Abstract
β-estradiol is the most potent estrogen of a group of endogenous estrogen steroids which includes estrone and estriol. This steroid hormone is the most potent natural estrogen, produced mainly by the ovary, placenta, and in smaller amounts by the adrenal cortex, and the male testes. Although β-estradiol protects the renal and cardiovascular systems, the mechanisms involved remain unclear. In this research, we performed the MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to evaluate the effect of β-estradiol on human T-lymphoma (Jurkat) cells upon 24 and 48 hours, respectively. Lipid peroxidation assay was also performed to estimate the levels of malondialdehyde (MDA) production in β-estradiol-treated cells. The results of MTT assay demonstrated that low, physiological levels of β-estradiol induce cellular proliferation in Jurkat T-cells. At higher dose of exposure, β-estradiol decreases the viability of Jurkat T-cells compared to the control cells. Data generated from lipid peroxidation assay resulted in a significant increase (p < 0.05) in MDA production in β-estradiol treated sample. Upon 48 h of exposure, MDA concentrations in the sample [µM] (mean ±SE, n = 3) compared to untreated control were 4.9 ± 1.7, 8.1 ± 1,6 11.5 ± 2.2, 21.1 ± 2.3, 19.5 ± 1.4, and 21.5 ± 2.6 in 0, 1, 2, 4, 8, and 16 µM β-estradiol, respectively. In summary, findings from this study demonstrated that high dose of β-estradiol is cytotoxic to Jurkat T-cells. This cytotoxicity is found to be associated with oxidative stress.
Keywords: β-estradiol, MTT assay, Jurkat T-cells, Lipid peroxidation assay
1. INTRODUCTION
Estradiol is a form of estrogen, a female sex hormone produced by the ovaries. Estrogen is necessary for many processes in the body. In vitro studies indicated that the addition of estradiol to cultures of human lymphocytes decreases in CD4+/CD8+ T-cell-subset ratios (Athreya et al., 1993), and enhances immunoglobulin secretion (Kanda and Tamaki, 1999). Studies indicated that estrogens can affect cells of the immune system and may play a role in modulating lymphocyte development and function (Smithson et al., 1998, Grimaldi et al., 2002). Published reports suggest that estrogens may have important actions at other steps in the atherogenic process (Nathan and Chaudhuri, 1997). However, the basis mechanisms by which β-estradiol-induced cytotoxic effects on cancer cells are not yet completely understood. In the present study, we use the human Jurkat T-lymphoma cell line as a test model to evaluate whether β-estradiol-induced cytotoxicity is associated with oxidative stress.
2. MATERIALS AND METHODS
2.1. Chemicals and Test Media
Growth medium RPMI 1640 containing 1 mmol/L L-glutamine was purchased from Gibco BRL products (Grand Island, NY). Fetal bovine serum (FBS), phosphate buffered saline (PBS) were obtained from Sigma Chemical Company (St. Louis, MO).
2.2. Tissue Culture
Human T-lymphoma (Jurkat) cells were from American Type Culture Collection (Manassas, VA, USA). In the laboratory, cells were stored in the liquid nitrogen until use. They were next thawed by gentle agitation of their containers (vials) for 2 minutes in a water bath at 37°C. After thawing, the content of each vial of cell was transferred to a 25 cm2 tissue culture flask, diluted with up to 10 mL of RPMI 1640 containing 1 mmol/L L-glutamine (GIBCO/BRL, Gaithersburg, MD) and supplemented with 10% (v/v) fetal bovine serum (FBS), and 1% (w/v) penicillin/streptomycin. The 25 cm2 culture flasks, each containing 2 × 106 viable cells, were observed under the microscope, followed by incubation in a humidified 5 % CO2 incubator at 37°C. Three times a week, they were diluted under same conditions to maintain a density of 5 × 105/mL, and harvested in the exponential phase of growth.
2.3. Cell treatment and biochemical test for cell viability
The in vitro cytotoxicity of β-estradiol against Jurkat T-cells was determined by MTT assay as previously described (Yedjou and Tchounwou, 2007). Briefly, to 180 µL aliquots in six replicates of the cell suspension (5 × 105 cells/mL) seeded to 96-well polystyrene tissue culture plates, 20 µL aliquots of stock solutions were added to each well using distilled water as solvent to make-up final β-estradiol concentrations of 1, 2, 4, 8, and 16 µM. Control cells received 20 µL of distilled water. Cells were placed in a humidified 5% CO2 incubator for 12 hr at 37°C. After incubation, 20 µL aliquots of MTT solution (5 mg/mL in PBS) were added to each well and re-incubated for 4 h at 37°C following by low centrifugation at 800 rpm for 5 min. Then, the 200 µL of supernatant culture medium were carefully aspirated and 200 µL aliquots of dimethylsulfoxide (DMSO) were added to each well to dissolve the formazan crystals, followed by incubation for 10 min to dissolve air bubbles. The culture plate was placed on a Biotek micro-plate reader and the absorbance was measured at 550 nm. All assays were performed in six replicates for each β-estradiol dose. Statistical analysis was done to determine the means ± SDs of cell viability. Cell viability rate was calculated as the percentage of MTT absorption as follows: % survival = (mean experimental absorbance/mean control absorbance×100).
2.4. Biochemical test for oxidative stress by lipid peroxidation assay
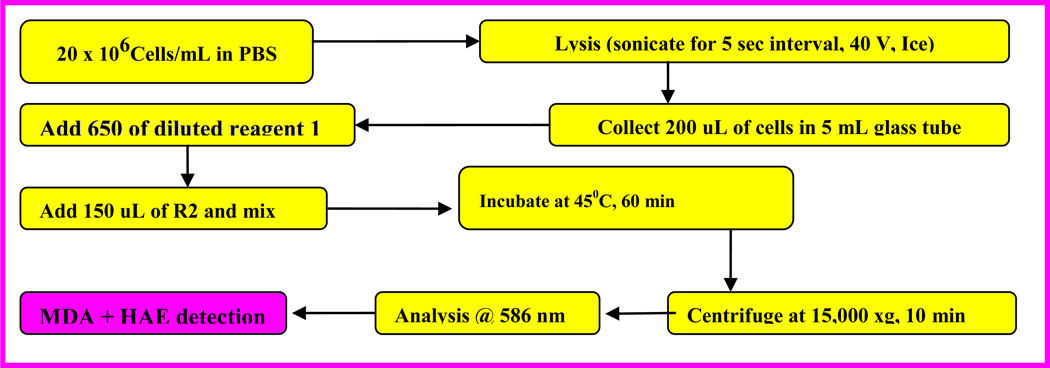
Lipid peroxidation is traditionally quantified to measure malondialdehyde (MDA) and 4-hydroxynonenal. The extraction procedure and measurement of the extracted MDA was performed according to the manufacturer's instructions (Calbiochem-Novabiochem, San Diego, CA). Malondialdehydes (MDA) are formed during lipid peroxidation (Halliwell and Gutteridge 1984). Briefly, 2 × 106 Jurkat T-cells/mL untreated as a control and cells treated with β-estradiol were incubated in a total volume of 10 ml growth medium for 48 hours. After the incubation period, cells were collected in 15 mL tubes, followed by low-speed centrifugation. The cell pellets were re-suspended in 0.5 ml of Tris-HCl, pH 7.4, and lysed using a sonicator (W-220; Ultrasonic, Farmingdale, NY) under the conditions of duty cycle-25% and output control-40% for 5 sec on ice. A 200 µl aliquot of the culture medium was assayed for MDA according to the lipid peroxidation assay kit protocol. The absorbance of the sample was read at 586 nm, and the concentration of MDA was determined from a standard curve.
2.5. Statistical analysis
Experiments were performed at least in triplicates. Data were represented as means ± SD. Where appropriate, one-way ANOVA test or paired t-test was performed using SAS Software available in the Bio-statistics Core Laboratory at Jackson State University.
3. RESULTS
3.1. β-estradiol Inhibits Cellular viability of Jurkat T-lymphoma cells
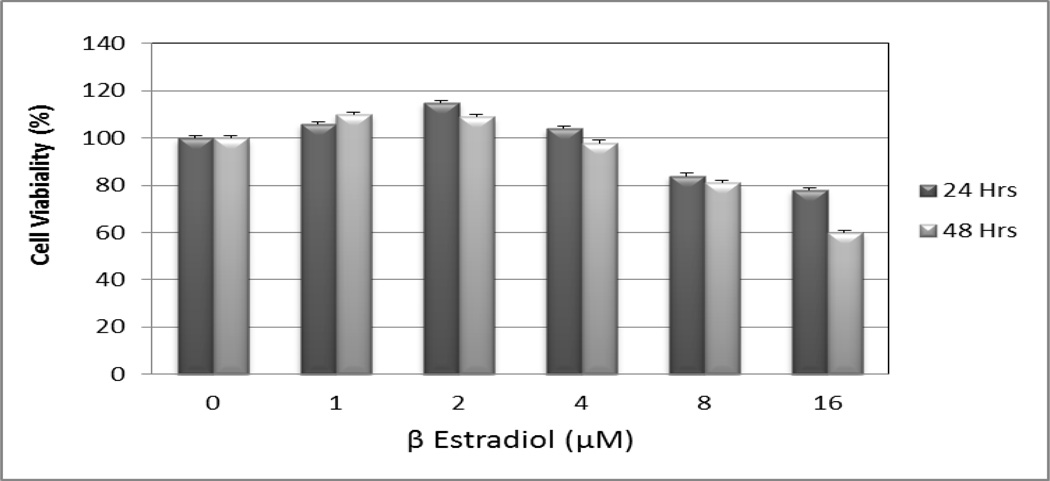
We used the MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to examine the cytotoxic effect of β-estradiol on Jurkat T-cells for 24 and 48 hours, respectively (Figure 2). Data generated from these studies clearly showed that β-estradiol exposure significantly reduces the viability of these cell lines. After 24 and 48 hours of exposure, β-estradiol exerted a significant cytotoxic effect on Jurkat T-cells in dose- and time-dependent, showing a response relationship with increasing doses of β-estradiol. We observed a biphasic response where physiological (low) doses of β-estradiol induce cell growth and cellular proliferation of Jurkat T-cells whereas higher doses inhibit cell growth and induce cell death.
Figure 2.
β-estradiol potently induces toxicity to human T-lymphoma (Jurkat) cells. Jurkat T-cells were treated for 24 and 48 hours with medium supplemented with solvent and various doses (1–16 µM) of β-estradiol.
3.2. β-estradiol Induces Oxidative Stress in Jurkat T-lymphoma cells
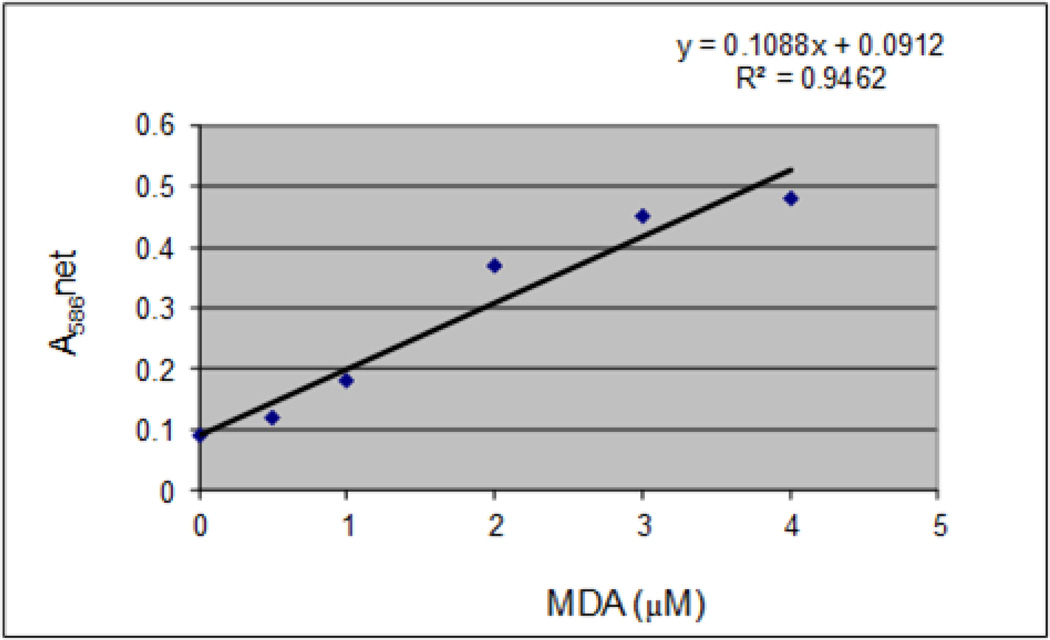
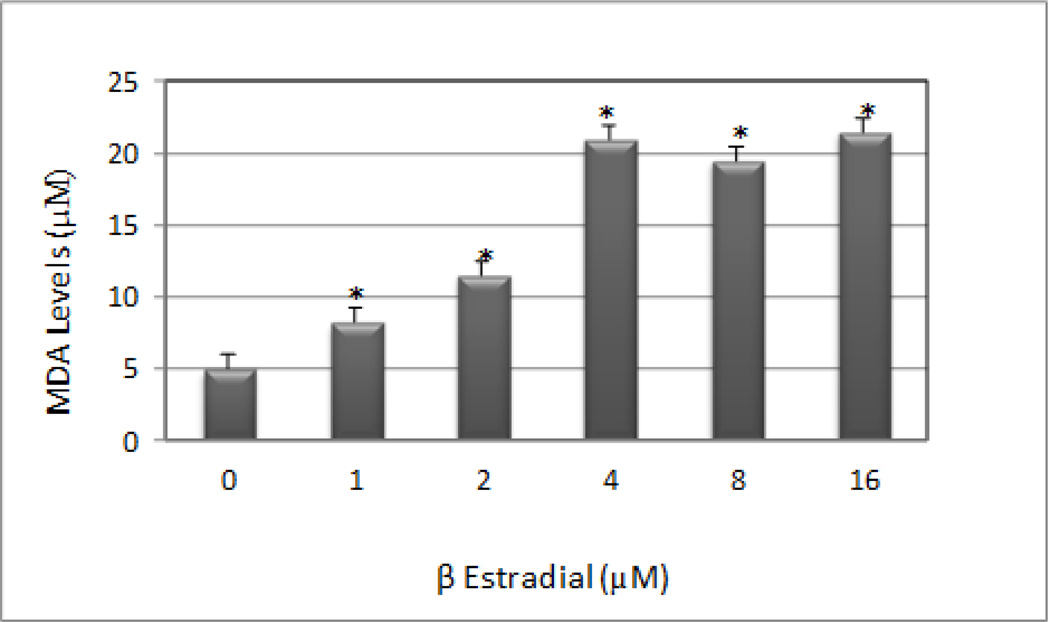
The standard curve generated from lipid peroxidation assay is presented in Figure 3, and the effect of β-estradiol on malondialdehyde (MDA) production in Jurkat T-cells is presented in Figure 4. MDA is an end product of lipid peroxidation which is a key determinant of cellular injury. As seen on figure 4, there is a significant increase in MDA level at all doses tested as result of oxidative stress, a biomarker of cellular injury. A maximum level (21.5 µM) of malondialdehyde (MDA) production was observed at the highest dose tested compared to (4.95 µM) in the control. This increase demonstrates that β-estradiol augments MDA production in Jurkat T-cells.
Figure 3.
MDA standard curve showing the net absorbance at 586 nm as a function of MDA concentration.
Figure 4.
β-estradiol-induced oxidative stress in human T-lymphoma (Jurkat) cells. Cells were incubated for 48 hours with increasing doses of β-estradiol (1, 2, 4, 8, and 16 µM). Malondialdehyde (MDA) concentrations were determined as described in Materials and Methods. *Significantly different from the control by ANOVA Dunnett's test; P < 0.05. Data are representative of 3 independent experiments.
4. DISCUSSIONS
Natural estrogens such as estradiol, estrone, and estriol are produced from natural sources such as the urine of pregnant mares and women, the adrenals and testes of stallions, and the human placenta and amniotic fluid (Roberts, 1986). To prove the cytotoxic efficacy of β-estradiol in Jurkat T-cell model, cell viability was determined by the MTT assay. As shown in Figure 2, β-estradiol treatment increased the percentage of dead cells in a dose- and time-dependent manner. Consistent with our results, in vitro studies indicated that the addition of estradiol to cultures of human lymphocytes causes a decrease in CD4+/CD8+ T-cell-subset ratios (Athreya et al., 1993), and enhances immunoglobulin secretion (Kanda and Tamaki, 1999). Several scientific reports indicated that estrogen toxicity resulted in the death of the patient (Suess et al., 1992; Sanpera et al., 2002) or in a long recovery period (Hall, 1992; Acke et al., 2003).
To test the hypothesis that oxidative stress may be involved in β-estradiol induced anti-tumor activity in Jurkat T-cells, we performed lipid peroxidation assay. Our result obtained from this assay showed a slight significant (p < 0.05) increase in malondialdehydes (a by-product of lipid peroxidation and biomarker of oxidative stress) production in β-estradiol-treated cells compared to the control. Similarly, a recent study in our laboratory indicated that garlic compounds generate MDA production in HL-60 cells leading to phosphatidylserine externalization, caspase-3 activation, nucleosomal DNA fragmentation, and cell death (Yedjou and Tchounwou, 2012). High level of MDA production detected in the Jurkat T-cells may be indicative of the therapeutic mechanisms of action of β-estradiol in human T-lymphoma (Jurkat) cells.
5. CONCLUSIONS
Results from this study indicate at the cellular level that β-estradiol significantly reduced the viability of human T-lymphoma (Jurkat) cells in a dose and time-dependent manner. At the molecular level, β-estradiol treatment resulted in a significant increase in lipid peroxidation generation in Jurkat T-cells. Findings from this study indicate that β-estradiol is highly cytotoxic to Jurkat T-cells. This cytotoxicity is found to be mediated through oxidative stress, a biomarker of cellular injury.
Figure 1.
Schematic representation of the steps in Lipid Peroxidation assay
ACKNOWLEDGEMENTS
This work was supported by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476. We also acknowledged the National Institutes of Health (NIH-NIMHD Grant No. G12MD07581) through the RCMI Center for Environmental Health at Jackson State University (Jackson, Mississippi, USA).
REFERENCES
- Acke E, Money CT, Jones BR. Estrogen toxicity in a dog. Ir Vet J. 2003;56:465–468. [Google Scholar]
- Athreya BH, Pletcher J, Zulian F, Weiner DB, Williams WV. Subset-specific effects of sex hormones and pituitary gonadotropins on human lymphocyte proliferation in vitro. Clinical Immunology and Immunopathology. 1993;66:201–211. doi: 10.1006/clin.1993.1026. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. Journal of Clinical Investigation. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ. Use of lithium for treatment of estrogen-induced bone marrow hypoplasia in a dog. J Am Vet Med Assoc. 1992;200:814–816. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan L, Chaudhuri G. 1997 Estrogen and atherosclerosis. Annual Review of Pharmacology and Toxicology. 1997;37:477–515. doi: 10.1146/annurev.pharmtox.37.1.477. [DOI] [PubMed] [Google Scholar]
- Roberts SJ. The estrogens. In: Roberts SJ, editor. Veterinary Obstetrics and Genital Diseases. Woodstock, Vermont: Roberts, SJ; 1986. pp. 403–405. [Google Scholar]
- Sanpera N, Masot N, Janer M, Romeo C, De Pedro R. Estrogen-induced bone marrow aplasia in a dog with a sertoli cell tumour. J Small Anim Pract. 2002;43:365–369. doi: 10.1111/j.1748-5827.2002.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. Journal of Immunology. 1998;161:27–34. [PubMed] [Google Scholar]
- Suess RP, Barr SC, Sacre BJ, French TW. Bone marrow hypoplasia in a feminized dog with interstitial cell tumor. J Am Vet Med Assoc. 1992;200:1346–1348. [PubMed] [PubMed] [Google Scholar]
- Yedjou CG, Tchounwou In vitro assessment of oxidative stress and apoptotic mechanisms of garlic extract in the treatment of acute promyelocytic leukemia. Journal of Cancer Science and Therapy. 2012;10(4):1948–1956. doi: 10.4172/1948-5956.S3-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedjou CG, Tchounwou PB. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoreis (comet) assays. Mol Cell Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]