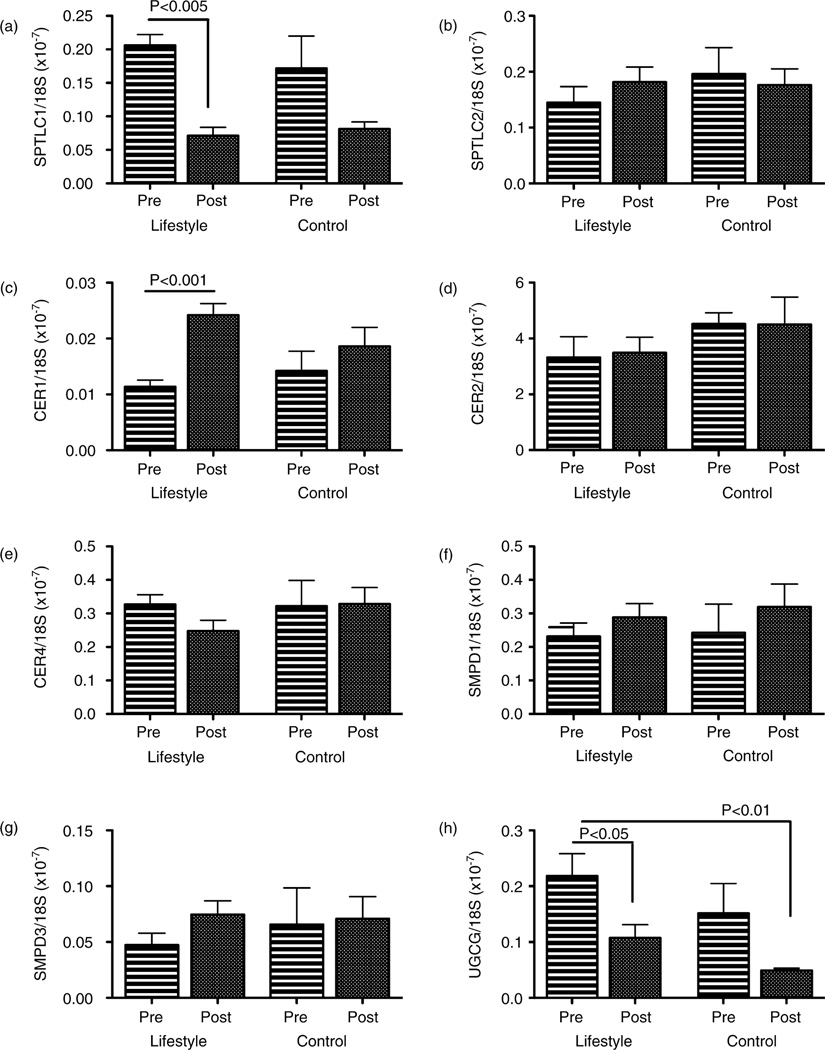
After weight loss (average 9.3% from baseline), hepatic SPTLC1 (P = 0.005) and UGCG (P = 0.001) mRNA levels significantly decreased while CER1 significantly increased (P = 0.001) compared to baseline levels in the lifestyle intervention group (Fig. 3). Moreover, those with greater improvements in liver histology (≥3 point reduction in NASH) had significant reductions in SPTLC1 (P = 0.0001) and UGCG (P = 0.004), and significant increases in CER1 (P = 0.001) mRNA.
Figure 3.
Effect of weight loss on ceramide-related gene expression in the liver. Graphs depict mean ± standard error of the mean of hepatic gene expression at baseline (Pre) and after 1 year of intervention (Post). Comparisons were made between lifestyle (n = 21) vs standard enrichment protocol controls (n = 10). (a) SPTLC1, (b) SPTLC2, (c) CER1, (d) CER2, (e) CER4, (f) SMPD-1, (g) SMPD-3, (h) UGCG. mRNA levels were normalized to 18S rRNA. CER, ceramide synthase; SMPD, sphingomyelin phosphodiesterase; SPTLC, serine palmitoyltransferase; UGCG, uridine diphosphate glucose ceramide glucosyltransferase.
Effects of dietary change on ceramide-related gene expression in the liver
Reduced daily calorie intake correlated with changes in expression of CER1 (r = 0.62, P = 0.02). Reduced daily fat consumption correlated with changes in SPTLC2 (r = 0.49, P = 0.04), CER4 (r = 0.57, P = 0.03) and SMPD3 (r = 0.47, P = 0.05) expression. Reductions in daily cholesterol (r = 0.57, P = 0.03) and saturated fat (r = 0.70, P = 0.006) consumption correlated with changes in CER1 expression.
Effects of weight loss on serum ceramide level
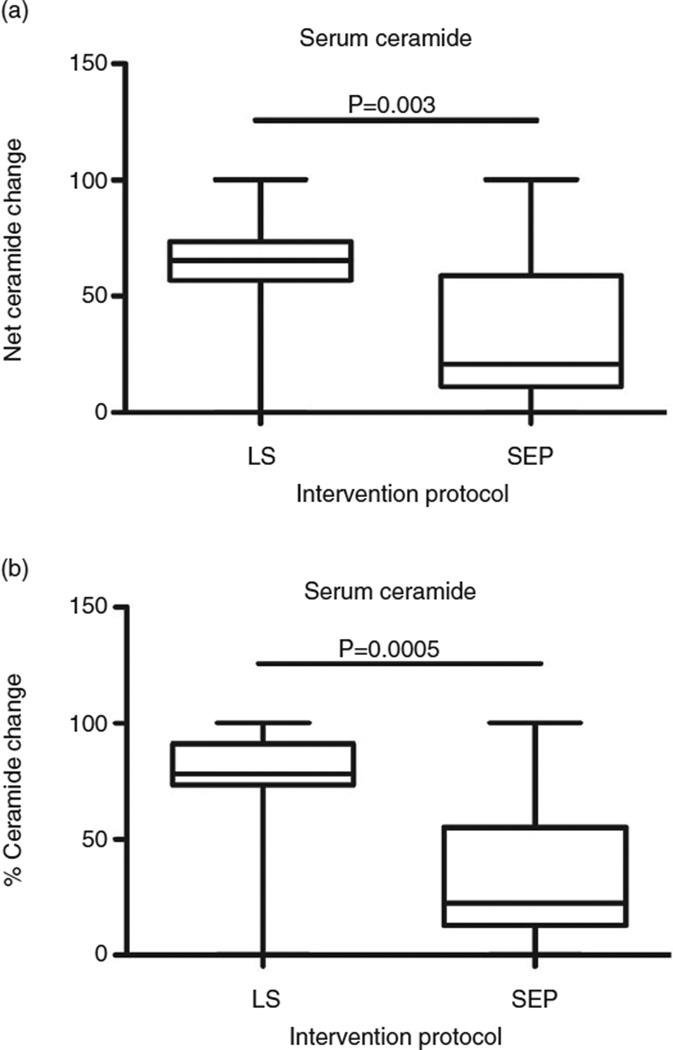
After participating for 1 year in the weight loss intervention programs, serum ceramide levels declined significantly in the LS relative to SEP control group (P = 0.01) (Fig. 4). Changes in serum ceramide levels were significantly correlated with changes in daily consumption of total fat (r = 0.46, P = 0.02) and saturated fat (r = 0.47, P = 0.02). In contrast, correlation analyses failed to demonstrate significant correlations between baseline serum ceramide level and biopsy score or Homeostatic Model of Assessment.
Figure 4.
Effect of weight loss on serum ceramide. Serum ceramide was measured by enzyme-linked immunosorbent assay. Box plots depict (a) net or (b) percentage reductions in mean (horizontal bar) ± 95% confidence limit interval limits (upper and lower boundaries of the boxes) and range (whiskers) of values in the lifestyle modification (LS, n = 21) vs standard enrichment protocol control (SEP, n = 10) groups.
DISCUSSION
Non-Alcoholic fatty liver disease is strongly correlated with obesity, insulin resistance and type 2 diabetes, and considered a hepatic manifestation of metabolic syndrome.35 The metabolic pathways leading to the development of fatty liver include increased fatty acid release from adipose tissue (lipolysis),36 increased de novo formation of fatty acids (lipogenesis)37 and decreased hepatic β-oxidation. Excess triacylglycerides accumulate in cytosol of hepatocytes leading to lipotoxicity, a critical component in progressive hepatocellular injury in NASH. Recent studies have demonstrated that other lipids accumulate in liver and contribute to insulin resistance and lipotoxicity. One candidate includes ceramides, which inhibit insulin signaling through suppression of IRS-1 phosphorylation, and cause lipotoxicity in various tissues.20 Plasma ceramide levels are elevated in type 2 diabetes and obesity, and closely correlate with severity of insulin resistance and pro-inflammatory cytokine, tumor necrosis factor-α.38 Increased expression of genes involved in ceramide metabolism (UGCG) and signaling (MAP2K4) correlates with hepatic lipid content in humans with fatty liver disease.19 In addition, ceramide levels in adipose tissue are higher in individuals with high liver lipid content compared with low liver lipid content, independent of obesity.39
Herein, we demonstrated that genes involved in de novo biosynthesis and degradation of ceramide were differentially expressed in hepatic tissue of subjects with NASH compared to controls. Weight reduction improved steatosis and hepatocellular injury and substantially changed the expression of ceramide-related genes in liver. Changes in calorie intake and fat consumption, in particular cholesterol and saturated fat, were significantly correlated with changes in ceramide-related gene expression.
Ceramide production is mediated by de novo synthesis via serine palmitoyltransferase and ceramide synthase, or by hydrolysis of membrane sphingomyelin by sphingomyelin phosphodiesterase.11 Rates of de novo ceramide synthesis depend on bioavailability of long-chain saturated fats, in particular palmitic acid.13 Previous studies conclusively showed that ceramide antagonizes insulin actions, and it mediates the inhibitory effects of long-chain saturated fatty acids on insulin signaling.21,40 Herein, we demonstrate that baseline expression of SPTLC2, which encodes an initial rate-limiting step enzyme in de novo synthesis of ceramide from condensation of serine and palmitoyl-CoA and CER1, which encodes the enzyme for incorporating the second fatty acyl-CoA, was significantly reduced in NASH compared to control. This finding suggests fundamental differences in the levels of ceramide intermediate substrates in the de novo biosynthesis pathway in individuals with NASH. Conceivably, negative feedback from other ceramide synthesis pathways (turnover of complex sphingolipids) or partitioning of saturated fatty acids between sphingolipids and TAG pools may play a role.41 Importantly, after weight loss (~9.3%), the level of SPTLC1 (P = 0.005) and CER1 (P = 0.001) expression in subjects with NASH increased significantly toward the level observed in controls. These molecular changes were accompanied by significant improvements in all key histological components of NASH, namely steatosis, parenchymal inflammation and hepatocellular injury.26 This suggests that weight loss leads to significant alteration in the regulation of ceramide biosynthesis which is likely important in the pathogenesis of liver damage in NASH. Potential mechanisms include better regulation of insulin signaling, reversal of mitochondrial dysfunction and reduced apoptosis.
Uridine diphosphate glucose ceramide glucosyltransferase (UGCG) is essential in the biosynthesis of glycosphingolipids (GSL). UGCG catalyzes the first glycosylation step in the conversion of ceramide to glucosylceramide42 which is the core structure of more than 300 GSL. Glycosphingolipids such as ganglioside GM3, are potent inhibitors of insulin signaling via suppression of tyrosine phosphorylation of insulin receptor and IRS-1.43 Microarray analysis demonstrated that UGCG gene expression was elevated in livers with NAFLD and that the degree of increased gene expression correlated with liver lipid content.19 Importantly, pharmacological inhibition of glycosphingolipid synthesis using specific small molecule inhibitors of UGCG improved insulin sensitivity and glucose homeostasis16,17 and reversed hepatic steatosis24,25 in rodent models of diabetes and obesity. Moreover, biliary lipid secretion doubles after UGCG inhibitor treatment in mice.44 Our study demonstrated that baseline hepatic UGCG expression was elevated in NASH, as observed in animal models of insulin resistance, and significantly decreased after weight loss, along with improvement in histological parameters of NASH. Together, these results suggest important roles for GSL metabolites of ceramide in the pathogenesis of insulin resistance and NASH in humans. Pharmacological interference with GSL biosynthesis may represent a novel approach for treating NASH.
Strengths of this study include: (i) selection of patients with clinically and histologically, well-characterized NASH; (ii) the use of standardized lifestyle intervention protocol; and (iii) availability of paired liver biopsies for molecular analyses in relation to weight loss and changes in calorie and macronutrient consumption over time. Most prior studies focused on severely obese subjects undergoing bariatric surgery.45 In summary, this study provides evidence that genes involved in ceramide biosynthesis and degradation are differentially expressed in hepatic tissue of subjects with NASH. Weight reduction improves steatosis and hepatocellular injury, and substantially changes the expression of ceramide-related genes in the liver. Our data suggest that ceramides play important roles in the pathogenesis of NASH.
Supplementary Material
ACKNOWLEDGMENTS
Research was supported by grants R03DK67263, CA-123544, AA-11431, AA-12908 and K24-AA-16126 from the National Institutes of Health.
Footnotes
Author contribution: K. P. conceived of the study and drafted the manuscript; L. L. performed the gene expression analyses; J. R. W. participated in the translational research and data interpretation; S. M. D. conceived of, directed, supervised and interpreted the molecular/translational studies and assisted with writing of the manuscript.
Conflicts and disclosures: None of the authors has anything to disclose and they have no conflicts of interest related to this research.
Additional Supporting Information may be found in the online version of this article:
Table S1 Quantitative reverse transcriptase polymerase chain reaction primers.
Table S2 Baseline characteristics of study participants.
Table S3 Ceramide-related genes and their function. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 3.Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:351–359. doi: 10.1055/s-0028-1091979. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 6.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 7.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 8.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 9.Puri P, Wiest MM, Cheung O, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 13.Merrill AH, Jr, Lingrell S, Wang E, Nikolova-Karakashian M, Vales TR, Vance DE. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J Biol Chem. 1995;270:13834–13841. doi: 10.1074/jbc.270.23.13834. [DOI] [PubMed] [Google Scholar]
- 14.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 16.Aerts JM, Ottenhoff R, Powlson AS, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Przybylska M, Wu IH, et al. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 18.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 20.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 22.Langeveld M, Aerts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res. 2009;48:196–205. doi: 10.1016/j.plipres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bijl N, Sokolovic M, Vrins C, et al. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 2009;50:1431–1441. doi: 10.1002/hep.23175. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Przybylska M, Wu IH, et al. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50:85–93. doi: 10.1002/hep.22970. [DOI] [PubMed] [Google Scholar]
- 26.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8:399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 30.Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The National Cancer Institute diet history questionnaire: validation of pyramid food servings. Am J Epidemiol. 2006;163:279–288. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- 31.Lyn-Cook LE, Jr, Lawton M, Tong M, et al. Hepatic Ceramide May Mediate Brain Insulin Resistance and Neurodegeneration in Type 2 Diabetes and Non-alcoholic Steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brade L, Vielhaber G, Heinz E, Brade H. In vitro characterization of anti-glucosylceramide rabbit antisera. Glycobiology. 2000;10:629–636. doi: 10.1093/glycob/10.6.629. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 34.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 35.Musso G, Gambino R, Bo S, et al. Should nonalcoholic fatty liver disease be included in the definition of metabolic syndrome? A cross-sectional comparison with Adult Treatment Panel III criteria in nonobese nondiabetic subjects. Diabetes Care. 2008;31:562–568. doi: 10.2337/dc07-1526. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 41.Deevska GM, Rozenova KA, Giltiay NV, et al. Acid Sphingomyelinase Deficiency Prevents Diet-induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J Biol Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci USA. 1996;93:4638–4643. doi: 10.1073/pnas.93.10.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tagami S, Inokuchi Ji J, Kabayama K, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 44.Bijl N, van Roomen CP, Triantis V, et al. Reduction of glycosphingolipid biosynthesis stimulates biliary lipid secretion in mice. Hepatology. 2009;49:637–645. doi: 10.1002/hep.22663. [DOI] [PubMed] [Google Scholar]
- 45.Younossi ZM, Gorreta F, Ong JP, et al. Hepatic gene expression in patients with obesity-related non-alcoholic steatohepatitis. Liver Int. 2005;25:760–771. doi: 10.1111/j.1478-3231.2005.01117.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.