Abstract
Alzheimer’s disease (AD) is the most common cause of dementia in North America. Growing evidence supports the concept that AD is fundamentally a metabolic disease that results in progressive impairment in the brain’s capacity to utilize glucose and respond to insulin and insulin-like growth factor (IGF) stimulation. Moreover, the heterogeneous nature of AD is only partly explained by the brain’s propensity to accumulate aberrantly processed, mis-folded and aggregated oligomeric structural proteins, including amyloid-β peptides and hyperphosphorylated tau. Evidence suggests that other factors, including impaired energy metabolism, oxidative stress, neuroinflammation, insulin and IGF resistance, and insulin/IGF deficiency in the brain should be incorporated into an overarching hypothesis to develop more realistic diagnostic and therapeutic approaches to AD. In this review, the interrelationship between impaired insulin and IGF signalling and amyloid-β pathology is discussed along with potential therapeutic approaches. Impairments in brain insulin/IGF signalling lead to increased expression of amyloid-β precursor protein (AβPP) and accumulation of AβPP-Aβ. In addition, they promote oxidative stress and deficits in energy metabolism, leading to the activation of pro-AβPP-Aβ-mediated neurodegeneration cascades. Although brain insulin/IGF resistance and deficiency can be induced by primary or secondary disease processes, the soaring rates of peripheral insulin resistance associated with obesity, diabetes mellitus and metabolic syndrome quite likely play major roles in the current AD epidemic. Both clinical and experimental data have linked chronic hyperinsulinaemia to cognitive impairment and neurodegeneration with increased AβPP-Aβ accumulation/reduced clearance in the CNS. Correspondingly, both the restoration of insulin responsiveness and the use of insulin therapy can lead to improved cognitive performance, although with variable effects on brain AβPP-Aβ load. On the other hand, experimental evidence supports the concept that the toxic effects of AβPP-Aβ can promote insulin resistance. Together, these findings suggest that a positive feedback loop of progressive neurodegeneration can develop whereby insulin resistance drives AβPP-Aβ accumulation, and AβPP-Aβ fibril toxicity drives brain insulin resistance. This phenomenon could explain why measuring AβPP-Aβ levels in cerebrospinal fluid or imaging of the brain has proven to be inadequate as a stand-alone biomarker for diagnosing AD, and why the clinical trial results of anti-AβPP-Aβ monotherapy have been disappointing. Instead, the aggregate data suggest that brain insulin resistance and deficiency must also be therapeutically targeted to halt AD progression or reverse its natural course. The positive therapeutic effects of different treatments that address the role of brain insulin/IGF resistance and deficiency, including the use of intranasal insulin delivery, incretins and insulin sensitizer agents are discussed along with potential benefits of lifestyle changes to modify risk for developing mild cognitive impairment or AD. Altogether, the data strongly support the notion that we must shift toward the implementation of multimodal rather than unimodal diagnostic and therapeutic strategies for AD.
1. Alzheimer’s Disease (AD) Diagnosis
Alzheimer’s disease (AD) is the most common cause of dementia in North America and, over the past several decades, the prevalence rates of sporadic AD have sky-rocketed, even after correcting for increasing longevity.[1] In standard clinical practice, a diagnosis of AD is rendered based on the National Institute of Neurological and Communicative Disorders and Stroke, the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA), and Diagnostic and Statistical Manual of Mental Disorders (4th edition) criteria.[2] However, more recently, consideration has been given to the inclusion of additional studies including neuropsychological and other performance-based assessments, genetic factors, and biochemical and neuroimaging biomarkers, which may more accurately correspond to AD pathology.[3] Although the revisions in diagnostic criteria enable the incorporation of data from more sophisticated tests, diagnosing AD remains challenging, particularly in the hands of non-specialists, institutions that lack ready access to additional diagnostic aids, or patients who cannot afford to undergo an extensive battery of tests. In addition, the long intervals (often years) required to demonstrate that the relevant signs and symptoms are indeed progressive in nature, delay diagnosis and treatment. Although many of the current limitations in diagnosing AD will eventually be overcome through the use of neuroimaging and biomarker panels,[4] one of the critical rate-limiting steps involves the selection of biomarkers. Unless the panels are sufficiently broad and take into consideration the varied pathophysiological and molecular mechanisms of neurodegeneration, significant improvements in AD diagnostics, therapeutics, and our ability to assess clinical responses to early intervention will remain stymied.
A major goal in the field of AD research is to devise better non-invasive tools to accurately and reliably detect hallmark indices of neurodegeneration, including (i) loss of neurons; (ii) intra-neuronal accumulations of abnormal, hyperphosphorylated cytoskeletal proteins and dystrophic neurites; (iii) increased expression and abnormal processing of amyloid-β precursor protein (AβPP); and (iv)AβPP-Aβ peptide deposition in neurons, plaques and vessels. For the most part, biomarker assays of AD are focused on detecting AD-associated lesions that harbour or are caused by accumulations of insoluble aggregates of abnormally phosphorylated and ubiquitinated tau, and neurotoxic AβPP-Aβ in the form of oligomers, fibrillar aggregates or extracellular plaques. Secreted AβPP-Aβ oligomers contribute to neurodegeneration because they are neurotoxic and can inhibit long-term potentiation, i.e. synaptic plasticity.[5] Undoubtedly, steady progress has been made in the applications of neuroimaging and non-invasive biomarker assays to detect, quantify and localize AβPP-Aβ deposits, biochemical indices of neurodegeneration, and functional impairments, but many aspects of AD remain inaccessible to objective, reliable and quantifiable examinations. The very fact that the gold standard for diagnosing AD continues to be the post-mortem exam doggedly reminds us of our limitations regarding rational drug design and early therapeutic intervention. Fortunately, in recent years, the field has shifted its focus away from the overwhelmingly dominant hypotheses that hyperphosphorylation of tau and excessive deposition of neurotoxic AβPP-Aβ are fundamentally causal in the neurodegeneration cascade, and opened the doors to exciting new lines of investigation and therapeutic strategies.
2. Metabolic Dysregulation in AD
Growing evidence supports the concept that AD is a degenerative metabolic disease in which brain glucose uptake and utilization are impaired.[6–10] Dysregulated brain glucose metabolism is associated with brain insulin and insulin-like growth factor (IGF) resistance and reduced signalling through pathways that mediate neuronal survival, energy production, gene expression and plasticity.[6] Impairments in brain insulin/IGF signalling contribute to neurodegeneration due to increased (i) activation of kinases that aberrantly phosphorylate tau; (ii) expression of AβPP and accumulation of AβPP-Aβ; (iii) oxidative and endoplasmic reticulum (ER) stress; (iv) generation of reactive oxygen and reactive nitrogen species that damage proteins, RNA, DNA and lipids; (v) mitochondrial dysfunction; (vi) activation of pro-inflammatory and pro-death cascades; and (vii) downregulation of target genes that mediate cholinergic homeostasis. Together, these adverse effects of brain insulin/IGF resistance significantly compromise systems that promote neuronal plasticity, memory and cognition.
The early stages of AD are marked by deficits in cerebral glucose utilization[11–16] and, as the disease progresses, the metabolic and physiological abnormalities worsen.[17,18] These early stages of metabolic dysfunction are associated with brain insulin resistance and insulin deficiency, and significant abnormalities in insulin/IGF-regulated gene expression and kinase activation.[6–10] The impairments in cerebral glucose utilization, deficits in insulin signalling, and declines in insulin/IGF-responsive gene expression, including choline acetyltransferase, tau and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which respectively mediate cholinergic/cognitive, neuronal cytoskeletal and metabolic functions, worsen with each stage of AD.[9] Brain insulin resistance promotes oxidative stress, reactive oxygen species (ROS) generation, DNA damage and mitochondrial dysfunction, all of which drive pro-apoptosis, pro-inflammatory and pro-AβPP-Aβ cascades. Experimental animals in which brain insulin receptor expression and function are suppressed exhibit cognitive impairment and neurodegeneration with features in common with AD.[19–23]
In AD, deficits in brain insulin/IGF signalling are due to combined effects of insulin/IGF resistance and deficiency. Insulin/IGF resistance is manifested by reduced levels of insulin/IGF receptor binding and decreased responsiveness to insulin/IGF stimulation, while trophic factor deficiencies are associated with reduced levels of insulin polypeptide and gene expression in brain and cerebrospinal fluid (CSF).[8–10,24–26] It is particularly noteworthy that brain regions that have the most abundant expression of insulin and IGF receptors, e.g. hippocampus, temporal lobe, diencephalon, are also the most vulnerable targets of AD neurodegeneration.[27–29] Therefore, impairments in insulin/IGF signalling due to receptor resistance would logically account for many of the clinical manifestations of AD. In essence, AD can be regarded as a form of brain diabetes that has elements of both insulin resistance and insulin deficiency. To consolidate this concept, we proposed that AD be referred to as ‘type 3 diabetes’.[9,10]
Although debate surrounding the concepts of primary (endogenous) versus secondary (exogenous) brain insulin resistance in AD continues with regard to which process is more important in fuelling the current epidemic, it is clear that chronic peripheral insulin resistance and hyperinsulinaemia, with or without diabetes mellitus, is a major risk factor for subsequent development of AD.[30] Correspondingly, experimental diabetes with chronic hyperinsulinaemia is associated with brain insulin resistance and impaired insulin uptake from the periphery;[31,32] the latter could contribute to the brain insulin deficiency observed in AD.[9] In addition, hyperinsulinaemia increases AβPP-Aβ and inflammatory indices in the brain,[33] and it promotes formation of advanced glycation end-products, which lead to increased generation of ROS.[34] Together, these observations highlight the deleterious effects of chronic hyperinsulinaemia as a mediator of neurodegeneration. On the other hand, there is seemingly paradoxical evidence that treatment with insulin, particularly via intranasal delivery, can be therapeutic. For example, clinical trials have demonstrated improved cognition and memory following intranasal treatment of people with mild cognitive impairment (MCI).[35,36] In addition, intranasal insulin therapy was demonstrated to reduce biomarker indices of neurodegeneration,[37] and improve memory, prevent cognitive decline and reduce AD-associated CSF biomarker indices in individuals with MCI or early AD.[38]
3. Impaired Insulin/Insulin-Like Growth Factor (IGF) Signalling and Tau Pathology in AD
The most significant neuronal cytoskeletal lesions that correlate with AD/dementia severity, including neurofibrillary tangles and dystrophic neurites, contain aggregated and ubiquitinated insoluble fibrillar tau.[39,40] The pathophysiological basis of the tau-associated lesions is that tau, a microtubule-associated protein, gets hyperphosphorylated due to inappropriate activation of several proline-directed kinases, including glycogen synthase kinase 3β (GSK-3β). As a result, tau misfolds and self-aggregates into insoluble fibrillar structures (paired helical and straight filaments) that eventually form neurofibrillary tangles, dystrophic neurites or neuropil threads.[41] Intraneuronal accumulations of fibrillar tau disrupt the cytoskeletal network and axonal transport, leading to synaptic disconnection and progressive neurodegeneration.[41] Besides fibrillar tau, pre-fibrillar tau can aggregate, forming soluble oligomers or insoluble granules that cause synaptic disconnection and neuronal death.[42] The eventual ubiquitination of hyperphosphorylated tau,[43] combined with dysfunction of the ubiquitin-proteasome system,[44] cause further accumulation of insoluble fibrillar tau, oxidative stress and ROS production, which together promote neuronal apoptosis, mitochondrial dysfunction and necrosis in AD.[45]
Growing evidence suggests that many of the aforementioned cellular aspects of AD may be caused by brain insulin/IGF resistance,[9,10] which, as in other brain insulin-resistance states, results in inhibition of downstream pro-growth and pro-survival signalling.[46–49] Tau gene expression and phosphorylation are regulated by insulin and IGF stimulation.[50,51] In AD, brain insulin and IGF resistances result in decreased signalling through phosphoinositol-3-kinase (PI3K), Akt[50,51] and Wnt/β-catenin,[52] and increased activation of GSK-3β.[53–57] GSK-3β over-activation is partly responsible for the hyperphosphorylation of tau, which leads to tau misfolding and fibril aggregation.[58] In addition, tau hyperphosphorylation in AD is mediated by increased activation of cyclin-dependent kinase 5 (cdk-5) and c-Abl kinases,[59,60] and inhibition of protein phosphatases 1 and 2A.[41,60,61] Besides hyperphosphorylation, tau pathology in AD is mediated by impaired tau gene expression due to reduced insulin and IGF signalling.[62] Consequences include failure to generate sufficient quantities of normal soluble tau protein vis-a-vis accumulation of hyperphosphorylated insoluble fibrillar tau, and attendant exacerbation of cytoskeletal collapse, neurite retraction and synaptic disconnection.
4. Insulin/IGF Resistance and Amyloid-Beta (Aβ) Neurotoxicity
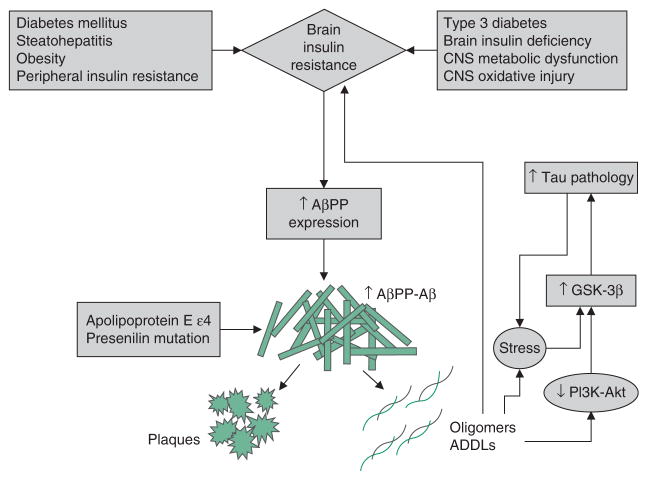
AD is associated with dysregulated expression and processing of AβPP, resulting in the accumulation of AβPP-Aβ oligomeric fibrils or insoluble larger aggregated fibrils (plaques) that are neurotoxic (figure 1). Pathophysiologically, increased AβPP gene expression, together with altered proteolysis, results in accumulation of 40 or 42 amino acid length AβPP-Aβ peptides that can self-aggregate. In familial forms of AD, mutations in the AβPP, presenilin (PS)-1 and PS2 genes, and inheritance of the apoliprotein E ε4 (ApoE-ε4) allele, are responsible for increased synthesis and deposition of AβPP-Aβ peptides in the brain. However, in sporadic AD, which accounts for 90% or more of the cases, the causes of AβPP-Aβ accumulation and toxicity are still under intense investigation. Over the past few years, interest in the role of impaired insulin/IGF signalling as either the cause or consequence of dysregulated AβPP processing and AβPP-Aβ protein accumulation has grown.
Fig. 1.
Brain insulin resistance and amyloid-β precursor protein (AβPP) Aβ-mediated neurotoxicity. Brain insulin resistance caused by peripheral insulin resistance diseases or intrinsic/genetic processes, toxic exposures or environmental factors contributing to neurodegeneration promote neuroinflammation and increased expression of AβPP. Through the action of β and γ secretases, AβPP is cleaved to generate 40–42 kD AβPP-Aβ peptides that aggregate and form insoluble fibrils and plaques, or oligomers and AβPP-Aβ-derived diffusible ligands (ADDLs), which are neurotoxic. AβPP-Aβ oligomers and ADDLs promote oxidative stress and activate kinases that lead to tau accumulation, hyperphosphorylation and eventual ubiquitination, misfolding and aggregation. AβPP-Aβ oligomers and ADDLs can block insulin-receptor function and contribute to insulin resistance. Carriers of the apoliprotein E ε4 allele or presenilin mutations are predisposed to abnormal AβPP cleavage, and AβPP-Aβ accumulation, aggregation and fibril formation, correlating with increased rates and familial occurrences of Alzheimer’s disease. This scenario depicts a positive feedback or reverberating loop linking AβPP-Aβ ADDL and oligomer accumulation/toxicity with brain insulin resistance, and vice versa. Reproduced from de la Monte,[63] with permission. GSK-3β = glycogen synthase kinase 3β; PI3K= phosphoinositol-3-kinase; ↓ indicates decrease; ↑ indicates increase.
The concept that AβPP-Aβ toxicity causes insulin resistance, and the opposing argument that brain insulin resistance with attendant oxidative stress and neuroinflammation promote AβPP-Aβ accumulation and toxicity are both supported by experimental data. For example, studies have established that insulin stimulation accelerates trafficking of AβPP-Aβ from the trans-Golgi network, where it is generated, to the plasma membrane, and that insulin stimulates AβPP-Aβ extracellular secretion[64] and inhibits its intracellular accumulation and degradation by insulin-degrading enzyme.[65,66] Although it remains uncertain as to whether these physiological actions of insulin on AβPP processing contribute to AβPP-Aβ burden, what is apparent is that impaired insulin signalling can disrupt both the processing of AβPP and clearance of AβPP-Aβ.[31] Accumulation of AβPP-Aβ exacerbates the problem because AβPP-Aβ disrupts insulin signalling by competing with insulin, or reducing the binding affinity of insulin for its own receptor.[67,68] In addition, AβPP-Aβ oligomers inhibit neuronal transmission of insulin-stimulated signals by desensitizing and reducing cell surface expression of insulin receptors. Furthermore, intracellular AβPP-Aβ directly interferes with PI3 kinase activation of Akt, which leads to impaired survival signalling, increased activation of GSK-3β and hyperphosphorylation of tau. Hyperphosphorylated tau is prone to misfold, aggregate and become ubiquitinated, leading to formation of dementia-associated paired-helical filament- containing neuronal cytoskeletal lesions. Since IGF-1 or IGF-2 suppression of GSK-3β[69] reduces the neurotoxic effects of AβPP,[70–73] the neuro-protective properties of these and related trophic factors could be exploited for therapeutic purposes.
5. Insulin/IGF Resistance, Oxidative Stress and Metabolic Dysfunction in AD
Insulin and IGF signalling pathways regulate glucose utilization, metabolism and adenosine triphosphate (ATP) synthesis needed for cellular homeostasis and dynamic modulation of a broad range of functions. Deficits in cerebral glucose utilization and energy metabolism occur very early in the course of AD, and are detectable prior to, or coincident with, initial stages of cognitive impairment.[26,74,75] Mitochondrial dysfunction exacerbates electron transport chain function, reducing ATP and increasing ROS production. Neuroinflammatory responses in microglia and astrocytes increase oxidative stress, organelle dysfunction and pro-apoptosis signalling. Moreover, stresses caused by inhibition of insulin/IGF signalling stimulate AβPP gene expression[76] and aberrant AβPP cleavage, with attendant increased AβPP-Aβ deposition and toxic fibril formation in the brain.[73,77–81] Persistent oxidative stress leads to constitutive activation of kinases, e.g. GSK-3β, that promote aberrant hyperphosphorylation of tau. Therefore, in AD, oxidative stress and impairments in energy metabolism stemming from brain insulin/IGF resistance quite likely contribute to neuronal loss, AβPP-Aβ toxicity, tau cytoskeletal pathology and neuroinflammation.[9,27,82] The degree to which these abnormalities can be effectively targeted for therapy in AD is actively under investigation.
6. Mechanisms of Brain Insulin/IGF Resistance in Neurodegeneration
Epidemiological data have demonstrated significant correlations between type 2 diabetes mellitus (T2DM), obesity and peripheral insulin resistance with MCI, dementia and neurodegeneration. Peripheral insulin resistance could promote cognitive impairment and neurodegeneration due to associated chronic hyperglycaemia, hyperinsulinaemia, oxidative stress, accumulation of advanced glycation end-products, increased expression and activation of insulin-degrading enzyme, increased production of pro-inflammatory cytokines, and cerebral microvascular disease.[83] A focus on vascular factors is justified because chronic hyperglycaemia, hyperinsulinaemia, oxidative stress, advanced glycation end-products and inflammation promote vascular disease. Moreover, the contributions of cerebral microvascular disease to AD progression have been recognized for years in that post-mortem studies showed similar degrees of dementia in subjects who had either severe AD neuropathology or moderate AD plus chronic ischaemic encephalopathy. The nature of ischaemic encephalopathy ranged from multifocal ischaemic lesions, to infarcts strategically localized in structures ordinarily targeted by AD, to leukoaraiosis with extensive attrition of white matter fibres.[84] Correspondingly, MRI studies showed that among older adults, the risks of developing lacunes and atrophy of medial temporal lobe structures (hippocampus and amygdala), i.e. targets of AD neurodegeneration, increased with duration and progression of T2DM.[85] Apart from diabetes-associated arteriosclerosis, hyperinsulinaemia, inflammation and oxidative stress, inheritance of the ApoE-ε4 allele can also contribute to cerebrovascular disease and increase the risk of AD progression. Although controversial, both insulin resistance and chronic hyperinsulinaemia can injure blood vessels, causing intimal thickening, scarring and leakiness.[86–91] Furthermore, the risk of developing AD among hyperinsulinaemic diabetic patients who carry at least one ApoE-ε4 allele is compounded relative to non-diabetic, ApoE4-ε4 negative individuals. Post-mortem studies demonstrated that the latter group has significantly lower densities of AβPP-Aβ plaques and neurofibrillary tangles than do ApoE-ε4-positive, hyperinsulinaemic, diabetic patients.
7. Strategies for Early Diagnosis and Evaluation of Treatment Responses
The combined use of clinical and post-mortem assessments provides the most accurate means of diagnosing AD. However, further advancements in detecting, monitoring and treating AD, particularly in its early stages, will require additional objective and standardized in vivo, non-invasive tools.[92] Although MRI of the brain can track progression of medial temporal lobe atrophy as MCI advances toward AD, and as AD worsens in severity,[93] the specificity of such single-pronged approaches is limited.[94] On the other hand, the combined use of brain structural and functional (flow plus metabolism) MRI (fMRI) could substantially improve diagnostic accuracy and the ability to monitor disease progression. For example, progressive cortical hypoperfusion and hypometabolism in AD are readily detected by single photon emission computed tomography (SPECT)[95,96] and positron-emission tomography (PET).[13,94,97–100] Furthermore, using fMRI to detect impairments in metabolism and blood flow in the posterior cingulate and parietal-temporal cortices would support an AD diagnosis.[101] However, neuroimaging will likely not stand alone as a diagnostic tool because, often, abnormal signals can overlap with other forms of neurodegeneration. The expectation is that the combined use of sensitive functional neuroimaging, structural imaging, neurobehavioral assessments and multi-modal biomarker panels, will be needed to significantly improve diagnostic accuracy and facilitate evaluation of treatment effects in the early stages of AD.[102–104]
8. Cerebrospinal Fluid and Peripheral Blood Diagnostic Biomarkers
Biomarkers for detecting and grading severity of AD have largely been focused on measuring AβPP-Aβ, tau and phospho-tau in CSF.[105,106] Changes in CSF levels of Aβ-42, total tau and phospho-tau[107] can be used to predict progression from MCI to dementia,[107] or aid in establishing a diagnosis of AD.[108] At least in some studies, the sensitivity and specificity of these CSF biomarkers approach 85%for diagnosing AD and distinguishing AD from MCI.[101,102,106,109–111] However, inter-laboratory variability and the lack of standardized measures to ensure quality assurance limit broad implementation of these assays.[112,113] Moreover, since protein misfolding and aggregation are at the core of the neurodegenerative process, biomarkers are needed to identify and quantify oligomeric neurotoxic aggregates of tau and Aβ-42.[114] Another matter of concern is that by confining our analyses to relatively few biomarkers, we effectively retard our own advancements in the field. Correspondingly, despite large-scale efforts, these somewhat restricted approaches have not proven sensitive enough to accurately diagnose AD or predict outcomes of MCI,[115] and certainly the use of a single biomarker such as AβPP-Aβ levels in CSF[116] or imaging of the brain[117] has proven to be inadequate as a stand-alone biomarker for diagnosing AD.
Given the spectrum of abnormalities that precede or accompany AD, it would seem prudent to utilize multimodal biomarker arrays for diagnosing and staging AD.[118] For example, indices of oxidative stress, neuroinflammation, mitochondrial dysfunction, metabolic derangements and impaired insulin/IGF signalling should be integrated into the overall equation to improve the sensitivity and specificity of diagnosis.[4,118,119] Multi-analyte profiling is particularly advantageous because it enables efficient capture of data and tracking of abnormalities as biomarker indices shift with disease progression.[120] For example, CSF pro-inflammatory cytokines, oxidative stress and redox-active iron levels are all elevated in the early stages of AD and MCI,[121,122] whereas in later stages of disease, oxidative stress and pro-inflammatory biomarkers, whether in plasma or CSF, seem to lack diagnostic utility.[123] Therefore, neuroinflammatory and oxidative stress responses should be measured to help gauge the presence and severity of neurodegeneration in the early stages. Alternatively, it could be argued that these factors may initiate or propagate early stages of the neurodegeneration cascade, and should instead be regarded as therapeutic targets. In addition, the persistently elevated CSF levels of oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxy-guaniosine suggest that mitochondrial and DNA oxidative damage mediate AD progression,[124,125] and therefore could be targeted therapeutically to slow the advancement of dementia.
Peripheral blood biomarkers in lymphocytes and plasma hold some promise as non-invasive screening tools, and may provide a means to study populations at increased risk for developing AD.[126] For example, abnormalities in AβPP-Aβ cleavage are detectable in peripheral blood lymphocytes in AD. In addition, protein kinase C (PKC), which has an important role in stimulating AβPP-Aβ peptide formation and tau hyperphosphorylation, could serve as a peripheral blood biomarker, since conformational changes in the PKC enzyme that promote AD pathology are detectable in erythrocytes.[127] Similarly, from the perspective that neuroinflammation promotes neurodegeneration, it may be possible to use elevated serum levels of acute phase proteins and pro-inflammatory cytokines to help gauge the likelihood of progression from MCI to dementia, particularly in the early stages of disease when neuroinflammation is likely to be a relevant biomarker.[128] Although the combined use of serum and CSF to measure AβPP-Aβ peptides, total tau and phosphorylated tau has been proposed for diagnosing and monitoring treatment responses,[129] this approach could be flawed because drug treatments may not produce detectable shifts in serum AβPP-Aβ or tau levels.[130] Again, these limitations highlight the importance of establishing multi-pronged diagnostic approaches that will include CSF and serum bioassays, together with functional and structural neuroimaging studies to diagnose AD, and predict progression from MCI to AD.[106]
In designing neurodegenerative disease biomarker panels, it should be possible to capitalize on the concept that AD is a metabolic disease with features that best correspond to a brain form of diabetes. CSF assays could be used to detect brain insulin resistance and insulin deficiency, while peripheral blood studies could be used to simultaneously assess peripheral insulin resistance status marked by reduced glucose tolerance, hyperglycaemia, hyperinsulinaemia, accumulation of advanced glycation endproducts, and ROS.[34] For example, AD is associated with significantly reduced CSF insulin levels, although in some cases, insulin levels were found to be elevated.[34,131] While the reduced CSF insulin levels most likely reflect insulin deficiency, elevated plasma and CSF insulin levels following a glucose challenge in AD subjects[12] suggest that insulin resistance is a feature of AD. These observations highlight the need to use dynamic functional/physiological tests rather than static assays. Understanding the time-course of progressive CNS insulin resistance and insulin deficiency in relation to AD severity and genotype, e.g. ApoE-ε4 allele,[132,133] could lead to better decisions about the timing and nature of therapeutic interventions, and possibly explain some of the discrepant results from different studies. Finally, post-mortem human brain and CSF studies indicate that AD is also associated with IGF-1 and IGF-2 resistance and deficiency,[134,135] highlighting the fact that function of the entire insulin/IGF family of genes is dysregulated in AD. This realization benefits how we consider therapeutic targets and monitor disease progression.
9. Managing Dementia Based on the Brain Insulin Resistance/Insulin Deficiency
The volume of literature supporting the concept that AD is associated with deficits in energy metabolism, glucose utilization and insulin/IGF responsiveness in the brain has grown rapidly, causing the paradigm of AD pathogenesis to shift away from the overwhelmingly dominant amyloid and tauopathy hypotheses. The attractiveness of the metabolic/brain insulin resistance hypothesis is that impairments in brain insulin and IGF signalling caused by insulin/IGF resistance, together with the eventual depletion of trophic factors, could account for nearly all other abnormalities that occur in AD, including increased oxidative stress and ROS generation, mitochondrial dysfunction, cell death, loss of synaptic plasticity, deficits in cholinergic homeostasis, increased expression of AβPP, hyperphosphorylation of tau, compromised myelin maintenance and neuroinflammation. Another attractive feature of the metabolic/brain insulin resistance hypothesis is that it demystifies the pathophysiology of AD by relating it to other well recognized systemic diseases, i.e. diabetes, non-alcoholic steatohepatitis and metabolic syndrome. If indeed these diseases only differ by the principal organs and tissues that they afflict, then the treatment and prevention approaches would likely overlap or possibly be identical.
Additional research is needed to determine the degree to which brain insulin resistance, cognitive impairment and neurodegeneration constitute an intrinsic brain form of diabetes, or reflect CNS components of peripheral insulin resistance diseases, including T2DM, obesity and metabolic syndrome. Epidemiological, clinical and postmortem studies have clearly shown that sporadic AD occurs primarily in the absence of obesity and T2DM. On the other hand, the risks of developing cognitive impairment are significantly higher in individuals with T2DM, obesity or metabolic syndrome relative to controls.[136–139] Moreover, in a recent study, investigators demonstrated that cognitive performance and AD biomarkers in CSF, including evidence of enhanced AβPP-Aβ clearance, improved significantly after a short period of dietary intervention that was designed to curb peripheral insulin resistance in overweight subjects.[140] Together, these observations support the concept that both primary and secondary mechanisms of brain insulin resistance and neurodegeneration exist; this phenomenon could very well account for the heterogeneity in the AD phenotype. Potential therapeutic strategies for addressing brain insulin resistance in relation to AD neurodegenertion are discussed in section 10.
10. AβPP-Aβ Accumulation and Production as Therapeutic Targets
Over the past 2 decades, AD research has been heavily focused on developing safe and effective means to deplete the brain of toxic AβPP-Aβ deposits, reduce AβPP-Aβ fibrillarization and aggregation, and prevent abnormal cleavage and processing of AβPP.[141] The overarching hypothesis is that AβPP-Aβ peptides are neurotoxic, promote amyloid plaque formation, and mediate tau hyperphosphorylation, fibrillarization and neurofibrillary tangle formation.[142] Efforts to deplete the brain of toxic AβPP-Aβ led to the development of AβPP-Aβ-targeted immunotherapy. Although AβPP-Aβ active immunization with AβPP-Aβ peptides, or passive delivery of AβPP-Aβ-specific antibodies can effectively clear AβPP-Aβ plaques from humans and experimental animal brains,[143] the net outcomes have not been very encouraging because (i) the AβPP-Aβ instead accumulates in vessels, increasing propensity for micro-haemorrhage;[144] (ii) the increased clearance of AβPP-Aβ has had modest or no detectable positive effects on cognitive function; and (iii) patients who received the vaccine still died with end-stage dementia, and extensive neurofibrillary tangle and neuritic pathology in their brains.[145,146] Although the therapeutic effects of increasing the clearance or preventing the formation and accumulation of toxic soluble oligomeric AβPP-Aβ on cognitive function and neurodegeneration have not yet been determined, the subtext in the discussion is that the single-pronged approach to treatment is not sufficient.
Insulin resistance can contribute to AβPP-Aβ toxicity because insulin accelerates trafficking of AβPP-Aβ from the trans-Golgi network to the plasma membrane, and promotes its extracellular secretion,[64] while impaired insulin signalling disrupts the processing of AβPP and clearance of AβPP-Aβ.[31] IGFs activate signalling pathways similar to those driven by insulin and, in addition to their neuroprotective properties, IGFs can reduce the neurotoxic effects of AβPP-Aβ.[70–73] On the other hand, the fact that AβPP-Aβ oligomers inhibit neuronal insulin-stimulated signals and block PI3K activation of Akt, which leads to impaired survival signalling, increased activation of GSK-3β and hyperphosphorylation of tau, supports the argument that AβPP-Aβ promotes insulin resistance and therefore should be targeted therapeutically to help restore brain insulin sensitivity. Therefore, it seems that by addressing the underlying causes of insulin/IGF resistance, we may be able to reduce AβPP-Aβ burden in the brain, while at the same time treat the other adverse effects of insulin/IGF resistance, e.g. impaired energy metabolism and cholinergic homeostasis. At the same time, evidence suggests that once the AβPP-Aβ cascade takes root, it promotes insulin resistance, and together with the factors that initially drive the brain insulin/IGF resistance, a reverberating loop of neurodegeneration gets established, necessitating therapeutic measures that halt AβPP-Aβ accumulation/toxicity and also enhance insulin/IGF sensitivity in the CNS.
10.1 Insulin Therapy
The proposed use of anti-diabetic, hypoglycaemic drugs to treat AD is based on the findings that (i) AD is associated with brain insulin resistance and insulin deficiency (reduced brain and CSF levels), with or without associated systemic insulin resistance or T2DM; (ii) diabetic patients who are well managed with insulin or hypoglycaemic medications exhibit significant improvements in memory and slowing of AD progression; (iii) treated elderly diabetic patients have lower densities of AD lesions than non-diabetic controls; (iv) insulin administration improves cognition and memory in AD, and insulin-stimulated cognition is correlated with increased levels of norepinephrine in both plasma and CSF;[147] (v) hyperinsulinaemic euglycaemic clamping enhances cognition and attention in patients with AD; and (vi) experimental intra-cerebral or intravenous treatments with insulin improve memory, cognition, evoked brain potentials and neurotransmitter function.[31] However, it is noteworthy that the effectiveness of insulin therapy may require increased availability of glucose, and may not be therapeutically effective in facilitating memory if CSF AβPP-Aβ42 levels are markedly elevated due to insulin resistance.[148]
The more recent trend toward intranasal insulin therapy for AD is advantageous because the treatment is safe and effective for increasing in brain insulin levels, and improving declarative memory,[149] attention and the CSF AβPP-Aβ40/42 ratio.[37] Reducing AβPP-Aβ42 is neuroprotective because AβPP-Aβ42 is the neurotoxic form of the secreted peptide. As exciting as this approach appears on the surface, its application is limited since, in a controlled clinical trial, only ApoE-ε4-negative individuals were demonstrated to benefit significantly from intranasal insulin, as manifested by improvements in cognitive performance.[149] The fact that ApoEε4-positive subjects did not benefit from the same treatment suggests that intranasal insulin, as well as other pro-metabolic therapies for AD, may have to be tailored according to genetic risk factors.
10.2 Insulin-Stimulating/-Releasing Hormones (Incretins)
As an alternative to insulin, another promising approach is therapeutic administration of incretins, such as glucagon-like peptide (GLP)-1. GLP-1 is an insulinotropic peptide that is generated by cleavage of proglucagon protein and secreted by small intestinal L cells following food intake. GLP-1 has a half-life of only a few minutes and is rapidly degraded by dipeptidyl peptidase (DPP)-4. GLP-1 stimulates insulin gene expression and secretion, and suppresses glucagon. GLP-1 lowers blood glucose in individuals with T2DM,[150,151] and it restores insulin sensitivity.
Like insulin, GLP-1 stimulates neuritic growth in CNS neurons and is neuroprotecive against glutamate-mediated excitotoxity, oxidative stress, trophic factor withdrawal and cell death.[152–154] In addition, inhibition of DPP-4, which degrades GLP-1, reduced oxidative and nitrosative stress, inflammation, memory impairment and AβPP-Aβ deposits in an AD transgenic mouse model.[155] Together, these results support the hypothesis that insulin resistance and deficiency play critical roles in the pathogenesis of AD, including AβPP-Aβ accumulation and neurotoxicity. Importantly, GLP-1 can cross the blood-brain barrier and may effectively reduce brain AβPP-Aβ burden in AD.[150,151,156] With the realization that GLP-1 has a short half-life and therefore limited practical use for long-term therapy, synthetic long-lasting analogues of GLP-1 have been generated and proven to be effective in preserving cholinergic neuron function.[157] For example, liraglutide, a novel GLP-1 receptor agonist used to achieve glycaemic control in individuals with T2DM, was demonstrated to promote long-term potentiation (synaptic plasticity) in hippocampal neurons.[158] Furthermore, in the APP/PS1 AD transgenic mouse model, liraglutide treatment prevented the impairments in memory, loss of synapses and deterioration of synaptic plasticity, it reduced AβPP-Aβ plaque and soluble oligomer burden and inflammation, and it increased neurogenesis in the hippocampal formation.[159] Therapeutic effects of synthetic GLP-1 receptor agonists on AβPP-Aβ burden and clearance have not yet been demonstrated in humans.
10.3 Anti-Hyperglycaemic Agents
Metformin is a biguanide anti-hyperglycaemic drug that is used to treat T2DM. Metformin suppresses gluconeogenesis and enhances glucose uptake and insulin sensitivity. Metformin protects against neurological complications of T2DM, including cognitive impairment and cerebral vascular disease.[160] Although metformin treatment may increase both intra- and extracellular AβPP-Aβ due to increased expression of β-secretase 1 (BACE1), when administered with insulin, metformin provides significant neuroprotection in that, AβPP-Aβ levels, including AβPP-Aβ neuritic plaques, and oligomeric AβPP-Aβ-mediated downregulation of the insulin receptor are reduced.[161] Therefore, metformin plus insulin may benefit patients in the early stages of AD by significantly improving cognitive performance and slowing the rate of neurodegeneration.
10.4 Insulin Sensitizers
Peroxisome proliferator-activated receptors (PPARs) are steroid hormone super family ligand-inducible transcription factors that enhance insulin sensitivity, modulate glucose and lipid metabolism, stimulate mitochondrial function and reduce inflammatory responses.[162–165] Three classes of PPARs are recognized, PPARα, PPARδ and PPARγ. All three are expressed in the adult brain, although PPARδ is most abundant, followed by PPARγ.[10,82,166] PPAR agonist treatments improve cognitive performance in experimental animal models,[82,167] and in humans with AD or MCI.[168–170] The PPARγ agonist, rosiglitazone, has been most widely studied in human clinical trials. In addition to its insulin sensitizing and anti-inflammatory properties, rosiglitazone, like metformin, increases expression of the glucose transporter type 4 (GLUT4) and glucose metabolism. Moreover, simultaneous treatment with PPAR agonists such as rosiglitazone enhances the therapeutic effects of metformin plus insulin.
In a small, double-blind, placebo-controlled trial, rosiglitazone treatment significantly preserved performance on delayed recall and attention tasks relative to placebo,[171] but later it was found that rosiglitazone mainly helped preserve cognition in subjects who were ApoE ε4-negative.[172] More recently, the results of a rosiglitazone monotherapy, randomized, double-blind, placebo-controlled, phase III study were reported as negative with respect to improvements in objective cognitive assessments, but highly statistically significant based on clinical and caregiver impressions.[173] Potential explanations for these non-encouraging results include (i) a different PPAR agonist isoform, i.e. PPARδ, may be needed to treat neurodegeneration since PPARδ is abundantly expressed in the brain, and previous studies showed that PPARδ agonists more effectively prevented neurocognitive deficits and AD-type neurodegeneration, including AβPP expression and AβPP-Aβ accumulation, compared with PPARα and PPARγ agonists;[82] and (ii) monotherapy may not be sufficient, and instead the combined administration of a PPAR agonist with insulin or GLP-1 and metformin may be needed to treat AD-associated brain insulin resistance and metabolic dysfunction. Although the data generated from rosiglitazone clinical trials were not as promising as anticipated based on the overarching hypothesis, we are reminded that therapeutic approaches for AD may have to be tailored to the CNS rather than simply borrowed from other treatment models, e.g. the use of PPARδ or hybrid PPAR-δ/γ/α drugs instead of PPARγ agonists. In addition, other strategies will have to be incorporated into the treatment paradigms, e.g. combined use of insulin sensitizers and GLP-1 receptor agonists.
11. Lifestyle Changes
Multi-institutional investigations on the roles of brain insulin resistance and deficiency as mediators of cognitive impairment and neurodegeneration led to hypothesis-driven treatment approaches that seem to hold promise for achieving significant therapeutic responses, while also demonstrating proof of principle. This convergence of ideas has added new excitement to the field, and opened Alzheimer’s research to a multidisciplinary community of researchers. But the concept of insulin resistance is not new to medicine, as there is a large literature concerning its prevention and treatment, particularly with regard to lifestyle measures. From a public health perspective, adjusting lifestyle would be the most logical and cost-effective means of ultimately reducing morbidity, disability and mortality from cognitive impairment and AD. To this end, previous studies have demonstrated that maintaining an active physical lifestyle can be protective against cognitive impairment in the elderly.[174] Similarly, in the APP-23 mouse model of AD, physical activity and environmental enrichment improved cognitive performance on learning and memory tasks, despite the high brain amyloid burdens.[175] Finally, recent studies demonstrated benefits in vigorous aerobic exercise for improving cognitive performance in individuals with MCI.[176]
12. Summary and Conclusions
This review relates metabolic abnormalities, principally insulin/IGF resistance and deficiency to AβPP-Aβ mediated toxicity and neurodegeneration in AD, and discusses why monodiagnostic and monotherapeutic strategies should be replaced by biomarker panels that detect and gauge severity of different stages of AD, and multi-modal therapy is needed to conquer different components of the cascade. While insulin and IGF resistance can directly account for the deficits in brain glucose utilization and energy metabolism that are detectable early in the course of AD, the consequences of impaired insulin/IGF signalling include aberrant activation of kinases that lead to tau hyperphosphorylation, increased oxidative stress, activation of proinflammatory cascades and ROS generation, all of which promote aberrant AβPP expression and cleavage, AβPP-Aβ42 accumulation due to reduced clearance, and fibrillarization and misfolding of both tau and AβPP-Aβ. Increased ROS production causes electrophilic attacks on proteins, lipids and nucleic acids, resulting in the formation of adducts that promote further structural and functional damage, oxidative stress, and ubiquitination of proteins, targeting them for degradation. Finally, brain insulin/IGF resistance can also explain the frequent co-existence of cerebral microvascular disease, which substantially contributes to the neuropathology of AD. The end-products of neurodegeneration, including hyperphosphorylated/ubiquitinated tau, and AβPP-Aβ fibrils themselves promote ROS, neuroinflammation and insulin resistance, and thereby help establish a reverberating loop of neurodegeneration. Whether the initiating factors are T2DM, as seems to be a growing problem throughout the world secondary to excessive calorically dense nutrient intake, or genetic factors such as ApoEe4 genotype or presenilin gene mutations, the constellation of molecular, biochemical and structural pathological abnormalities is similar. However, the very fact that multiple cellular functions become deranged in a progressive manner indicates that our treatments must be 100% preventative or multi-pronged and address the spectrum of pathological processes that contribute to AD neurodegeneration.
Acknowledgments
Research support was provided by the National Institutes of Health through grants AA11431, AA12908 and AA16260.
Footnotes
The author has no conflicts of interest that are directly relevant to the content of this review.
References
- 1.de la Monte SM, Neusner A, Chu J, et al. Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. doi: 10.3233/JAD-2009-1070. Epub 2009 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings JL. Definitions and diagnostic criteria. In: Gauthier S, editor. Clinical diagnosis and management of Alzheimer’s disease. 3. London: Informa UK Limited; 2007. [Google Scholar]
- 3.DeKosky ST, Carrillo MC, Phelps C, et al. Revision of the criteria for Alzheimer’s disease: a symposium. Alzheimers Dement. 2011;7 (1):e1–12. doi: 10.1016/j.jalz.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Gustaw-Rothenberg K, Lerner A, Bonda DJ, et al. Biomarkers in Alzheimer’s disease: past, present and future. Biomark Med. 2010;4 (1):15–26. doi: 10.2217/bmm.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416 (6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 6.Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105 (4–5):423–38. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J Neural Transm. 2002;109 (3):341–60. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490 (1–3):115–25. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Rivera EJ, Goldin A, Fulmer N, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8 (3):247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 10.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease: is this type 3 diabetes? J Alzheimers Dis. 2005;7 (1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 11.Adolfsson R, Bucht G, Lithner F, et al. Hypoglycemia in Alzheimer’s disease. Acta Med Scand. 1980;208 (5):387–8. doi: 10.1111/j.0954-6820.1980.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa Y, Sasaki K, Akiyama K. Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry. 1991;30 (12):1219–28. doi: 10.1016/0006-3223(91)90158-i. [DOI] [PubMed] [Google Scholar]
- 13.Caselli RJ, Chen K, Lee W, et al. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65 (9):1231–6. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 14.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–95. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36 (5):811–22. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langbaum JB, Chen K, Caselli RJ, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67 (4):462–8. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer S, Nitsch R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J Neural Transm. 1989;75 (3):227–32. doi: 10.1007/BF01258634. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3 (1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 19.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, et al. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101 (3):757–70. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer S, Lee SK, Loffler T, et al. Inhibition of the neuronal insulin receptor: an in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–8. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 21.Labak M, Foniok T, Kirk D, et al. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease. Acta Neurochir Suppl. 2010;106:177–81. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- 22.Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112 (5):1199–208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 23.Lester-Coll N, Rivera EJ, Soscia SJ, et al. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9 (1):13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 24.Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer’s disease. J Alzheimers Dis. 2002;4 (3):225–32. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- 25.Blum-Degen D, Frolich L, Hoyer S, et al. Altered regulation of brain glucose metabolism as a cause of neurodegenerative disorders? J Neural Transm Suppl. 1995;46:139–47. [PubMed] [Google Scholar]
- 26.Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–52. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- 27.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7 (1):45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 28.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Curr Alzheimer Res. 2009;6 (3):213–23. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- 29.Gammeltoft S, Fehlmann M, Van OE. Insulin receptors in the mammalian central nervous system: binding characteristics and subunit structure. Biochimie. 1985;67 (10–11):1147–53. doi: 10.1016/s0300-9084(85)80113-9. [DOI] [PubMed] [Google Scholar]
- 30.Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63 (7):1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 31.Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 2005;12 (4):311–28. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006;20 (4):298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 33.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4 (2):147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 34.Roriz-Filho SJ, Sa-Roriz TM, Rosset I, et al. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792 (5):432–43. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Reger MA, Watson GS, Frey WH, 2nd, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27 (3):451–8. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Hallschmid M, Benedict C, Born J, et al. Targeting metabolic and cognitive pathways of the CNS by intranasal insulin administration. Expert Opin Drug Deliv. 2007;4 (4):319–22. doi: 10.1517/17425247.4.4.319. [DOI] [PubMed] [Google Scholar]
- 37.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates {beta}-amyloid in early AD. Neurology. 2008;70 (6):440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 38.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. doi: 10.1001/archneurol.2011.233. Epub 2011 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118 (1):5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 40.Takashima A. Amyloid-beta, tau, and dementia. J Alzheimers Dis. 2009;17 (4):729–36. doi: 10.3233/JAD-2009-1090. [DOI] [PubMed] [Google Scholar]
- 41.Iqbal K, Liu F, Gong CX, et al. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118 (1):53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashima A. Drug development for tauopathy and Alzheimer’s disease [in Japanese] Nihon Shinkei Seishin Yakurigaku Zasshi. 2010;30 (4):177–80. [PubMed] [Google Scholar]
- 43.Arnaud L, Robakis NK, Figueiredo-Pereira ME. It may take inflammation, phosphorylation and ubiquitination to ‘tangle’ in Alzheimer’s disease. Neurodegener Dis. 2006;3 (6):313–9. doi: 10.1159/000095638. [DOI] [PubMed] [Google Scholar]
- 44.Oddo S. The ubiquitin-proteasome system in Alzheimer’s disease. J Cell Mol Med. 2008;12 (2):363–73. doi: 10.1111/j.1582-4934.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandelkow EM, Stamer K, Vogel R, et al. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24 (8):1079–85. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 46.de la Monte SM, Ganju N, Banerjee K, et al. Partial rescue of ethanol-induced neuronal apoptosis by growth factor activation of phosphoinositol-3-kinase. Alcohol Clin Exp Res. 2000;24 (5):716–26. [PubMed] [Google Scholar]
- 47.de la Monte SM, Neely TR, Cannon J, et al. Ethanol impairs insulin-stimulated mitochondrial function in cerebellar granule neurons. Cell Mol Life Sci. 2001;58 (12–13):1950–60. doi: 10.1007/PL00000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sci. 2002;59:882–93. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Eun Yeon J, Chang H, et al. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003;278 (29):26929–37. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- 50.Schubert M, Brazil DP, Burks DJ, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23 (18):7084–92. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubert M, Gautam D, Surjo D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101 (9):3100–5. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116 (Pt 7):1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Brain Res Rev. 2000;33 (1):1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 54.Fraser PE, Yu G, Levesque L, et al. Presenilin function: connections to Alzheimer’s disease and signal transduction. Biochem Soc Symp. 2001;67:89–100. doi: 10.1042/bss0670089. [DOI] [PubMed] [Google Scholar]
- 55.Grilli M, Ferrari Toninelli G, Uberti D, et al. Alzheimer’s disease linking neurodegeneration with neurodevelopment. Funct Neurol. 2003;18 (3):145–8. [PubMed] [Google Scholar]
- 56.Mudher A, Chapman S, Richardson J, et al. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci. 2001;21 (14):4987–95. doi: 10.1523/JNEUROSCI.21-14-04987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimura M, Yu G, Levesque G, et al. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nat Med. 1999;5 (2):164–9. doi: 10.1038/5526. [DOI] [PubMed] [Google Scholar]
- 58.Bhat R, Xue Y, Berg S, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278 (46):45937–45. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 59.Lebouvier T, Scales TM, Williamson R, et al. The microtubule-associated protein tau is also phosphorylated on tyrosine. J Alzheimers Dis. 2009;18 (1):1–9. doi: 10.3233/JAD-2009-1116. [DOI] [PubMed] [Google Scholar]
- 60.Morales I, Farias G, Maccioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17 (3):202–4. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- 61.Hanger DP, Seereeram A, Noble W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Expert Rev Neurother. 2009;9 (11):1647–66. doi: 10.1586/ern.09.104. [DOI] [PubMed] [Google Scholar]
- 62.de la Monte SM, Chen GJ, Rivera E, et al. Neuronal thread protein regulation and interaction with microtubule-associated proteins in SH-Sy5y neuronal cells. Cell Mol Life Sci. 2003;60 (12):2679–91. doi: 10.1007/s00018-003-3305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. doi: 10.2174/156720512799015037. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson GS, Peskind ER, Asthana S, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60 (12):1899–903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 65.Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21 (8):2561–70. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gasparini L, Netzer WJ, Greengard P, et al. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23 (6):288–93. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 67.Ling X, Martins RN, Racchi M, et al. Amyloid beta antagonizes insulin promoted secretion of the amyloid beta protein precursor. J Alzheimers Dis. 2002;4 (5):369–74. doi: 10.3233/jad-2002-4504. [DOI] [PubMed] [Google Scholar]
- 68.Xie L, Helmerhorst E, Taddei K, et al. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22(10):RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng WH, Kar S, Dore S, et al. Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J Neural Transm Suppl. 2000;(60):261–72. doi: 10.1007/978-3-7091-6301-6_17. [DOI] [PubMed] [Google Scholar]
- 70.Dore S, Bastianetto S, Kar S, et al. Protective and rescuing abilities of IGF-I and some putative free radical scavengers against beta-amyloid-inducing toxicity in neurons. Ann N Y Acad Sci. 1999;890:356–64. doi: 10.1111/j.1749-6632.1999.tb08015.x. [DOI] [PubMed] [Google Scholar]
- 71.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94 (9):4772–7. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Abeta amyloid peptides. Peptides. 2002;23 (7):1285–97. doi: 10.1016/s0196-9781(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 73.Tsukamoto E, Hashimoto Y, Kanekura K, et al. Characterization of the toxic mechanism triggered by Alzheimer’s amyloid-beta peptides via p75 neurotrophin receptor in neuronal hybrid cells. J Neurosci Res. 2003;73 (5):627–36. doi: 10.1002/jnr.10703. [DOI] [PubMed] [Google Scholar]
- 74.Iwangoff P, Armbruster R, Enz A, et al. Glycolytic enzymes from human autoptic brain cortex: normal aged and demented cases. Mech Ageing Dev. 1980;14 (1–2):203–9. doi: 10.1016/0047-6374(80)90120-7. [DOI] [PubMed] [Google Scholar]
- 75.Sims NR, Bowen DM, Smith CC, et al. Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer’s disease. Lancet. 1980;I(8164):333–6. doi: 10.1016/s0140-6736(80)90884-3. [DOI] [PubMed] [Google Scholar]
- 76.Chen GJ, Xu J, Lahousse SA, et al. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5 (3):209–28. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 77.Blasko I, Stampfer-Kountchev M, Robatscher P, et al. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3 (4):169–76. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 78.Eikelenboom P, van Gool WA. Neuroinflammatory perspectives on the two faces of Alzheimer’s disease. J Neural Transm. 2004;111 (3):281–94. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- 79.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37 (2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Lorenzo A, Yankner BA. Amyloid fibril toxicity in Alzheimer’s disease and diabetes. Ann N Y Acad Sci. 1996;777:89–95. doi: 10.1111/j.1749-6632.1996.tb34406.x. [DOI] [PubMed] [Google Scholar]
- 81.Niikura T, Hashimoto Y, Tajima H, et al. Death and survival of neuronal cells exposed to Alzheimer’s insults. J Neurosci Res. 2002;70 (3):380–91. doi: 10.1002/jnr.10354. [DOI] [PubMed] [Google Scholar]
- 82.de la Monte SM, Tong M, Lester-Coll N, et al. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10 (1):89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 83.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7 (5):373–80. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 84.Etiene D, Kraft J, Ganju N, et al. Cerebrovascular pathology contributes to the heterogeneity of Alzheimer’s disease. J Alzheimers Dis. 1998;1 (2):119–34. doi: 10.3233/jad-1998-1205. [DOI] [PubMed] [Google Scholar]
- 85.Korf ES, White LR, Scheltens P, et al. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care. 2006;29 (10):2268–74. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- 86.Huang K, Zou CC, Yang XZ, et al. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Arch Pediatr Adolesc Med. 164(9):846–51. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 87.Hotta O, Taguma Y, Chiba S, et al. Possible relationship between hyperinsulinemia and glomerular hypertrophy in nephrosclerosis. Ren Fail. 1996;18 (2):271–8. doi: 10.3109/08860229609052797. [DOI] [PubMed] [Google Scholar]
- 88.Haudenschild CC, Van Sickle W, Chobanian AV. Response of the aorta of the obese Zucker rat to injury. Arteriosclerosis. 1981;1 (3):186–91. doi: 10.1161/01.atv.1.3.186. [DOI] [PubMed] [Google Scholar]
- 89.Kubota T, Kubota N, Moroi M, et al. Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation. 2003;107 (24):3073–80. doi: 10.1161/01.CIR.0000070937.52035.25. [DOI] [PubMed] [Google Scholar]
- 90.Kincaid-Smith P. Hypothesis: obesity and the insulin resistance syndrome play a major role in end-stage renal failure attributed to hypertension and labelled ‘hypertensive nephrosclerosis’. J Hypertens. 2004;22 (6):1051–5. doi: 10.1097/00004872-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto H, Nakao T, Okada T, et al. Insulin resistance contributes to obesity-related proteinuria. Intern Med. 2005;44 (6):548–53. doi: 10.2169/internalmedicine.44.548. [DOI] [PubMed] [Google Scholar]
- 92.Allan CL, Sexton CE, Welchew D, et al. Imaging and biomarkers for Alzheimer’s disease. Maturitas. 2010;65 (2):138–42. doi: 10.1016/j.maturitas.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 93.Meyer JS, Huang J, Chowdhury M. MRI abnormalities associated with mild cognitive impairments of vascular (VMCI) versus neurodegenerative (NMCI) types prodromal for vascular and Alzheimer’s dementias. Curr Alzheimer Res. 2005;2 (5):579–85. doi: 10.2174/156720505774932241. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt SL, Correa PL, Tolentino JC, et al. Value of combining activated brain FDG-PET and cardiac MIBG for the differential diagnosis of dementia: differentiation of dementia with Lewy bodies and Alzheimer disease when the diagnoses based on clinical and neuroimaging criteria are difficult. Clin Nucl Med. 2008;33 (6):398–401. doi: 10.1097/RLU.0b013e3181708244. [DOI] [PubMed] [Google Scholar]
- 95.Lim SM, Katsifis A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50 (10):1638–45. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 96.O’Brien JT. Role of imaging techniques in the diagnosis of dementia. Br J Radiol. 2007;80(Spec no 2):S71–7. doi: 10.1259/bjr/33117326. [DOI] [PubMed] [Google Scholar]
- 97.Ibanez V, Deiber MP. Functional imaging in mild cognitive impairment and early Alzheimer’s disease: is it pertinent? Front Neurol Neurosci. 2009;24:30–8. doi: 10.1159/000197882. [DOI] [PubMed] [Google Scholar]
- 98.Finelli PF. Positron emission tomography in diagnosis of visual variant Alzheimer disease. J Neuroophthalmol. 2009;29 (2):149–50. doi: 10.1097/WNO.0b013e3181a57cf8. [DOI] [PubMed] [Google Scholar]
- 99.Morbelli S, Piccardo A, Villavecchia G, et al. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37 (1):36–45. doi: 10.1007/s00259-009-1218-6. [DOI] [PubMed] [Google Scholar]
- 100.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68 (7):501–8. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 101.Krolak-Salmon P. What use of biological markers for the diagnosis of Alzheimer’s disease and associated disorders? [in French] Psychol Neuropsychiatr Vieil. 2010;8 (1):25–31. doi: 10.1684/pnv.2009.0189. [DOI] [PubMed] [Google Scholar]
- 102.Pauwels EK, Volterrani D, Mariani G. Biomarkers for Alzheimer’s disease. Drug News Perspect. 2009;22 (3):151–60. doi: 10.1358/dnp.2009.22.3.1354128. [DOI] [PubMed] [Google Scholar]
- 103.Hampel H, Burger K, Teipel SJ, et al. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4 (1):38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 104.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461 (7266):916–22. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsubara E. Biological marker for Alzheimer’s disease [in Japanese] Brain Nerve. 2010;62 (7):769–75. [PubMed] [Google Scholar]
- 106.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer’s disease neuroimaging initiative subjects. Alzheimers Dement. 2010;6 (3):230–8. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18 (2):413–7. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 108.Roher AE, Maarouf CL, Sue LI, et al. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers. 2009;14 (7):493–501. doi: 10.3109/13547500903108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Monge-Argiles JA, Sanchez-Paya J, Munoz-Ruiz C, et al. Biomarkers in the cerebrospinal fluid of patients with mild cognitive impairment: a meta-analysis of their predictive capacity for the diagnosis of Alzheimer’s disease [in Spanish] Rev Neurol. 2010;50 (4):193–200. [PubMed] [Google Scholar]
- 110.van Rossum IA, Vos S, Handels R, et al. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J Alzheimers Dis. 2010;20 (3):881–91. doi: 10.3233/JAD-2010-091606. [DOI] [PubMed] [Google Scholar]
- 111.Ringman JM, Younkin SG, Pratico D, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71 (2):85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mattsson N, Blennow K, Zetterberg H. Inter-laboratory variation in cerebrospinal fluid biomarkers for Alzheimer’s disease: united we stand, divided we fall. Clin Chem Lab Med. 2010;48 (5):603–7. doi: 10.1515/CCLM.2010.131. [DOI] [PubMed] [Google Scholar]
- 113.Zhou B, Teramukai S, Yoshimura K, et al. Validity of cerebrospinal fluid biomarkers as endpoints in early-phase clinical trials for Alzheimer’s disease. J Alzheimers Dis. 2009;18 (1):89–102. doi: 10.3233/JAD-2009-1124. [DOI] [PubMed] [Google Scholar]
- 114.Carter MD, Simms GA, Weaver DF. The development of new therapeutics for Alzheimer’s disease. Clin Pharmacol Ther. 2010;88 (4):475–86. doi: 10.1038/clpt.2010.165. [DOI] [PubMed] [Google Scholar]
- 115.Forstl H, Werheid K, Ulm K, et al. MCI-plus: mild cognitive impairment with rapid progression. Part II: Biomarkers and research methods [in German] Dtsch Med Wochenschr. 2009;134 (3):88–91. doi: 10.1055/s-0028-1105896. [DOI] [PubMed] [Google Scholar]
- 116.Fagan AM, Shaw LM, Xiong C, et al. Comparison of analytical platforms for cerebrospinal fluid measures of {beta}-amyloid 1–42, total tau, and P-tau181 for Identifying Alzheimer Disease Amyloid Plaque Pathology. Arch Neurol. 2011;68 (9):1137–44. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimada H, Ataka S, Takeuchi J, et al. Pittsburgh Compound B-Negative Dementia: a possibility of misdiagnosis of patients with non-Alzheimer disease-type dementia as having AD. J Geriatric Psychiatry Neurol. 2011;24 (3):123–6. doi: 10.1177/0891988711409410. [DOI] [PubMed] [Google Scholar]
- 118.Mattsson N, Blennow K, Zetterberg H. CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann N Y Acad Sci. 2009;1180:28–35. doi: 10.1111/j.1749-6632.2009.04944.x. [DOI] [PubMed] [Google Scholar]
- 119.Hampel H, Broich K, Hoessler Y, et al. Biological markers for early detection and pharmacological treatment of Alzheimer’s disease. Dialogues Clin Neurosci. 2009;11 (2):141–57. doi: 10.31887/DCNS.2009.11.2/hhampel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu WT, Chen-Plotkin A, Arnold SE, et al. Biomarker discovery for Alzheimer’s disease, frontotemporal lobar degeneration, and Parkinson’s disease. Acta Neuropathol. 2010;120 (3):385–99. doi: 10.1007/s00401-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galimberti D, Fenoglio C, Scarpini E. Inflammation in neurodegenerative disorders: friend or foe? Curr Aging Sci. 2008;1 (1):30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 122.Lavados M, Guillon M, Mujica MC, et al. Mild cognitive impairment and Alzheimer patients display different levels of redox-active CSF iron. J Alzheimers Dis. 2008;13 (2):225–32. doi: 10.3233/jad-2008-13211. [DOI] [PubMed] [Google Scholar]
- 123.Korolainen MA, Pirttila T. Cerebrospinal fluid, serum and plasma protein oxidation in Alzheimer’s disease. Acta Neurol Scand. 2009;119 (1):32–8. doi: 10.1111/j.1600-0404.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 124.Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J Neurol. 2010;257 (3):399–404. doi: 10.1007/s00415-009-5333-x. [DOI] [PubMed] [Google Scholar]
- 125.Isobe C, Abe T, Terayama Y. Increase in the oxidized/total coenzyme Q-10 ratio in the cerebrospinal fluid of Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2009;28 (5):449–54. doi: 10.1159/000256209. [DOI] [PubMed] [Google Scholar]
- 126.Schneider P, Hampel H, Buerger K. Biological marker candidates of Alzheimer’s disease in blood, plasma, and serum. CNS Neurosci Ther. 2009;15 (4):358–74. doi: 10.1111/j.1755-5949.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Barry J, Liegeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45 (1):64–9. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 128.Eikelenboom P, van Exel E, Hoozemans JJ, et al. Neuroinflammation- an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010;7 (1–3):38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 129.Siemers E, DeMattos RB, May PC, et al. Role of biochemical Alzheimer’s disease biomarkers as end points in clinical trials. Biomark Med. 2010;4 (1):81–9. doi: 10.2217/bmm.09.85. [DOI] [PubMed] [Google Scholar]
- 130.Thompson PW, Lockhart A. Monitoring the amyloid beta-peptide in vivo: caveat emptor. Drug Discov Today. 2009;14 (5–6):241–51. doi: 10.1016/j.drudis.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 131.Neumann KF, Rojo L, Navarrete LP, et al. Insulin resistance and Alzheimer’s disease: molecular links and clinical implications. Curr Alzheimer Res. 2008;5 (5):438–47. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 132.Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50 (1):164–8. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 133.Molina JA, Jimenez-Jimenez FJ, Vargas C, et al. Cerebro-spinal fluid levels of insulin in patients with Alzheimer’s disease. Acta Neurol Scand. 2002;106 (6):347–50. doi: 10.1034/j.1600-0404.2002.01326.x. [DOI] [PubMed] [Google Scholar]
- 134.Tham A, Nordberg A, Grissom FE, et al. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect. 1993;5 (3):165–76. doi: 10.1007/BF02257671. [DOI] [PubMed] [Google Scholar]
- 135.Salehi Z, Mashayekhi F, Naji M. Insulin like growth factor-1 and insulin like growth factor binding proteins in the cerebrospinal fluid and serum from patients with Alzheimer’s disease. Biofactors. 2008;33 (2):99–106. doi: 10.1002/biof.5520330202. [DOI] [PubMed] [Google Scholar]
- 136.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64 (4):570–5. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 137.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20 (3):723–36. doi: 10.3233/JAD-2010-091687. [DOI] [PubMed] [Google Scholar]
- 138.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11 (2):111–28. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, et al. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4 (2):103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 140.Bayer-Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnesticmild cognitive impairment. Arch Neurol. 2011;68 (6):743–52. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hull M, Berger M, Heneka M. Disease-modifying therapies in Alzheimer’s disease: how far have we come? Drugs. 2006;66 (16):2075–93. doi: 10.2165/00003495-200666160-00004. [DOI] [PubMed] [Google Scholar]
- 142.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110 (4):1129–34. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 143.Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine. Prog Brain Res. 2009;175:83–93. doi: 10.1016/S0079-6123(09)17506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilcock DM, Colton CA. Immunotherapy, vascular pathology, and microhemorrhages in transgenic mice. CNS Neurol Disord Drug Targets. 2009;8 (1):50–64. doi: 10.2174/187152709787601858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vasilevko V, Head E. Immunotherapy in a natural model of Abeta pathogenesis: the aging beagle. CNS Neurol Disord Drug Targets. 2009;8 (2):98–113. doi: 10.2174/187152709787847333. [DOI] [PubMed] [Google Scholar]
- 146.Kerchner GA, Boxer AL. Bapineuzumab. Expert Opin Biol Ther. 2010;10 (7):1121–30. doi: 10.1517/14712598.2010.493872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Watson GS, Bernhardt T, Reger MA, et al. Insulin effects on CSF norepinephrine and cognition in Alzheimer’s disease. Neurobiol Aging. 2006;27 (1):38–41. doi: 10.1016/j.neurobiolaging.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 148.Galasko D. Insulin and Alzheimer’s disease: an amyloid connection. Neurology. 2003;60 (12):1886–7. doi: 10.1212/wnl.60.12.1886. [DOI] [PubMed] [Google Scholar]
- 149.Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13 (3):323–31. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer’s disease. Curr Alzheimer Res. 2005;2 (3):377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- 151.Li L. Is Glucagon-like peptide-1, an agent treating diabetes, a new hope for Alzheimer’s disease? Neurosci Bull. 2007;23 (1):58–65. doi: 10.1007/s12264-007-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu J, Yin F, Zheng X, et al. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51 (6–7):361–9. doi: 10.1016/j.neuint.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 153.Biswas SC, Buteau J, Greene LA. Glucagon-like peptide-1 (GLP-1) diminishes neuronal degeneration and death caused by NGF deprivation by suppressing Bim induction. Neurochem Res. 2008;33 (9):1845–51. doi: 10.1007/s11064-008-9646-4. [DOI] [PubMed] [Google Scholar]
- 154.Liu JH, Yin F, Guo LX, et al. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol Sin. 2009;30 (2):159–65. doi: 10.1038/aps.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.D’Amico M, Di Filippo C, Marfella R, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol. 2010;45 (3):202–7. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease: recent patents on CNS drug discovery. 2010;5 (2):109–17. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- 157.Perry T, Haughey NJ, Mattson MP, et al. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302 (3):881–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 158.McClean PL, Gault VA, Harriott P, et al. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer’s disease. Eur J Pharmacol. 2010;630 (1–3):158–62. doi: 10.1016/j.ejphar.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 159.McClean PL, Parthsarathy V, Faivre E, et al. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31 (17):6587–94. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Correia S, Carvalho C, Santos MS, et al. Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem. 2008;8 (13):1343–54. doi: 10.2174/138955708786369546. [DOI] [PubMed] [Google Scholar]
- 161.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106 (10):3907–12. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug News Perspect. 2010;23 (4):241–56. doi: 10.1358/dnp.2010.23.4.1437710. [DOI] [PubMed] [Google Scholar]
- 163.Strum JC, Shehee R, Virley D, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11 (1):45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 164.Hanyu H, Sato T. Alzheimer’s disease [in Japanese] Nippon Rinsho. 2010;68 (2):330–4. [PubMed] [Google Scholar]
- 165.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112 (12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771 (8):1031–45. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 167.Pedersen WA, McMillan PJ, Kulstad JJ, et al. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199 (2):265–73. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 168.Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2 (3):159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 169.Landreth G. PPARgamma agonists as new therapeutic agents for the treatment of Alzheimer’s disease. Exp Neurol. 2006;199 (2):245–8. doi: 10.1016/j.expneurol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 170.Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer’s disease. Curr Alzheimer Res. 2007;4 (2):159–64. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- 171.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13 (11):950–8. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 172.Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6 (4):246–54. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 173.Gold M, Alderton C, Zvartau-Hind M, et al. Rosiglitazone monotherapy in mild-to-moderate alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30 (2):131–46. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nash DT, Fillit H. Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol. 2006;97 (8):1262–5. doi: 10.1016/j.amjcard.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 175.Wolf SA, Kronenberg G, Lehmann K, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60 (12):1314–23. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 176.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67 (1):71–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]