Abstract
Background
Streptozotocin (STZ) is a nitrosamine-related compound that causes Alzheimer disease (AD)-type neurodegeneration with cognitive impairment, brain insulin resistance, and brain insulin deficiency. Nitrosamines and STZ mediate their adverse effects by causing DNA damage, oxidative stress, lipid peroxidation, pro-inflammatory cytokine activation, and cell death, all of which occur in AD.
Objective
We tested the hypothesis that exposure to N-nitrosodiethylamine (NDEA), which is widely present in processed/preserved foods, causes AD-type molecular and biochemical abnormalities in central nervous system (CNS) neurons.
Results
NDEA treatment of cultured post-mitotic rat CNS neurons (48 h) produced dose-dependent impairments in ATP production and mitochondrial function, and increased levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), 4-hydroxy-2-nonenal (HNE), phospho-Tau, amyloid precursor protein-amyloid beta (AβPP-Aβ), and ubiquitin immunoreactivity. These effects were associated with decreased expression of the insulin, IGF-I, and IGF-II receptors, and choline acetyltransferase.
Conclusions
Nitrosamine exposure causes neurodegeneration with a number of molecular and biochemical features of AD including impairments in energy metabolism, insulin/IGF signaling mechanisms, and acetylcholine homeostasis, together with increased levels of oxidative stress, DNA damage and AβPP-Aβ immunoreactivity. These results suggest that environmental exposures and food contaminants may play critical roles in the pathogenesis of sporadic AD.
Key Phrases: Alzheimer disease, diabetes mellitus, nitrosamine, environmental toxin, neurodegeneration
INTRODUCTION
Nitrosamines and N-nitroso compounds are potent, broad acting carcinogens. Nitrosamine-mediated injury and mutagenesis is heavily influenced by route of administration, dose, chemical nature of the compound, and frequency of exposure. Nitrosamines (R1N(-R2)-N=O) are formed by chemical reactions between nitrites and secondary amines or proteins. Sodium nitrite is added to meat and fish to prevent toxin production by Clostridium botulinum, and also to color, preserve, and flavor meat. Heating, acidification, or oxidation of nitrite leads to nitrous acid formation, and the resulting nitrosonium cation (N=O+) reacts with dimethylamine to generate nitrosamines. Ground beef, cured meats, and bacon in particular, contain abundant amines due to their high protein content, and they also have high levels of nitrates and nitrites [1]. Other important sources of nitrosamine exposure include, cheese products, fish byproducts, nonfat dry milk, tobacco, and water [2]. Moreover, nitrosamines are easily generated under strong acid conditions, as exist in the stomach, or with high temperatures associated with frying or flame broiling [3].
Nitrosamines exert their toxic and mutagenic effects by alkylating N-7 of guanine, leading to destabilization and increased breakage of DNA [4–9]. Activated nitrosamines also generate reactive oxygen species such as superoxide (O2−) and hydrogen peroxide (H2O2), and thereby increase oxidative stress, DNA damage, lipid peroxidation, and protein adduct formation [10–13]. Oxidative stress and DNA damage activate pro-inflammatory cytokines and promote insulin resistance, both of which are key elements in the pathogenesis of Alzheimer’s Disease (AD), and experimental models of AD-type neurodegeneration [14–23]. Although nitrosamine-related research has been largely focused on mutagenesis, the cellular and molecular alterations caused by nitrosamine exposures are fundamentally similar to those associated with aging and Alzheimer’s Disease (AD) [14, 16].
An important clue regarding the probable connection between nitrosamine exposure and AD was provided by experimental data demonstrating that treatment with Streptozotocin [2-deoxy-2-(3-methyl-3-nitrosoureido-D-glucopyranose (C8H15N3O7)] (STZ), a glucosamine-nitrosourea compound and derivative of N-methyl-N-nitrosourea (MNU), caused AD-type neurodegeneration with cognitive impairment [15, 17, 24, 25]. Structurally, STZ is quite similar to nitrosamines. Like other N-nitroso compounds, including N-nitrosodiethylamine (NDEA) and N-Nitrosodimethylamine (NDMA) [7], STZ’s MNU causes cellular injury and disease by functioning as: 1) an alkylating agent and potent mutagen resulting in cancer development in various organs [26]; 2) an inducer of DNA adducts, most significantly N7-methylguanine, which lead to increased apoptosis [27]; 3) a mediator of unscheduled DNA synthesis that triggers cell death [26]; 4) an inducer of single-strand DNA breaks; 5) a stimulus for nitric oxide (NO) formation following breakdown of its nitrosamine group [25]; and 6) an enhancer of the xanthine oxidase system leading to increased production of superoxide anion, H2O2, and OH− radicals [28]. In essence, STZ-induced cellular injury is mediated by the generation of reactive oxygen species with attendant increased levels of superoxide, nitric oxide, and lipid peroxidation, all of which cause DNA damage. Radical ion accumulation leads to inhibition of oxidative metabolism, mitochondrial dysfunction [28], decreased ATP production [29], activation of poly-ADP ribosylation, and finally cell death. The present study was designed to test the hypothesis that brief and relatively low-level exposures to NDEA, a nitrosamine present in processed food, can cause neurodegeneration similar to the effects of STZ, and that is associated with sporadic AD.
MATERIALS AND METHODS
Source of Reagents
QuantiTect SYBR Green PCR Mix was obtained from (Qiagen Inc, Valencia, CA). MitoTracker, JC-1, and H33342 fluorescent dyes were purchased from Molecular Probes (Eugene, OR). ATPLite reagents were purchased from PerkinElmer (Boston, MA). Antibodies to ubiquitin, tau, phospho-tau, 4-hydroxy-2-nonenal (HNE), choline acetyltransferase (ChAT), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and β-actin were purchased from Chemicon (Tecumsula, CA), CalBiochem (Carlsbad, CA), or Molecular Probes (Eugene, OR). All other fine chemicals and antibodies were purchased from either CalBiochem (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO).
In Vitro Characterization of NDEA-Mediated Neurodegeneration
We examined the effects of NDEA exposure on viability, mitochondrial mass, mitochondrial function, ATP content, and oxidative stress in post-mitotic cerebellar granule neuron cultures that were generated from postnatal day 8 (P8) Long Evans rat pups as previously described [30, 31]. Five-day-old 96-well micro-cultures were treated with 15–250 μg/ml of NDEA or vehicle for 48 hours. We then measured neuronal viability, mitochondrial mass, mitochondrial function, and ATP content. Viability was assessed with the CyQuant assay (Molecular Probes, Eugene, OR). ATP content was measured with the ATPLite assay kit, and luminescence was measured in TopCount machine (Packard Instrument Co., Meriden, CT). For mitochondrial mass and function, cells were labeled respectively, with MitoTracker Green FM and MitoTracker Red mitochondria-specific cell permeable fluorescent dyes, and fluorescence light units (FLU; Ex560/Em590) were measured with a Spectramax M5 microplate reader (Molecular Dynamics, Inc. Sunnyvale, CA) [32, 33]. Subsequently, the cells were stained with Hoechst 33342 (H33342) and fluorescence intensity was again measured (Ex 360 nm/Em 460 nm) with a Spectramax M5 to assess cell density [32, 33]. Results from 16–24 replicate cultures are expressed as mean ± S.E.M. of the MitoTracker/H33342 ratios. Mitochondrial permeability, an index of mitochondrial dysfunction, was measured using the JC-1 assay [34–36]. Fluorescence was measured at Ex485/Em530 to detect J-monomers and at Ex560/Em590 to detect J-aggregates. Results are expressed as JC1 aggregate-to-monomer ratios [32, 36]. Relative increases in J-monomers correlate with increased mitochondrial membrane permeability.
Cellular enzyme-linked immunosorbant assay (ELISA)
To examine effects of NDEA on proteins relevant to AD, 96-well cultures were treated for 48 hours with 15–250 μg/ml of NDEA. The cells were fixed over night in 4% buffered paraformaldehyde solution, and immunoreactivity was quantified in situ using a cellular ELISA [37]. Briefly, fixed cells were permeabilized with 0.05% Tween 20 in Tris-buffered saline, pH 7.5, then treated with 0.3% H2O2 in 60% methanol to quench endogenous peroxidase, and blocked with SuperBlock-TBS (Pierce Chemical Company, Rockford, IL) to adsorb non-specific binding sites. Cells were incubated over night at 4°C with 0.5–1 μg/ml primary antibody. Immunoreactivity was detected with horseradish peroxidase conjugated Amplex Red soluble fluorophore (Molecular Probes, Eugene, OR), and fluorescence intensity (Ex 530/Em 590) was measured with a Spectramax M5 microplate reader. To assess cell density, cells were then labeled with H33342 and fluorescence intensity (Ex360 nm/Em460 nm) was measured in the M5 Spectromax. Immunoreactivity was normalized to H33342 fluorescence. At least 8 replicate cultures were analyzed in each experiment. All experiments were repeated 2 or 3 times.
Quantitataive Reverse Transcriptase Polymerase Chain Reaction (QRT-PCR) Assays of Gene Expression
To examine effects of NDEA on genes pertinent to insulin and insulin-like growth factor (IGF) signaling and AD, the cells were treated for 48 hours with 30 μg/ml NDEA, and mRNA expression was measured by qRT-PCR analysis as previously described [17]. Briefly, total RNA isolated from cultured cells was reverse transcribed, and the resulting cDNA templates were used in PCR amplification reactions with gene specific primer pairs. The primer sequences have already been published [17]. Amplified signals were detected and analyzed in triplicate using the Mastercycler ep realplex instrument and software (Eppendorf AG, Hamburg, Germany). Relative mRNA abundance was calculated from the ratios of specific mRNA to 18S measured in the same samples, and those data were used for inter-group statistical comparisons. Control studies included template-free reactions, and reactions in which RNA or genomic DNA was used instead of cDNA.
Statistical Analysis
Graphs depict the means ± S.E.M.’s for each group, with 8–10 samples included per group. Inter-group comparisons were made with Student t-tests using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA). We used ANOVA to examine NDEA dose-dependent effects on viability, mitochondrial function, oxidative stress, and immunoreactivity, and the Deming (Model II) linear regression analysis to determine if the trends (regression lines) were significantly different from zero. Significant P-values are indicated within the graph panels.
RESULTS
NDEA Impairs Mitochondrial Function and Increases Oxidative Stress and DNA Damage
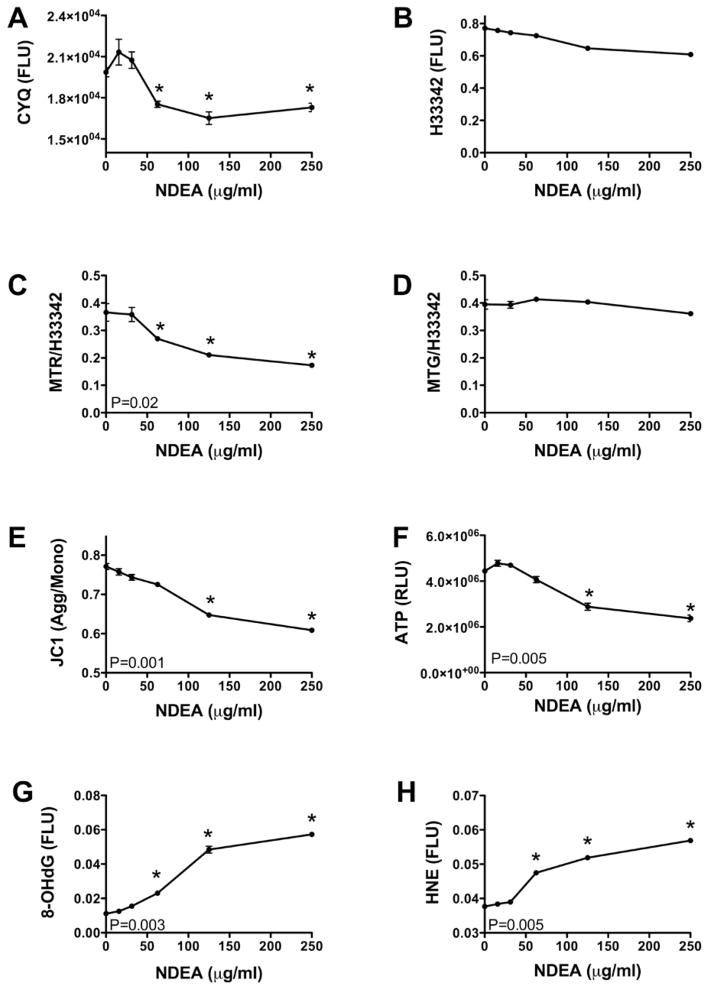
Primary cerebellar neuron cultures treated for 48 h with NDEA in the range of 15 to 250 μg/ml, were used to measure viability, ATP content, mitochondrial mass, and mitochondrial function. The CyQuant assay demonstrated that mean cell density was reduced when neurons were treated with greater than 50 μg/ml of NDEA (Figure 1A). In contrast, H33342 fluorescence, which correlates with cell density, was not significantly changed as a function of NDEA dose (Figure 1B). NDEA exposure caused dose-dependent reductions in mitochondrial function/oxidative phosphorylation, as demonstrated with the MitoTracker Red fluorescence assay (Figure 1C), reduction in mitochondrial potential, as shown by the progressive decline in JC1 aggregate/monomer ratio (Figure 1E), and energy depletion with loss of ATP content, as demonstrated with the ATP-Lite luminescence assay (Figure 1F). In contrast, mitochondrial mass, assessed by MitoTracker Green fluorescence, was not significantly altered by the NDEA treatments (Figure 1D).
Figure 1.
NDEA treatment impairs neurons mitochondrial function and causes oxidative stress and DNA damage. Post-mitotic rat cerebellar granule neurons were treated with 0–250 μg/ml NDEA for 48 h. The cultures were analyzed for: (A) viability using the CyQuant assay; (B) cell density by measuring Hoechst H33342 fluorescence; (C) mitochondrial function and (D) mitochondrial mass using the MitoTracker Red and Green assays, respectively; (E) mitochondrial membrane permeability using the JC-1 assay; (F) ATP content by the ATP-Lite luminescence assay; and (G) DNA damage and (H) oxidative stress/lipid peroxidation, by measuring 8-OHdG and HNE immunoreactivity separately. All assays except ATP-Lite were fluorescence-based, and quantified with a M-5 Spectromax microplate reader (FLU=fluorescence light units). MitoTracker fluorescence and 8-OHdG and HNE immunoreactivity were normalized against H33342 fluorescence. ATP luminescence was measured in a TopCount machine (RLU=relative light units). Each data point reflects the mean ± S.E.M. for 16 replicate cultures. P-values correspond to levels of significance for dose-effect increasing or decreasing linear trends. Asterisks indicate values that significantly differ from control by ANOVA (P<0.05 or better).
To examine the consequences of impaired mitochondrial function and ATP production, we assessed oxidative stress and DNA damage by measuring 4-hydroxy-2-nonenal (HNE) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) immunoreactivities by cellular ELISA. HNE is a highly toxic lipid-derived aldehyde that is generated by lipid peroxidation. HNE covalently modifies and cross-links cytoskeletal proteins and forms adducts with proteins [38, 39]. 8-OHdG is an abnormal nucleoside that becomes progressively incorporated into damaged DNA, leading to destabilization and increased breakage of DNA [40, 41]. Increased levels of both HNE and 8-OHdG have been detected in AD [42, 43] and in brains treated by intracerebral injection of STZ to generate an experimental model of AD [17]. NDEA treatment caused dose-dependent increases in the levels of both 8-OHdG and HNE in CNS neurons (Figures 1G–1H).
NDEA-Impaired Insulin/IGF Signaling Mechanisms
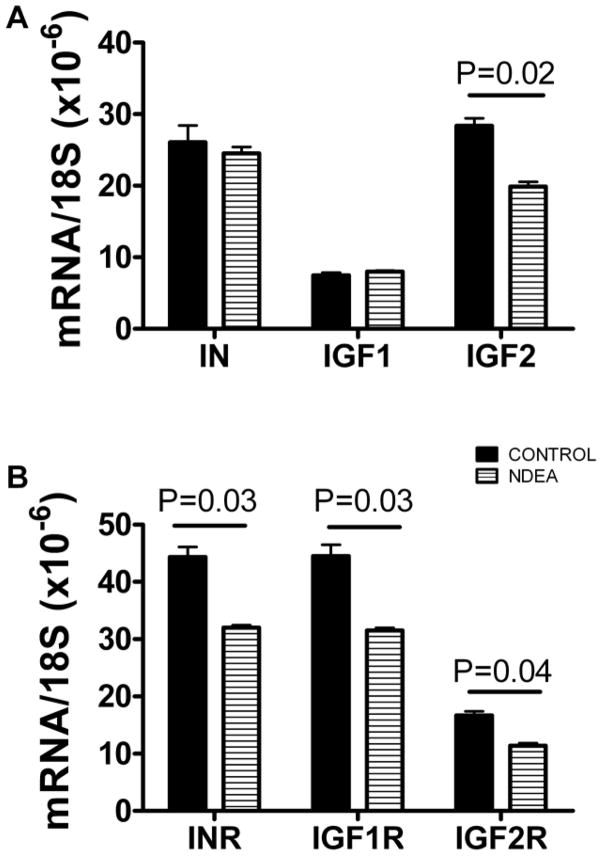
We examined the effects of NDEA treatment on the expression of genes that are required for insulin and insulin-like growth factor (IGF) signaling in CNS neurons, by measuring mRNA levels of insulin, IGF-I, and IGF-II polypeptides, and insulin, IGF-I, and IGF-II receptors using qRT-PCR analysis. For the qRT-PCR analyses, cultures were treated with 50 μg/ml NDEA for 48 h. The mRNA levels were normalized to 18S rRNA, which was measured simultaneously in parallel reactions. NDEA-treated and control cells had similar expression levels of insulin and IGF-I. In contrast, NDEA-treated neurons had significantly reduced mean levels of IGF-II polypeptide, and insulin, IGF-I, and IGF-II receptors (Figure 2). Therefore, NDEA treatment could cause insulin and IGF resistance, and IGF-II deficiency in CNS neurons.
Figure 2.
NDEA exposure inhibits expression of genes required for insulin and IGF signaling. Post-mitotic rat cerebellar granule neurons were treated with 50 μg/ml NDEA for 48 h. RNA was reverse transcribed and cDNA templates were used in qRT-PCR amplification assays with gene-specific primer pairs to detect (A) insulin, (B) IGF-I, and (C) IGF-II polypeptide, and (D) insulin, (E) IGF-I, and (F) IGF-II receptor genes. Results were normalized to 18S rRNA measured in parallel reactions. Graphs depict the mean ± S.E.M. corresponding to 6 replicate cultures. Inter-group statistical comparisons were made using Student T-tests and significant P-values are indicated over the bars.
Effects of NDEA on AD-Associated Genes
We used qRT-PCR analysis to examine the effects of NDEA treatment (50 μg/ml for 48 h) on the mRNA levels of amyloid precursor protein (AβPP), Tau, choline acetyltransferase (ChAT), and acetylcholinesterase (AChE) (Table 1), because these genes are highly relevant to the AD neurodegeneration cascade. Similar mean levels of AβPP, Tau, and 18S RNA were measured in vehicle-and NDEA-treated neuronal cells. In contrast, NDEA treatment significantly reduced the mean levels of ChAT. Although the mean levels of AChE were also lower in the NDEAtreated cultures, the inter-group difference was not statistically significant due to the large standard errors.
Table 1.
Effects of NDEA Treatment on AD-Associated Genes
| Gene* | Control | NDEA | P-Value |
|---|---|---|---|
| Tau (×10−6) | 6.03 ± 0.72 | 6.20 ± 0.74 | NS |
| AβPP(×10−6) | 2.64 ± 0.32 | 2.29 ± 0.28 | NS |
| AChE (×10−6) | 4.14 ± 0.74 | 2.65 ± 0.26 | NS |
| ChAT (×10−6) | 9.88 ± 0.98 | 6.83 ± 0.34 | 0.02 |
| 18S rRNA | 0.95 ± 0.04 | 0.98 ± 0.02 | NS |
Tau, amyloid precursor protein (AβPP), acetylcholinesterase (AChE), and choline acetyltransferase (ChAT) mRNA levels were normalized to 18S rRNA (ng input) and values shown represent mean ± S.E.M. calculated from 5 replicate samples. Inter-group statistical comparisons were made using Student T-tests. NS=not significant.
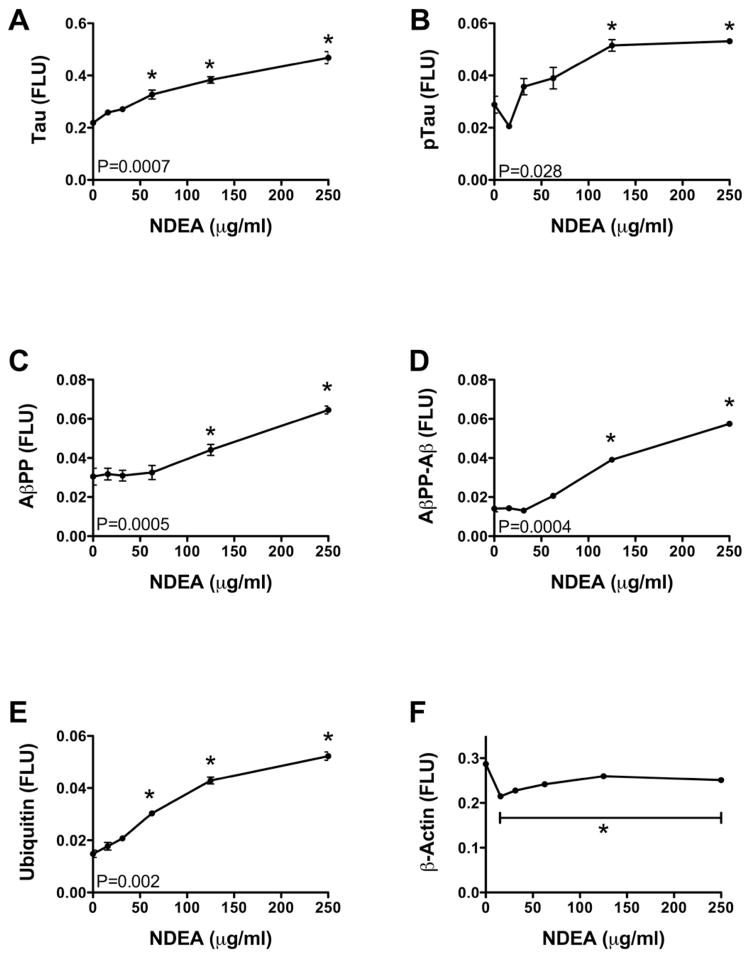
We further examined the effects of NDEA treatment on the levels of AD-associated proteins. We measured Tau, phospho-Tau (p-Tau), AβPP, AβPP-amyloid-β (AβPP-Aβ), ubiquitin, and β-actin immunoreactivity by cellular ELISA after 48-hours treatment with 15–250 μg/ml NDEA. Tau (Figure 3A), p-Tau (Figure 3B), and ubiquitin (Figure 3E) immunoreactivity increased progressively in a dose-dependent manner. AβPP(Figure 3C) and AβPP-Aβ (Figure 3D) levels remained unchanged relative to control following treatment with less than 50 μg/ml NDEA, but at higher concentrations, the levels of each of the proteins increased significantly. β-actin immunoreactivity was sharply reduced by 48-hours treatment with the lowest dose of NDEA used, but the levels did not decline further with increasing NDEA concentration (Figure 3F).
Figure 3.
NDEA treatment causes AD-type molecular abnormalities. Post-mitotic rat cerebellar granule neurons were treated with 0–250 μg/ml NDEA for 48 h. We used cellular ELISAs to measure immunoreactivity corresponding to: (A) Tau; (B) phospho-Tau (p-Tau); (C) amyloid beta precursor protein (AβPP); (D) AβPP-Aβ; (E) ubiquitin; (F) and β-actin. Immunoreactivity was detected with the horseradish peroxidase conjugated Amplex Red soluble fluorophore and quantified in an M-5 Spectromax microplate reader (FLU=fluorescence light units) with results normalized to H33342 fluorescence. Each data point reflects the mean ± S.E.M. for 16 replicate cultures. P-values correspond to levels of significance for dose-effect increasing or decreasing linear trends. Asterisks indicate values that significantly differ from control by ANOVA (P<0.05 or better).
DISCUSSION
Recent studies have linked the molecular and biochemical pathology of AD to impairments in insulin and IGF signaling mechanisms in the brain [14, 16, 44, 45]. Because of the similarities between AD and diabetes mellitus, experimental models were generated to test the hypothesis that AD represents a brain-specific form of diabetes, i.e. Type 3 diabetes [14, 16, 45]. Intracerebral injection with Streptozotocin (STZ), which is a glucosamine-nitrosourea compound and derivative of N-methyl-N-nitrosourea (MNU) and routinely used to produce models of diabetes mellitus, was found to cause AD-type neurodegeneration with cognitive impairment in rats [17]. Those results prompted us to question whether related compounds in the environment could mediate similar neurodegenerative effects. We considered the potential role of nitrosamines, including NDEA and NDMA, because of their structural similarity to STZ [7], 2) the fact that STZ, like NDEA and NDMA, causes cellular injury and disease by functioning as an alkylating agent, mutagen, and inducer of DNA adducts, single-strand DNA breakage, and nitric oxide, superoxide anion, H2O2, and OH− radical formation [25, 28]. Although NDMA and NDEA are aqueous soluble, they are found in lipid fractions, are distributed in adipose tissue in vivo [46–48], and cause lipid peroxidation [10], indicating potential for penetrating and/or disrupting the blood-brain barrier. Moreover, experimental and epidemiological studies linking nitrosamine exposures from foods to the pathogenesis of primary brain tumors [49–54] provide evidence that these compounds partition into the CNS and cause disease. Radical ion accumulation impairs oxidative metabolism, mitochondrial function, ATP production, and cell survival [25, 29]. In the present study, we examined the hypothesis that relatively brief and low level (sub-mutagenic) exposure to NDEA, a nitrosamine compound commonly present in processed food, can produce neurodegeneration similar to that caused by intracerebral STZ injection and associated with sporadic AD in humans.
Post-mitotic CNS neuronal cultures were briefly (48 h) exposed to a range in concentration of NDEA to examine dose-effects on viability, energy metabolism, mitochondrial function, DNA damage, and oxidative stress. The studies demonstrated that NDEA treatment causes dose-dependent decreases in mitochondrial function and ATP production, and dose-dependent increases in DNA damage and oxidative stress, similar to the effects of STZ treatment and AD neurodegeneration [15, 17]. The NDEA-induced impairments in energy metabolism and mitochondrial function, coupled with increased DNA damage and lipid peroxidation observed in CNS neurons are consistent with nitrosamine’s, including NDEA’s, known alkylating properties and capacity to induce DNA adducts, single-strand DNA breakage, and radical formation in other cell types [6, 10, 11, 25, 28, 29, 55, 56]. Therefore, it’s important to emphasize that post-mitotic neurons are susceptible to nitrosamine-mediated injury and degeneration.
Modest levels of NDEA treatment significantly reduced expression of insulin, IGF-I, and IGF-II receptors, which are needed to transmit signals that mediate neuronal survival, energy metabolism, plasticity, and neurotransmitter function [16, 57]. Therefore, it is likely that the NDEA-associated impairments in energy metabolism and mitochondrial function were mediated in part by decreased expression of these critical receptors. Inhibition of insulin/IGF signaling increases oxidative stress and DNA damage [15, 20, 25, 26, 58–60], and correspondingly, the NDEA-treated neuronal cells had significantly increased levels of HNE and 8-OHdG immunoreactivity over vehicle-treated control cells. Other important consequences of both impaired insulin/IGF signaling and oxidative stress include increased activation of kinases that phosphorylate Tau, increased expression of AβPP, accumulation of AβPP-Aβ, and decreased expression of ChAT [32], which has a critical role in CNS cognitive and motor functions. Moreover, the hypometabolic state induced by neuronal insulin resistance could itself promote tau hyperphosphorylation in brain [61]. Altogether, the abnormalities produced by NDEA exposure in vitro correspond with the critical features of AD neurodegeneration. Finally, the NDEA-mediated increases in ubiquitin immunoreactivity most likely correspond with increased oxidative stress, cytoskeletal collapse, and adduct formation.
In aggregate, these results demonstrate that NDEA can cause molecular and biochemical abnormalities in post-mitotic CNS neurons that resemble the effects of STZ treatment and AD neurodegeneration. NDEA and other nitrosamines, are widely present in processed/preserved foods. Nitrosamines readily form in amine-rich (protein-containing) foods, such as meat and fish that have been preserved or flavored with nitrites. In addition, ingested nitrites and nitrates in food and water can lead to endogenous production of nitrosamines in the highly acidic gastric environment [62]. Given the widespread and ever-increasing presence of NDEA, related compounds, and their precursors in foods, particularly processed foods, beverages, and tobacco [63–66], it is conceivable that these exposures have contributed to the growing prevalence of AD and possibly other forms of neurodegeneration.
Acknowledgments
Supported by AA-02666, AA-02169, AA-11 431, AA-12908, and K24-AA-16126 from the National Institutes of Health
References
- 1.Lijinsky W. N-Nitroso compounds in the diet. Mutat Res. 1999;443:129–138. doi: 10.1016/s1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss JH. Preformed N-nitroso compounds in foods and beverages. Cancer Surv. 1989;8:295–321. [PubMed] [Google Scholar]
- 3.Kearney PC. Nitrosamines and pesticides: A special report on the occurrence of nitrosamines as terminal residues resulting from agricultural use of certain pesticides. Pure Appl Chem. 1980;52:499–526. [Google Scholar]
- 4.Swann PF, Magee PN. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968;110:39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegg AE. Metabolism of N-nitrosodimethylamine. IARC Sci Publ. 1980:3–22. [PubMed] [Google Scholar]
- 6.Fournier P. Biotransformation of dimethylnitrosamine. J Toxicol Clin Exp. 1990;10:283–296. [PubMed] [Google Scholar]
- 7.Robbiano L, Mereto E, Corbu C, Brambilla G. DNA damage induced by seven N-nitroso compounds in primary cultures of human and rat kidney cells. Mutat Res. 1996;368:41–47. doi: 10.1016/s0165-1218(96)90038-5. [DOI] [PubMed] [Google Scholar]
- 8.Kyrtopoulos SA, Anderson LM, Chhabra SK, Souliotis VL, Pletsa V, Valavanis C, Georgiadis P. DNA adducts and the mechanism of carcinogenesis and cytotoxicity of methylating agents of environmental and clinical significance. Cancer Detect Prev. 1997;21:391–405. [PubMed] [Google Scholar]
- 9.Otteneder M, Lutz WK. Correlation of DNA adduct levels with tumor incidence: carcinogenic potency of DNA adducts. Mutat Res. 1999;424:237–247. doi: 10.1016/s0027-5107(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 10.Chuang CH, Hu ML. Synergistic DNA damage and lipid peroxidation in cultured human white blood cells exposed to 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone and ultraviolet A. Environ Mol Mutagen. 2006;47:73–81. doi: 10.1002/em.20168. [DOI] [PubMed] [Google Scholar]
- 11.Yadav AS, Bhatnagar D. Chemo-preventive effect of Star anise in N-nitrosodiethylamine initiated and phenobarbital promoted hepato-carcinogenesis. Chem Biol Interact. 2007;169:207–214. doi: 10.1016/j.cbi.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Espey MG, Miranda KM, Thomas DD, Xavier S, Citrin D, Vitek MP, Wink DA. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen oxide species. Ann N Y Acad Sci. 2002;962:195–206. doi: 10.1111/j.1749-6632.2002.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 13.Lim IK. Spectrum of molecular changes during hepatocarcinogenesis induced by DEN and other chemicals in Fisher 344 male rats [Mechanisms of Ageing and Development 123 (2002) 1665–1680] Mech Ageing Dev. 2003;124:697–708. doi: 10.1016/s0047-6374(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 14.de la Monte SM, Wands JR. Alzheimer’s Disease is Type 3 Diabetes: Evidence Reviewed. J Diabetes Science Tech. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 16.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 17.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 18.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 19.Grunblatt E, Koutsilieri E, Hoyer S, Riederer P. Gene expression alterations in brain areas of intracerebroventricular streptozotocin treated rat. J Alzheimers Dis. 2006;9:261–271. doi: 10.3233/jad-2006-9305. [DOI] [PubMed] [Google Scholar]
- 20.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer S, Lee SK, Loffler T, Schliebs R. Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–258. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 22.Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 23.Tahirovic I, Sofic E, Sapcanin A, Gavrankapetanovic I, Bach-Rojecky L, Salkovic-Petrisic M, Lackovic Z, Hoyer S, Riederer P. Reduced brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. Neurochem Res. 2007;32:1709–1717. doi: 10.1007/s11064-007-9410-1. [DOI] [PubMed] [Google Scholar]
- 24.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977;74:2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 26.Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–134. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Murata M, Takahashi A, Saito I, Kawanishi S. Site-specific DNA methylation and apoptosis: induction by diabetogenic streptozotocin. Biochem Pharmacol. 1999;57:881–887. doi: 10.1016/s0006-2952(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 28.Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J. Enhancement by streptozotocin of O2- radical generation by the xanthine oxidase system of pancreatic beta-cells. FEBS Lett. 1988;239:295–298. doi: 10.1016/0014-5793(88)80938-4. [DOI] [PubMed] [Google Scholar]
- 29.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 30.Soscia SJ, Tong M, Xu XJ, Cohen AC, Chu J, Wands JR, de la Monte SM. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–2056. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Eun Yeon J, Chang H, Tison G, Jun Chen G, Wands JR, De La Monte SM. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: Role of PTEN phosphatase. J Biol Chem. 2003 doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- 32.Chen GJ, Xu J, Lahousse SA, Caggiano NL, de la Monte SM. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5:209–228. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 33.de la Monte SM, Neely TR, Cannon J, Wands JR. Ethanol impairs insulin-stimulated mitochondrial function in cerebellar granule neurons. Cell Mol Life Sci. 2001;58:1950–1960. doi: 10.1007/PL00000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl. 2000;21:895–902. [PubMed] [Google Scholar]
- 35.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran V, Perez A, Chen J, Senthil D, Schenker S, Henderson GI. In utero ethanol exposure causes mitochondrial dysfunction, which can result in apoptotic cell death in fetal brain: a potential role for 4-hydroxynonenal. Alcohol Clin Exp Res. 2001;25:862–871. [PubMed] [Google Scholar]
- 37.de la Monte SM, Ganju N, Wands JR. Microtiter Immunocytochemical ELISA Assay: A Novel and Highly Sensitive Method of Quantifying Immunoreactivity. Biotecniques. 1999;26:1073–1076. doi: 10.2144/99266bm15. [DOI] [PubMed] [Google Scholar]
- 38.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 39.Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem. 1999;72:1617–1624. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- 40.Angerer J, Ewers U, Wilhelm M. Human biomonitoring: state of the art. Int J Hyg Environ Health. 2007;210:201–228. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Pilger A, Rudiger HW. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int Arch Occup Environ Health. 2006;80:1–15. doi: 10.1007/s00420-006-0106-7. [DOI] [PubMed] [Google Scholar]
- 42.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 44.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 45.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 46.Pylypiw HM, Zubroff JR, Magee PN, Harrington GW. The metabolism of N-nitrosomethylaniline. J Cancer Res Clin Oncol. 1984;108:66–70. doi: 10.1007/BF00390975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray JI, Skrypec DJ, Mandagere AK, Booren AM, Pearson AM. Further factors influencing N-nitrosamine formation in bacon. IARC Sci Publ. 1984:301–309. [PubMed] [Google Scholar]
- 48.Tricker AR, Perkins MJ, Massey RC, McWeeny DJ. Some nitrosoamino acids in bacon adipose tissue and their contribution to the total N-nitroso compound concentration. Z Lebensm Unters Forsch. 1985;180:379–383. doi: 10.1007/BF01027770. [DOI] [PubMed] [Google Scholar]
- 49.Dietrich M, Block G, Pogoda JM, Buffler P, Hecht S, Preston-Martin S. A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes Control. 2005;16:619–635. doi: 10.1007/s10552-005-0168-y. [DOI] [PubMed] [Google Scholar]
- 50.McKinney PA. Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 2):ii12–17. doi: 10.1136/jnnp.2004.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Ward MH, Tucker KL, Graubard BI, McComb RD, Potischman NA, Weisenburger DD, Heineman EF. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control. 2002;13:647–655. doi: 10.1023/a:1019527225197. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan S, Novikov I, Modan B. Nutritional factors in the etiology of brain tumors: potential role of nitrosamines, fat, and cholesterol. Am J Epidemiol. 1997;146:832–841. doi: 10.1093/oxfordjournals.aje.a009201. [DOI] [PubMed] [Google Scholar]
- 53.Ivankovic S, Druckrey H. Carcinogenesis in the progeny after exposure of pregnant animals. Food Cosmet Toxicol. 1968;6:584–585. doi: 10.1016/0015-6264(68)90304-0. [DOI] [PubMed] [Google Scholar]
- 54.Druckrey H, Preussmann R, Ivankovic S, Schmahl D. Organotropic carcinogenic effects of 65 various N-nitroso-compounds on BD rats. Z Krebsforsch. 1967;69:103–201. [PubMed] [Google Scholar]
- 55.Cao L, Xu X, Cao LL, Wang RH, Coumoul X, Kim SS, Deng CX. Absence of full-length Brca1 sensitizes mice to oxidative stress and carcinogen-induced tumorigenesis in the esophagus and forestomach. Carcinogenesis. 2007;28:1401–1407. doi: 10.1093/carcin/bgm060. [DOI] [PubMed] [Google Scholar]
- 56.Iwai S, Murai T, Makino S, Min W, Morimura K, Mori S, Hagihara A, Seki S, Fukushima S. High sensitivity of fatty liver Shionogi (FLS) mice to diethylnitrosamine hepatocarcinogenesis: comparison to C3H and C57 mice. Cancer Lett. 2007;246:115–121. doi: 10.1016/j.canlet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 57.LeRoith D. Insulin-like growth factors and the brain. Endocrinology. 2008;149:5951. doi: 10.1210/en.2008-1190. [DOI] [PubMed] [Google Scholar]
- 58.Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- 59.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 60.Kang D, Hamasaki N. Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr Med Chem. 2005;12:429–441. doi: 10.2174/0929867053363081. [DOI] [PubMed] [Google Scholar]
- 61.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scanlan RA. Formation and occurrence of nitrosamines in food. Cancer Res. 1983;43:2435s–2440s. [PubMed] [Google Scholar]
- 63.Jakszyn P, Agudo A, Berenguer A, Ibanez R, Amiano P, Pera G, Ardanaz E, Barricarte A, Chirlaque MD, Dorronsoro M, Larranaga N, Martinez C, Navarro C, Quiros JR, Sanchez MJ, Tormo MJ, Gonzalez CA. Intake and food sources of nitrites and N-nitrosodimethylamine in Spain. Public Health Nutr. 2006;9:785–791. doi: 10.1079/phn2005884. [DOI] [PubMed] [Google Scholar]
- 64.Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del Giudice G, Palli D, Saieva C, Krogh V, Sacerdote C, Tumino R, Panico S, Berglund G, Siman H, Hallmans G, Sanchez MJ, Larranaga N, Barricarte A, Chirlaque MD, Quiros JR, Key TJ, Allen N, Lund E, Carneiro F, Linseisen J, Nagel G, Overvad K, Tjonneland A, Olsen A, Bueno-de-Mesquita HB, Ocke MO, Peeters PH, Numans ME, Clavel-Chapelon F, Trichopoulou A, Fenger C, Stenling R, Ferrari P, Jenab M, Norat T, Riboli E, Gonzalez CA. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–1501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 65.Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol. 2006;12:4296–4303. doi: 10.3748/wjg.v12.i27.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scanlan RA, Barbour JF, Hotchkiss JH, Libbey LM. N-nitrosodimethylamine in beer. Food Cosmet Toxicol. 1980;18:27–29. doi: 10.1016/0015-6264(80)90006-1. [DOI] [PubMed] [Google Scholar]