Abstract
Introduction and hypothesis
The use of polypropylene meshes for surgical repair of pelvic organ prolapse (POP) has been limited by complications, including mesh exposure, encapsulation, and pain. Numerous products are available with a wide array of textile and structural properties. It is thought that complications may be related, in part, to mesh structural properties. However, few descriptions of these properties exists to directly compare products. The aim of this study was to determine the textile and structural properties of five commonly used prolapse mesh products using a ball-burst failure protocol.
Methods
Porosity, anisotropic index, and stiffness of Gynemesh PS (n=8), the prototype polypropylene mesh for prolapse repair, was compared with four newer-generation mesh produces: UltraPro (n=5), SmartMesh (n=5), Novasilk (n=5), and Polyform (n=5).
Results
SmartMesh was found to be the most porous, at 78 %±1.4 %. This value decreased by 21 % for Gynemesh PS (p<0.001), 14 % for UltraPro and Novasilk (p<0.001), and 28 % for Polyform (p<0.001). Based on the knit pattern, SmartMesh and Polyform were the only products considered to be geometrically isotropic, whereas all other meshes were anisotropic. Comparing the structural properties of these meshes, Gynemesh PS and Polyform were the stiffest: 60 % and 42 % stiffer than SmartMesh (p<0.001) and Novasilk (p<0.001), respectively. However, no significant differences were found between these two mesh products and UltraPro.
Conclusions
Porosity, anisotropy, and biomechanical behavior of these five commonly used polypropylene mesh products were significantly different. This study provides baseline data for future implantation studies of prolapse mesh products.
Keywords: Ball-burst, Prolapse, Polypropylene mesh, Structural properties
Introduction
Pelvic organ prolapse (POP) is a condition that is reaching epidemic levels, affecting 50 % of women >50 years [1]. Although not all women become symptomatic, 225,000–00,000 women annually in the United States alone elect to proceed with surgery to repair prolapse and related conditions, with a cost of > $1 billion in the United States per year [2, 3]. There is good evidence that patients with prolapse have weaker tissue and require some type of surgical repair to improve anatomical symptoms [4, 5]. For this reason, many urogynecologists have turned to mesh to re-inforce native tissues to re-establish failed soft tissue support, and improve anatomical outcomes. Mesh is used in 40–70 % of prolapse repairs, with the majority (~75 %) being placed transvaginally [6]. However, there is a wide variety of mesh products available, with polypropylene being the material most commonly used. However, little is known about the properties of these mesh products before implantation, which likely impacts their behavior following surgery. In addition, as mesh use has increased, the number of related complications—including anatomical failure, mesh exposure into the vaginal lumen, erosion into an adjacent structure, infection, pain, and dyspareunia—have also increased [7, 8]. Due to this high rate of complications, the US Food and Drug Administration (FDA) recently released a warning against their use in urogyne-cological surgeries [6, 9].
This action fueled the development of the next-generation mesh products, which tend to be more lightweight and have larger pores in order to lessen the amount of foreign material in contact with host tissues. Thus, decreased stiffness, increased pore size, and lower mesh density or specific weight, are thought to be factors that may increase incorporation into host tissues and decrease complication rates [10-12]. At the same time, specific weight, pore size, and porosity are all textile properties that will alter mesh structural properties. In addition, the specific knit pattern can impact structural properties and cause these properties to be directionally dependent (anisotropic). Anisotropic materials display different biomechanical properties when tested along different axes, whereas isotropic materials are directionally independent or display similar biomechanical properties when loaded in different directions. Thus, textile alterations implemented by companies during product design have the potential to vastly impact mechanical behavior. As mesh structural properties are associated with reported complication rates after implantation, better characterization of these properties could improve our understanding of clinical outcomes [13].
The objective of this study was to characterize the textile and ex vivo structural properties of four types of new-generation synthetic mesh products: UltraPro (Gynecare, Somerville, NJ, USA), SmartMesh (Mpathy Medical, Raynham, MA, USA), Novasilk (Coloplast, Minneapolis, MN, USA) and Polyform (Boston Scientific, Natick, MA, USA) and compare them with Gynemesh PS, the prototype polypropylene mesh used in prolapse repair. Our aims were to: (1) describe porosity and geometric anisotropy of these prolapse mesh products, (2) characterize structural properties using a ball-burst load to failure protocol, and (3) correlate the textile properties (specific weight, pore size, porosity, and anisotropy) with the ex vivo structural properties.
Methods
For this study, each mesh product was removed from the sterile packaging and divided for imaging and structural analysis. Ex vivo properties ofthe sterile mesh was studied prior to implantation; therefore, this study was exempt from requiring approval from an ethical committee. Specific weight (g/m2) measurements and industry reported pore sizes (μm) were provided from each company (Table 1). To measure porosity, a 10 × 10-mm square section of mesh (n=4 per mesh) was removed. Images were taken with a custom camera (Sony XCD-SX910, Lens: Computar 55 mm telecentric). Images of each mesh were uploaded into a custom-designed program (Matlab Version 8.0, Natick, MA, USA). Porosity of each mesh was then calculated by taking the average points that were labeled as background to the total number of pixels in the image. The porosity value is reported as a percentage (%).
Table 1.
Industry-reported specific weight and pore size presented with the experimentally calculated porosity and anisotropy index
| Industry reported |
Experimental results |
|||
|---|---|---|---|---|
| Synthetic mesh | Specific weight (g/m2) |
Pore size (μm) |
Porosity (%) |
Anisotropy index |
| Gynemesh PS (n=4) | 42 | 2,440 | 64±2.1 | 1.5±0.12 |
| UltraPro (n=4) | 28 | 4,000 | 69±1.8 | 1.6±0.18 |
| SmartMesh (n=4) | 19 | 2,370 | 78±3.0 | 1.4±0.31 |
| Novasilk (n=4) | 21 | 1,121 | 72±3.0 | 1.2±0.11 |
| Polyform (n=4) | 41 | 1,730 | 58±4.6 | 1.4±0.41 |
To determine geometric anisotropy of each mesh, a 6.5 × 5.2-mm image of each mesh (n=4 per mesh) was taken via light microscopy (Olympus MVX10 MacroView). Briefly, images were imported into ImageJ (1.36b NIH, USA), and a material tensor analysis was performed as previously described [14, 15]. With the assumption that the examined materials were isotropic, the degree of material symmetry based on structural arrangement of the isotropic material, ranging from orthotropic to isotropic, can be determined [14, 15]. From this analysis, a fabric tensor was calculated and the eigenvalues of this fabric tensor were related to the magnitude of the microstructure distribution along the principle direction [14]. For the two-dimensional cases, eigenvalues of fabric tensors were denoted τ1 and τ2 (τ1≥τ2). The anisotropy index (AI) was determined from the ratio of the first and second eigenvalues (τ1/τ2), with a value of 1 representing an isotropic material. This measurement is an indicator of geometric anisotropy of the mesh and for this study is limited to categorizing the material as either isotropic or anisotropic.
Ball-burst testing protocol
To obtain structural properties in response to a ball-burst test, Gynemesh PS (n=8), UltraPro (n=5), SmartMesh (n=5), Novasilk (n=5), and Polyform (n=5) were cut into square samples 25 × 25 mm and examined using a scaled American Society of Testing and Materials (ASTM) standard ball-burst apparatus with a ball diameter of 9.52 mm and a sample surface area of ~216 mm2. This scaled ball-burst test was developed to measure structural properties of the mesh–tissue complex after implantation and tissue incorporation on sample sizes that would be too small for the ASTM standard ball-burst apparatus. A preliminary study was performed to examine the effects of scaling down the ASTM standard ball-burst apparatus. Briefly, each mesh type was examined (n=5 per mesh) on the ASTM standard ball-burst apparatus and compared with the scaled ball-burst results. There was no significant difference in stiffness between the ASTM standard and scaled ball-burst protocols (p=0.86). Gynemesh PS and Novasilk had the largest variability, with an 11 % and 9.8 % difference between ball-burst setups, respectively. Polyform had a 5 % difference and SmartMesh only 0.6 %. The ASTM standard failure load was significantly higher (p<0.001) compared with loads resulting from the scaled ball-burst protocol. The difference in the reported failure load was roughly 2.5 and could be attributed to the scaling factor. A significant difference was also observed in extension (p<0.001) and energy absorbed to failure (p<0.001; data not shown). These differences could also be accounted for by the scaling factor.
Using the scaled apparatus, each sample was soaked in physiological saline solution [0.9 % sodium chloride (NaCl)] at room temperature prior to testing. The mesh was secured between two flat clamps with interlocking triangular grooves and mounted onto a custom stand, which attaches to the base of an Instron™ 4502 (Instron, Norwood, MA). The ball was connected in series with a load cell (Honeywell, 5 kN) and a moveable crosshead. Prior to testing, a small preload of 0.5 N was applied to ensure contact between the mesh and the ball while minimizing mesh deformation. Each sample was then loaded to failure at a rate of 10 mm/min. The resulting load-elongation curves were analyzed to determine the structural properties of each mesh. Failure load (N) and maximum extension (mm) corresponding to the ball breaking through the specimen were recorded. To calculate stiffness (N/mm), the maximum slope over a running window of 20 % of the failure elongation was used. Lastly, the energy absorbed to failure was calculated as the area under the curve (AUC) until failure (J).
Statistics
A power analysis of structural properties was done on a preliminary data set (G*Power 3.1.2), for which we needed a minimum of three samples per group to detect a minimum difference of 4 % in stiffness, 21 % in failure load, 2 % in maximum extension, and 5 % in the energy absorbed to failure at 80 % power. A one-way analysis of variance (ANOVA) was used to assess differences in porosity, and different structural properties were determined from the ball-burst testing protocols using either a Bonferroni or Dunnett’s T3 post hoc test. The anisotropic index was examined using a one-sample t test, and each mesh was compared to a test value of 1 (AI = 1), which represents an isotropic material. A Pearson correlation test was performed to examine whether the textile properties (specific weight, pore size, or porosity) correlated to the ex vivo structural properties. Correlations are defined as strong (R2=0.7–1.0), mild (R2=0.4–0.7), or weak (R2=0.2–0.4). All statistical tests were performed with a significance of p<0.05 using a statistical software package (PASW Statistics 18.0, Chicago, IL, USA).
Results
Gross examination revealed that each mesh had a unique knit pattern (Fig. 1). SmartMesh had a porosity of 78 %±1.4 %, which was significantly higher than Gynemesh PS (62 %± 3.2 %;p<0.001), UltraPro (67 %±1.5 %;p<0.001), Novasilk (67 %±3.8 %, p<0.001), and Polyform (56 %±3.2 %; p< 0.001). Polyform was the least porous and significantly less porous than UltraPro (p=0.001), SmartMesh (p<0.001), and Novasilk (p<0.001). Further, when comparing to the reported pore sizes, there was not a one-to-one correlation between pore size and porosity, indicating that these metrics are distinct from one another. The latter describes mesh burden, or the amount of material in contact with tissue, which is a common misinterpretation among clinicians using mesh.
Fig. 1.

Gross fiber network for Gynemesh PS (a), UltraPro (b), SmartMesh (c), Novasilk (d), and Polyform (e)
In terms of geometric anisotropy, SmartMesh and Polyform were not significantly different from the assumption of isotropy (p=0.1 and p=0.12, respectively). However, Gynemesh PS (p=0.004), UltraPro (p=0.007), and Novasilk (p= 0.02) all had some degree of anisotropy (Table 1). This information, along with their planar geometry, indicates that Gynemesh PS, UltraPro, and Novasilk behave like a transversely isotropic material. Thus, the direction in which these mesh products are implanted and way they are secured may affect the host response and the underlying tissue incorporation.
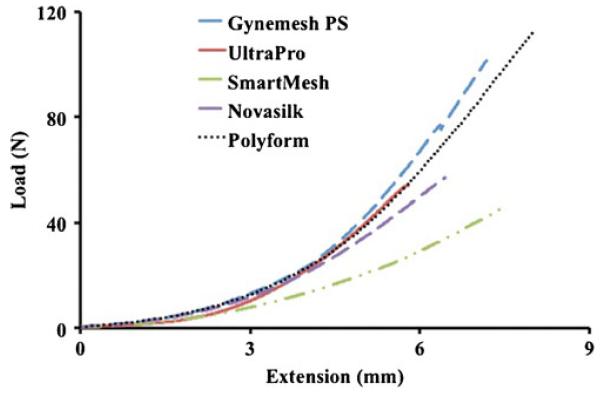
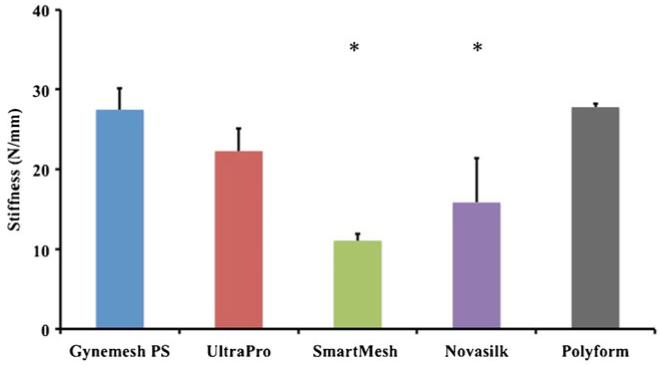
From the ball-burst-testing protocol, each mesh followed a common nonlinear load-to-failure profile, displaying a low-stiffness toe region followed by a linear and failure region (Fig. 2). Comparisons between structural properties of each of mesh revealed that Gynemesh PS and Polyform were roughly 60 % and 42 % stiffer than SmartMesh (p < 0.001) and Novasilk (p<0.001), respectively (Fig. 3). No significant differences between UltraPro were found when compared with Gynemesh PS (p=0.1) or Polyform (p=0.12; Table 2). SmartMesh, the least stiff mesh, failed at the lowest load. SmartMesh failed at a load 143 % smaller than that of Gynemesh PS (p<0.001), 70 % lower than UltraPro (p=0.003), and 144 % lower than Polyform (p<0.001). Gynemesh PS and Polyform failed at comparable loads (0.4 % difference), and both were significantly higher than UltraPro (p=0.001 and p=0002, respectively) and Novasilk (p<0.001). The energy absorbed to failure displayed a trend similar to that of the ball-burst stiffness data, and the data are displayed in Table 2.
Fig. 2.
Representative load-extension failure curves from the ball-burst protocol
Fig. 3.
Stiffness of Gynemesh PS (n=8), UltraPro (n=5), SmartMesh (n=5), Novasilk (n=5), and Polyform (n=5) calculated from the ball-burst protocol. *Significant difference from Gynemesh PS, the prototype polypropylene prolapse mesh
Table 2.
Structural properties of individual mesh products calculated from the load–elongation relationship from the ball-burst protocol
| Synthetic mesh | Stiffness (N/mm) | Failure load (N) | Extension (mm) | Energy absorbed (J) |
|---|---|---|---|---|
| Gynemesh PS (n=8) | 28±2.7 | 108±8.6 | 7.3±0.31 | 288±37 |
| UltraPro (n=4) | 22±2.8 | 76±12 | 7.3±0.21 | 170±11 |
| SmartMesh (n=5) | 11±0.89 | 45±3.8 | 6.7±0.45 | 109±11 |
| Novasilk (n=5) | 16±5.5 | 54±19 | 6.3±0.56 | 113±43 |
| Polyform (n=5) | 28±0.43 | 108±5.7 | 7.8±0.05 | 261±27 |
Textile properties correlated with structural properties determined from the ball-burst test. Specific weight (reported by the manufacture) strongly correlated with stiffness (R2=0.78, p<0.001), failure load (R2=0.85, p<0.001), and energy absorbed to failure (R2=0.89, p<0.001). However, it was only weakly related with extension (R2=0.38, p=0.001). The manufacturer-provided pore-size values of each mesh did not correlate with any of the structural properties; however, porosity measurements correlated with stiffness (R2=0.64, p<0.001), failure load (R2=0.6, p<0.001), and energy absorbed to failure (R2=0.57, p<0.001). A weak negative correlation between porosity and extension was also found (R2=0.34, p=0007). Interestingly, AI weakly correlated with extension (R2=0.22, p=0.037).
Discussion
We examined porosity, geometric AI, and performed an ex vivo analysis to determine structural properties via a ball-burst testing protocol of five commonly used types of polypropylene prolapse mesh products. It has become increasingly important to study these mesh products in light of the recent public concern over their use for prolapse repair. In addition, as the US FDA has become more involved in regulating these devices, several mesh products have been removed from the market. Most notably, Ethicon has stopped the sale of many of its gynecologic mesh products including the UltraPro mesh examined in this study (sale stopped after this study was completed), while Gynemesh PS, the original polypropylene mesh for prolapse repair, is currently still available but is now being labeled for a more restricted purpose.
Synthetic prolapse mesh products are designed to have large pores and a high degree of porosity to reduce the risk of infection and facilitate tissue ingrowth after implantation. Porosity, but not necessarily pore size, can be utilized as a metric for mesh burden on the tissue, or the amount the synthetic mesh that is in contact with tissue. A more porous mesh is thought to equate with a lower mesh burden. Compared to Gynemesh PS and Polyform, UltraPro, SmartMesh and Novasilk all have a potentially smaller mesh burden on the tissue. In addition, these values of porosity were within 5 % of those published utilizing another method [16], which helped confirm the methods of this study.
We also determined mesh geometric anisotropy by examining the architectural microstructure of each mesh utilizing the mean intercept length theorem. Gynemesh PS, UltraPro, and Novasilk all exhibited significant anisotropy as compared with SmartMesh and Polyform, which may be considered transversely isotropic due to the planar structure of these materials. This study and previous research have highlighted the difference in the structural properties [11, 13, 16, 17] and the importance of understanding the implantation direction prior to mesh placement. The present study offers insight as to why this finding may have been observed. In addition, this method offers us the ability to determine if a mesh is anisotropic without performing structural testing. In the future, this anisotropic index may help us better predict the behavior of these mesh products and why particular types lead to different clinical outcomes. Therefore, it is important to determine and compare the anisotropic index of mesh products prior to implantation.
In addition, our correlations illustrate that the specific weight and porosity are related to the structural properties of these mesh products and are factors to consider as manufacturers and researchers begin to develop the next generation of prolapse mesh products. Previously, Afonso et al. found a similar correlation between the geometry and textile properties to the stiffness of mesh products used for incontinence procedures, confirming the relationship between their textile and structural properties [18]. Therefore, the previous results combined with our findings strongly suggest that mesh architectural and textile properties can significantly influence the structural properties of these synthetic prolapse mesh products.
Although the structural properties of prolapse mesh products prior to implantation provide valuable baseline data, as shown above, they should by no means be considered a complete description of mesh mechanical behavior. The free edges of the uniaxial test allow for more initial geometric rearrangement of the mesh at lower loads, which is more likely to be influenced by geometric anisotropy than for the ball-burst protocol. In vivo, different boundary conditions are imposed depending on location and number of fixation sites used by the surgeon relative to the direction of the applied loads. Whereas, neither a uniaxial testing protocol nor ball-burst protocol alone will be able to specifically describe how a mesh will behave in vivo in any particular surgeons’ hands, an understanding of these properties and an appropriate interpretation based on boundary conditions of the testing protocol can form the basis for correlations with clinical findings and the development of more rigorous computational models for predicting mesh behavior in vivo.
This study established porosity, geometric anisotropy, and structural properties of five synthetic prolapse mesh products and is important for better characterizing these products and relating them to clinical outcomes. In addition, these mesh products have significantly different textile and structural properties, indicating that all polypropylene meshes are not similar and that their textile and structural properties prior to implantation likely have a critical impact on their behavior in vivo. Future studies by our group will investigate the relationship between these different properties acquired from the ball-burst test, the host response, and surgical outcomes in an animal model and use these findings as baseline data for our implantation studies.
Acknowledgements
National Institutes of Health (NIH) Support R01 HD061811-01
Footnotes
Conflicts of Interest None
Contributor Information
Andrew Feola, Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA.
William Barone, Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA.
Pamela Moalli, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee-Womens Hospital, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA, USA.
Steven Abramowitch, Musculoskeletal Research Center and Magee-Womens Research Institute, Departments of Bioengineering and Obstetrics, Gynecology, Reproductive Sciences University of Pittsburgh, Pittsburgh, PA, USA; Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, 405 Center for Bioengineering, 300 Technology Drive, Pittsburgh, PA 15219, USA, sdast9@pitt.edu.
References
- 1.Drutz HP, Alarab M. Pelvic organ prolapse: demographics and future growth prospects. Int Urogynecol J Pelvic Floor Dys-funct. 2006;17(Suppl 7):S6–S9. doi: 10.1007/s00192-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188(1):108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Brown JS, et al. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;186(4):712–6. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 4.Feola AJ, et al. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am J Obstet Gynecol. 2010;203(6):595e1–8. doi: 10.1016/j.ajog.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chermansky C, Winters J. Complications of vaginal mesh surgery. Curr Opin Urol. 2012;22:287–291. doi: 10.1097/MOU.0b013e32835480b2. [DOI] [PubMed] [Google Scholar]
- 6.FDA . In: Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse. E. F.a.D. Administration, editor. 2011. [Google Scholar]
- 7.Haylen BT, et al. An International Urogynecological Association (IUGA)/Intemational Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogyn J. 2010;21(1):5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 8.Fenner DE. New surgical mesh. Clin Obstet Gynecol. 2000;43(3):650–8. doi: 10.1097/00003081-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 9.FDA . In: Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. E. F.a.D. Administration, editor. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cardozo L, Staskin D, editors. Textbook of female urology and urogynecology. 2nd Edition Vol. 1. Informa Healthcare; 2006. [Google Scholar]
- 11.Jones KA, et al. Tensile properties of commonly used prolapse meshes. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):847–53. doi: 10.1007/s00192-008-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deffieux X, et al. Vaginal mesh erosion after transvaginal repair of cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J Pelvic Floor Dys-funct. 2007;18(1):73–9. doi: 10.1007/s0192-005-0041-2. [DOI] [PubMed] [Google Scholar]
- 13.Ozog Y, et al. Persistence of polypropylene mesh anisotropy after implantation: an experimental study. BJOG. 2011;118(10):1180–5. doi: 10.1111/j.1471-0528.2011.03018.x. [DOI] [PubMed] [Google Scholar]
- 14.Cowin SC, Doty SB. Tissue mechanics. Springer; New York: 2007. [Google Scholar]
- 15.Dalstra M, et al. Mechanical and textural properties of pelvic trabecular bone. J Biomech. 1993;26(4-5):523–35. doi: 10.1016/0021-9290(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd JP, et al. Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Intl Urogyn J. 2012;23(5):613–20. doi: 10.1007/s00192-011-1616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause H, et al. Biomechanical properties of raw meshes used in pelvic floor reconstruction. Int Urogyn J. 2008;19(12):1677–1681. doi: 10.1007/s00192-008-0711-y. [DOI] [PubMed] [Google Scholar]
- 18.Afonso JS, et al. Structural and thermal properties of polypropylene mesh used in treatment of stress urinary incontinence. Acta Bioeng Biomech. 2009;11(3):27–33. [PubMed] [Google Scholar]