Abstract
Fibrillins constitute the backbone of extracellular multi-functional assemblies present in elastic and non-elastic matrices, termed microfibrils. Assembly of fibrillins into microfibrils and their homeostasis is poorly understood and is often compromised in connective tissue disorders such as Marfan syndrome and other fibrillinopathies. Using interaction mapping studies, we demonstrate that fibrillins require the complete gelatin-binding region of fibronectin for interaction, which is comprised of domains FNI6-FNI9. However, the interaction of fibrillin-1 with the gelatin-binding domain of fibronectin is not involved in fibrillin-1 network assembly mediated by human skin fibroblasts. We further show that the fibronectin network is essential for microfibril homeostasis in early stages. Fibronectin is present in extracted mature microfibrils from tissue and cells as well as in some in situ microfibrils observed at the ultra-structural level, indicating an extended mechanism for the involvement of fibronectin in microfibril assembly and maturation.
Keywords: fibrillin, fibronectin, microfibrils, homeostasis, assembly
INTRODUCTION
Fibrillin-1, -2 and -3 are extracellular matrix glycoproteins constituting the fibrillin protein family. Each member of the family has a typical modular organization primarily composed of calcium-binding epidermal growth factor-like domains, transforming growth factor-β binding domains and hybrid domains [1,2]. The three fibrillin isoforms are highly homologous to each other on the amino acid sequence (~60–70%). They differ in their spatio-temporal expression patterns with fibrillin-1 being expressed throughout life whereas fibrillin-2 and -3 are mainly developmentally expressed [3–5]. Fibrillins form the backbone of highly ordered extended structures termed microfibrils, which have a characteristic bead-on-a-string ultra-structure with a 50–55 nm periodicity after extraction from tissues or cell culture sources [6–8]. Microfibrils are found in many tissues including the cardiovascular system, bones, eyes, skin and other tissues, where they fulfill a wide range of physiological functions [9]. They form the scaffold for elastic fiber assembly, act as stress-bearing entities (i.e. in ciliary zonules of the eye) [10–12], and serve as reservoirs and regulators for growth factors of the TGF-β superfamily [13–15]. Proper microfibril assembly and function is crucial as demonstrated by the wide range of clinical symptoms associated with fibrillinopathies [2,16]. Mutations in fibrillins can result in several connective tissue disorders including Marfan syndrome, stiff skin syndrome, autosomal dominant Weill-Marchesani syndrome, autosomal dominant geleophysic dysplasia, acromicric dysplasia all caused by mutations in fibrillin-1, and congenital contractural arachnodactyly caused by fibrillin-2 mutations [17–21].
Fibronectin is an extracellular matrix protein which is involved in fundamental processes such as cell adhesion, migration and proliferation during development and physiological processes (for review see [22]). Like fibrillins, fibronectin is a modular protein but with a different set of domains called type I, II and III repeats (FNI, FNII, FNIII). Fibronectin domains confer self-assembly and ligand binding properties to a number of proteinaceous and non-proteinaceous ligands [22,23]. Two forms of fibronectin exist: i) cellular fibronectin is secreted by mesenchymal cells and assembled into an insoluble fibrillar network; ii) soluble plasma fibronectin is synthesized by hepatocytes and secreted into the blood where it polymerizes during blood clotting upon vascular injury. Both forms are secreted as soluble dimers linked by two disulfide bonds [24,25]. Upon interaction with α5β1 integrin and other surface components, the protein is extended through forces originating from the intracellular contractile actin cytoskeleton [26]. This extension causes the exposure of cryptic self-assembly sites which enables the fibronectin dimers to multimerize and to form an extracellular fibronectin network [27]. Fibronectin network is formed and disassembled at the same rate as shown in vivo and in vitro. Therefore, the fibronectin network is constitutively turned-over [28,29].
In the recent years, a number of studies provided evidence that fibronectin is a “master organizer” of extracellular matrix assembly. Several studies demonstrated the requirement of a continuous polymerization and supply of fibronectin for the assembly of extracellular proteins such as collagen type I, thrombospondin-1, latent transforming growth factor-binding protein-1 (LTBP-1) and fibulin-1 [30–34]. In previous studies, we and others demonstrated an essential role of fibronectin in fibrillin-1 network assembly by human dermal fibroblasts cultures [35,36]. In our previous study, we also have shown that C-terminal halves of fibrillin-1, -2 and -3 as well as the N-terminal half of fibrillin-1 can interact directly with fibronectin in solid phase binding assays. More precisely, the relevant fibrillin fragments interact strongly with the collagen/gelatin-binding domain of fibronectin. These interactions are inhibited by gelatin. We have further demonstrated that cell-associated multimerization of the fibrillin C-termini generate high-affinity binding sites for both fibronectin and the fibrillin N-termini [36,37].
Remodeling of the extracellular matrix is an important process during development, wound healing and pathological processes. Therefore, it is important to understand how matrix deposition, homeostasis and degradation are regulated. Improper matrix remodeling by preventing turnover of collagen I or by alteration of regulation of matrix-degrading proteases and their inhibitors result in fibrosis, arthritis and developmental abnormalities [38–41].
Sottile and Hocking demonstrated the requirement of a continual fibronectin matrix polymerization for the retention of collagen I and thrombospondin-1 in fibrillar structures [31]. These authors have shown that an intact fibronectin matrix is essential to maintain the composition of cell-matrix adhesion sites, whereby integrin α5β1 is unable to localize to cell-matrix adhesion sites in the absence of fibronectin polymerization. These data suggest that fibronectin is not only a “master organizer” of extracellular matrix but also a “master stabilizer”. In order to do so, it is essential that fibronectin polymerization is maintained as inhibition of fibronectin assembly causes destabilization of fibronectin matrix [31].
This study aims to further characterize the interaction of fibrillins with fibronectin and the role fibronectin plays in microfibril homeostasis. We show that the entire collagen/gelatin-binding domain of fibronectin is required for optimal interaction with fibrillins, but that this interaction is not necessary for fibrillin-1 network assembly. We demonstrate that the fibronectin network is required for homeostasis of an immature but not a mature fibillin-1 network, even though fibronectin is present in mature microfibrils.
EXPERIMENTAL
Antibodies
The production and characterization of rabbit polyclonal antibodies against the N-terminal and C-terminal halves of human fibrillin-1 (anti-rFBN1-N, anti-rFBN1-C) [42,43], fibrillin-2 (anti-rFBN2-N, anti-rFBN2-C) [44], and fibrillin-3 (anti-rFBN3-C) [36] have been previously described. The mouse monoclonal antibody (mouse ascites, anti-FN clone 15) against human fibronectin was purchased (Sigma, St Louis, MO, USA; product F7387). The mouse monoclonal antibody 5C3 (anti-FN 5C3) was generated against the recombinant fibronectin fragment FN70K which includes the fibrin/heparin- and collagen/gelatin-binding domain and the epitope was previously mapped to FNI9 [45]. The rabbit polyclonal anti-human fibronectin antibody (anti-hFN poly IgG) was made in-house to plasma fibronectin isolated by affinity chromatography on gelatin-agarose: the antibodies recognized only fibronectin in immune-blots of plasma or fibroblast lysates. The rabbit polyclonal anti-mouse fibronectin antibody (anti-mFN poly IgG) was purchased (Millipore; product AB2033). Cy3-conjugated AffiniPure goat anti-rabbit IgG (H+L) and Alexa-488- conjugated AffiniPure goat anti-mouse IgG (H+L), Peroxidase-conjugated AffiniPure goat anti-rabbit and anti-mouse IgG (H+L), 12 nm colloidal gold-AffiniPure Donkey anti-mouse IgG (H+L) and 18 nm colloidal gold-AffiniPure Donkey anti-rabbit IgG (H+L) antibodies were purchased from Jackson ImmunoReseach Laboratories (West Grove, PA, USA).
Proteins
The production and the purification of the N- and C-terminal halves of fibrillin-1 (rFBN1-N, rFBN1-C) [46], fibrillin-2 (rFBN2-N, rFBN2-C) [44] and fibrillin-3 (rFBN3-C) [36] have been previously described in detail. An overview of these fragments is presented in Fig. 1A. Fibronectin was purified from human plasma as previously described [47]. Recombinant FNI4-FNII2 (amino acid residues 184 to 467), FNI6-FNII2 (amino acid residues 291–467), FNI6-FNI9 (amino acid residues 291–608), and FNI7-FNI9 (amino acid residues 468–608) were expressed as secreted His-tagged proteins using the baculovirus vector pAcGP67.coco (COCO) and purified from conditioned medium as described [45,48]. Residues are numbered beginning with the start methionine of the fibronectin signal peptide. The purity of the recombinant fibrillin and fibronectin fragments are shown in Supplemental Figure S1. Porcine gelatin (Sigma, St. Louis, MO, USA; product G1890) was resuspended in 1% (v/v) acetic acid at 37°C. For some experiments, gelatin was labeled with FITC (Sigma, St. Louis, MO, USA; product F7250) according to manufacturer’s protocol followed by extensive dialysis against 50 mM Tris-HCl, pH 7.4, 150 mM NaCl (TBS).
Figure 1. Characterization of the fibrillin-fibronectin interaction.
A: Schematic overview of previously produced recombinant halves of fibrillin-1, -2 and -3 used in this study. B: Shown is a representative solid phase binding assay. Human plasma fibronectin was immobilized and various fibrillin-1, -2 and -3 halves as indicated were used at constant concentrations (50 µg/ml) as soluble ligands in the presence of increasing NaCl concentrations. The interaction of rFBN1-N with fibronectin is typically much weaker compared to the interaction of the fibrillin C-terminal halves as previously shown [36]. Note that the presence of NaCl did not inhibit the interaction of all fibrillin fragments with fibronectin. Data represent means of duplicates; standard deviations are indicated.
Cell culture
Human skin fibroblasts (HSFs) were isolated from foreskin obtained from standard circumcision procedures in agreement with local ethics regulations (ethics approval PED-06-054, Montreal Children Hospital). The tissue donors were healthy individuals between 2 and 14 years of age. Consent was obtained from their parents. HSFs between passage 2 and 10 were used in experiments. Cells were cultured at 37°C in a 5 % CO2 atmosphere, in DMEM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FCS (Wisent, St. Bruno, QC, Canada). Depletion of fibronectin from FCS was performed by loading FCS twice on a Gelatin Sepharose 4B column according to manufacturer’s instructions (GE Healthcare, Waukesha, WI). The flow-through consisting of FCS depleted of fibronectin was used for fibrillin-1 assembly inhibition studies in cell culture.
Solid phase binding assays
Solid phase binding assays were performed as previously described [44]. In brief, 10 µg/ml in TBS (100 µl) of full-length or recombinant fragments of fibronectin were immobilized for 16 h at 4°C in 96-well plates (Maxisorp; Nalge Nunc International, Naperville, IL). Wells were blocked with 5 % (w/v) non-fat milk in TBS including 2 mM CaCl2 for 1 h at room temperature. Washing was performed with 0.05 % Tween-20 in TBS (TBST). Serial dilutions (1/2 starting at 100 µg/ml) of fibrillin fragments were prepared in 2 % (w/v) non-fat milk, 2 mM CaCl2 in TBS (binding buffer) and were incubated with immobilized proteins for 2 h. Similarly, for ionic strength dependency experiments, fibrillin fragments were incubated in binding buffer containing various concentrations of NaCl as indicated in the figure. Three 5 min washes with TBST were then performed between each subsequent step. Bound ligands were detected by incubation with polyclonal antisera against rFBN1-N, rFBN1-C, rFBN2-N, rFBN2-C and rFBN3-C, diluted 1/1000 in binding buffer for 90 min, followed by incubation with Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L) (1/800 diluted) for 90 min and color reaction with 5-aminosalicylic acid.
For inhibition experiments, the above procedure was used with modifications of the incubation with fibrillin fragments. After blocking non-specific binding sites, serial dilutions (1/2 starting at 1/400) of anti-FN 5C3 or anti-FN clone 15 diluted in binding buffer containing constant concentrations (50 µg/ml) of fibrillin fragments were incubated with immobilized full-length fibronectin for 2 h. For inhibition experiments with the functional upstream domain (FUD) peptide from Streptococcus pyogenes F1 adhesin protein [49], after the blocking step, constant concentrations (50 µg/ml) of fibrillin fragments were incubated with immobilized full-length fibronectin for 90 min with or before incubation with serial dilutions (1/2 starting at 50 nM in binding buffer) of FUD in binding buffer.
Immunofluorescence microscopy
For double immunofluorescence experiments, HSFs were seeded at 7.5 × 104 cells/well in eight-well chamber slides in DMEM supplemented with fibronectin depleted FCS. Cells were grown for 4 days until robust fibronectin and fibrillin-1 networks developed. Cells were washed twice with 137 mM NaCl, 207 mM KCl, 4.3 mM Na2HPO4 and 1.47 mM KH2PO4, pH 7.4 (PBS, standard washing buffer). Cells were then fixed with ice-cold 70 % methanol/ 30 % acetone for 5 min, followed by 3 washes with PBS. Cells were blocked for 30 min with 10 % normal goat serum in PBS (PBS-G, Jackson ImmunoReseach Laboratories) and incubated for 90 min with primary antibodies anti-rFBN1-C and anti-FN clone 15 diluted 1/500 in PBS-G. Three washes were performed followed by a 60 min incubation with secondary Cy3-conjugated AffiniPure goat anti-rabbit and Alexa-488-conjugated AffiniPure goat anti-mouse, or Cy3-conjugated AffinitiPure goat anti-mouse antibodies (1/100 in PBS-G). Cells were washed thrice. Cell nuclei were counterstained with DAPI (1 µg/ml in water) for 5 min before slides were washed and cover-slipped. Fluorescent images were recorded with an Axioskop 2 microscope equipped with an Axiocam camera and AxioVision software version 3.1.2.1 (Zeiss), or in some cases with an Axiovert 135 microscope (Zeiss) equipped with a Retiga EXI camera and the Northern Eclipse imaging software.
Gelatin inhibition of fibrillin-1 network formation
HSFs were seeded at 7.5 × 104 cells/well in eight-well chamber slides in DMEM supplemented with fibronectin-depleted FCS in the presence of 100 µg/ml gelatin, FITC-gelatin or equivalent volumes of TBS. Cells were grown for 5 days and immunofluorescence was performed as described under Immunofluorescence microscopy.
FUD pulse-chase experiment in cell culture
For short term experiments, HSFs were prepared as described under Immunofluorescence microscopy. Following 4 days of growth, the cells were then pulsed with 500 nM of FUD peptide for two days. A chase was then performed with the same medium in the absence of the FUD peptide for two and four additional days. Indirect immunofluorescence was performed as described under Immunofluorescence microscopy. For long-term experiments, HSFs were cultivated for 3 weeks. A pulse was performed with 2 µM FUD for three days and the cells were analyzed by indirect immunofluorescence.
Microfibril extraction
Microfibrils were extracted from HSFs or mouse tissues using collagenase or guanidine extraction as previously described with small modifications [50,51]. For microfibril extraction from cells, HSFs were grown for 3 weeks for subsequent collagenase extraction and for 1 month for subsequent guanidine extraction on a 1,000 cm2 total cell culture area. Cells were scraped off in 50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 5 mM CaCl2 (extraction buffer), followed by centrifugation at 6,000 × g for 15 min. The pellet was then resuspended in extraction buffer containing either 1 mg/ml crude collagenase (from Clostridium histolyticum, Sigma, St Louis, MO, USA; product C0130), 2 mM phenylmethylsulfonyl fluoride and 5 mM N-ethylmaleimide; or 6M guanidine, followed by incubation for 4 h at 4°C. The extract was centrifuged at 7,000 × g for 20 min. The supernatant was then separated on a Sephacryl S-500 HR gel filtration column (void volume= 47.52 ml) equilibrated with extraction buffer at a flow rate of 0.5 ml/min using an ÄktaPurifier 10 chromatography system (GE Healthcare). For microfibrils extracted from mouse tissue, 8 one month old mice were anesthetized. Intracardiac perfusion was performed with PBS followed by extraction buffer. The lungs, aorta and dorsal skin were dissected, homogenized in extraction buffer and centrifuged at 6,000 × g for 15 min. The pellet was divided in two equal amounts and extraction buffer with either 1 mg/ml crude collagenase, 2 mM phenylmethylsulfonyl fluoride and 5 mM N-ethylmaleimide, or 6M guanidine was added followed by incubation for 3 days at 4°C. The samples were then processed as described for microfibril extraction from cells.
Immunoprecipitation
Monoclonal anti-FN clone 15 (2.9 mg) was coupled to 2.5 ml cyanogen bromide-activated Sepharose (GE Healthcare) as instructed by the manufacturer and packed in a 2 ml chromatography column. High molecular weight microfibril and intermediate molecular weight gel filtration fractions from HSFs guanidine microfibril extractions were extensively dialyzed against TBS, 2 mM CaCl2. Aliquots (780 µl) of high molecular weight microfibril and intermediate molecular weight gel filtration fractions from the HSFs collagenase and guanidine microfibril extractions were loaded under low flow (10 µl/min) onto the antibody column equilibrated in TBS, 2 mM CaCl2. The unbound material was collected. The bound material was eluted with 0.1 M glycine pH 3.0 at 0.5 ml/min and immediately neutralized with 2 M Tris.
Immunoblotting
100 µl of each major peak fraction from the microfibril extraction were diluted in 400 µl TBS, 2 mM CaCl2 and dot-blotted onto a 0.45 µm nitrocellulose membrane (BioRad). Non-specific binding sites were blocked with 5 % non-fat milk (w/v) in TBS for 1 h at room temperature. Blots were then incubated with primary antibodies (1/500 diluted in 5 % bovine serum albumin in TBS) overnight at 4°C. After washing 3 × 10 min with TBS including 0.05 % (v/v) Tween-20, blots were incubated with Peroxidase-conjugated AffiniPure goat anti-rabbit antibody (1/800 diluted in TBS) for 1.5 h at room temperature. Color reaction was performed with TBS, including 0.5 mg/ml 4-chloro-1-naphthol, 17% (v/v) methanol and 0.02% (v/v) H2O2.
For the immunoprecipitation experiments, the immunoblotting procedure was performed with slight modifications. To compensate for the differences in optical density at 280 nm of the different peaks, 5 µl of start material, 50 µl of unbound and 200 µl of the bound fractions were dot-blotted onto a 0.45 µm nitrocellulose membrane (BioRad). The membrane was incubated with a 1/3,500 dilution of the peroxidase-conjugated AffiniPure goat anti-rabbit antibody and then developed with enhanced chemiluminescence (Thermo Scientific) using Hyblot CL autoradiography film.
Immunogold staining and electron microscopy
HSFs were prepared as described under Immunofluorescence microscopy and grown for 7 days. Cells were washed three times with PBS and then fixed for 1 h on ice with 3 % paraformaldehyde in PBS, followed by 3 washes with PBS. Cells were blocked for 1 h with 5 % normal donkey serum in PBS (Jackson ImmunoResearch Laboratories, Inc.). The primary anti-fibrillin-1 antibody (anti-rFBN1-C, 1/100) and anti-fibronectin (anti-FN clone 15, 1/100) were diluted in PBS and incubated overnight at 4°C. Following 3 washes with PBS, 12- and 18-nm gold-conjugated secondary antibodies were used diluted at 1/20 in PBS. Cells were washed with 0.1 M sodium cacodylate (cacodylate buffer) and then fixed with 2 % glutaraldehyde in cacodylate buffer. Cells were washed 4 times with cacodylate buffer, fixed for 20 min with 1 % OsO4 in cacodylate buffer. Cells were dehydrated and embedded in EPON. Ultrathin sections were processed and grids were contrasted with 1 % uranyl acetate and enhanced with Reynold’s lead for 3 min. Sections were then examined with a FEI Tecnai 12, 120 kV electron microscope equipped with a Gatan 792 Bioscan 1k × 1k Wide Angle Multiscan CCD camera.
RESULTS
Characterization of the fibrillin-fibronectin interaction
We have previously shown that fibrillin-1, -2, -3 C-terminal halves and the fibrillin-1 N-terminal half interact directly with fibronectin in solid phase binding assays [36]. To determine if fibrillin-fibronectin interaction is of ionic nature, various fibrillin fragments were tested for binding to immobilized full-length fibronectin in the presence of increasing NaCl concentrations (Fig. 1B). The presence of salt up to 1 M NaCl did not decrease the fibrillin interaction with fibronectin. Instead, the interactions increased slightly. These data indicate that the fibrillin-fibronectin interaction is of non-ionic nature. In control experiments, we verified that high NaCl concentrations did not affect the multimerization state of fibrillin C-terminal fragments, which is a pre-requisite for the interaction with fibronectin (data not shown).
Characterization of the fibrillin binding site in fibronectin
We have previously reported that the fibrillin-1, -2 and -3 C-terminal halves and the fibrillin-1 N-terminal half interact with the collagen/gelatin binding domain of fibronectin comprised of FNI6-FNI9 [36]. To map the fibrillin binding site in this region, we performed inhibition experiments with a monoclonal antibody, anti-FN 5C3, which epitope is located in the FNI9 domain of fibronectin [45]. This antibody inhibited the interaction of rFBN1-C, rFBN2-C and rFBN3-C with full-length fibronectin, whereas the control anti-FN clone 15 antibody which epitope is located in the region between FNIII1 and the C-terminus of fibronectin did not exert an inhibitory effect (Fig. 2A). Several other monoclonal antibodies which epitopes are located outside of the collagen/gelatin-binding domain of fibronectin also did not inhibit this interaction (data not shown). These data show that FNI9 is a critical determinant of the fibrillin-fibronectin interaction in the solid phase binding assay.
Figure 2. Mapping of the fibrillin binding site on fibronectin.
Shown are typical solid phase inhibition and binding assays. A: Full-length fibronectin was immobilized and incubated with constant concentrations (50 µg/ml) of rFBN1-C, rFBN2-C and rFBN3-C in the presence of serial 1/2 dilutions of monoclonal anti-FN 5C3 and anti-FN clone 15 antibodies which epitopes are located in the collagen/gelatin-binding domain (FNI9) [45], and in the region between FNIII1 and the C-terminus of fibronectin, respectively. Note that the anti-FN 5C3 antibody completely inhibited the interaction of the fibrillin C-terminal halves with fibronectin whereas anti-FN clone 15 had no inhibitory effect. Data represent means of duplicates; standard deviations are indicated. B: Recombinant fibronectin subfragments of the collagen/gelatin-binding domain were immobilized. Schematic representations of each fragment and corresponding graphical symbols are indicated. Soluble rFBN1-C, rFBN2-C, rFBN3-C and rFBN1-N were used as soluble ligands at the indicated concentrations. Note that fibrillin fragments interact with recombinant FNI6-FNI9 which spans the entire collagen/gelatin-binding domain of fibronectin. Interactions of fibrillins with the subfragments were weak or absent. Data represent means of duplicates; standard deviations are indicated.
To map the fibrillin binding site on fibronectin further, we used recombinant fibronectin subfragments spanning the collagen/gelatin-binding domain in solid phase binding assays with fibrillin fragments. Proper folding of those recombinant fragments was verified through interaction with monoclonal antibodies sensitive to conformational changes (ref [45] and data not shown). We previously published that rFBN1-C, rFBN2-C, rFBN3-C and rFBN1-N interacted strongly with a fibronectin proteolytic fragment FN40K spanning the entire collagen/gelatin binding domain FNI6-FNI9 [36]. In the present study, we demonstrated that fibrillin fragments interact with recombinant FNI6-FNI9 in a manner similar to that of FN40K (Fig. 2B). However, interaction with smaller subfragments of this region was either greatly reduced or completely absent (Fig. 2B). From these experiments, we conclude that the fibrillin C-terminal halves and the fibrillin-1 N-terminal half require the complete collagen/gelatin binding domain of fibronectin for efficient interaction with FNI9 providing potentially initial protein-protein contacts.
The collagen/gelatin binding domain of fibronectin is not involved in fibrillin network assembly
We have shown previously that gelatin inhibits fibrillin interaction with fibronectin in solid phase binding assays [36]. In the present study, we tested if gelatin inhibits the fibrillin-1 network assembly by skin fibroblasts. HSFs were grown for 4 days in the presence of various concentrations of gelatin (shown is 100 µg/ml) or TBS as a control (Fig. 3A). The formation of both networks, fibrillin-1 and fibronectin, by HSFs were not affected by the addition of gelatin. To ensure that gelatin was not endocytosed by the cells and thus was available to interact with fibronectin in the experimental system, FITC-labeled gelatin was added to HSFs under identical conditions. FITC-gelatin colocalized with the fibronectin network, indicating that it occupies the binding sites on fibronectin within the collagen/gelatin binding region (Fig. 3B) as was previously observed [52]. FITC-gelatin also colocalized with the fibrillin-1 network, which in turn colocalizes with fibronectin as was previously shown (Fig. 3B) [36]. We verified in solid phase binding assays that gelatin does not directly interact with fibrillin-1 (data not shown). Several concentrations of gelatin as well as addition of gelatin at different time points were tested but did not result in any effect on fibrillin-1 network formation. The monoclonal anti-FN 5C3 antibody which inhibits the fibrillin-fibronectin interaction in solid phase binding assay (see Fig. 2A), and the FNI6-FNI9 fibronectin fragment also did not affect fibrillin-1 network formation when added to HSFs (data not shown). From these experiments, we conclude that the interaction of fibrillin-1 with the collagen/gelatin binding domain of fibronectin, that dominates the solid phase binding assay, is not involved in fibrillin-1 network assembly. However, these experiments do not rule out involvement of other fibrillin-fibronectin interactions in fibrillin-1 assembly.
Figure 3. Analysis of gelatin treatment of fibrillin-1 network formation by HSFs.
A: Shown is an indirect immunofluorescence labeling of fibronectin (green) and fibrillin-1 (red) networks assembled by HSFs in the presence of TBS as a control or 100 µg/ml gelatin. As described in Experimental Procedures, DAPI staining of cell nuclei validated similar cell densities present in each field. Note that gelatin did not affect the fibronectin and fibrillin-1 network assembly. Scale bar = 100 µm. B: HSFs were cultured in the presence of TBS as a control or 100 µg/ml FITC-gelatin. No fluorescence was observed when FITC-gelatin was not present (TBS treatment, green channel). Fibronectin or fibrillin-1 networks were visualized through indirect immunofluorescence (red staining). DAPI staining of cell nuclei verified similar cell densities in each field. The presence of FITC-gelatin did not affect fibronectin (row 3) and fibrillin-1 (row 4) network formation. FITC-gelatin colocalized with both fibronectin (row 3) and fibrillin-1 (row 4) networks. Scale bar = 100 µm.
Fibronectin network is required for fibrillin-1 network homeostasis
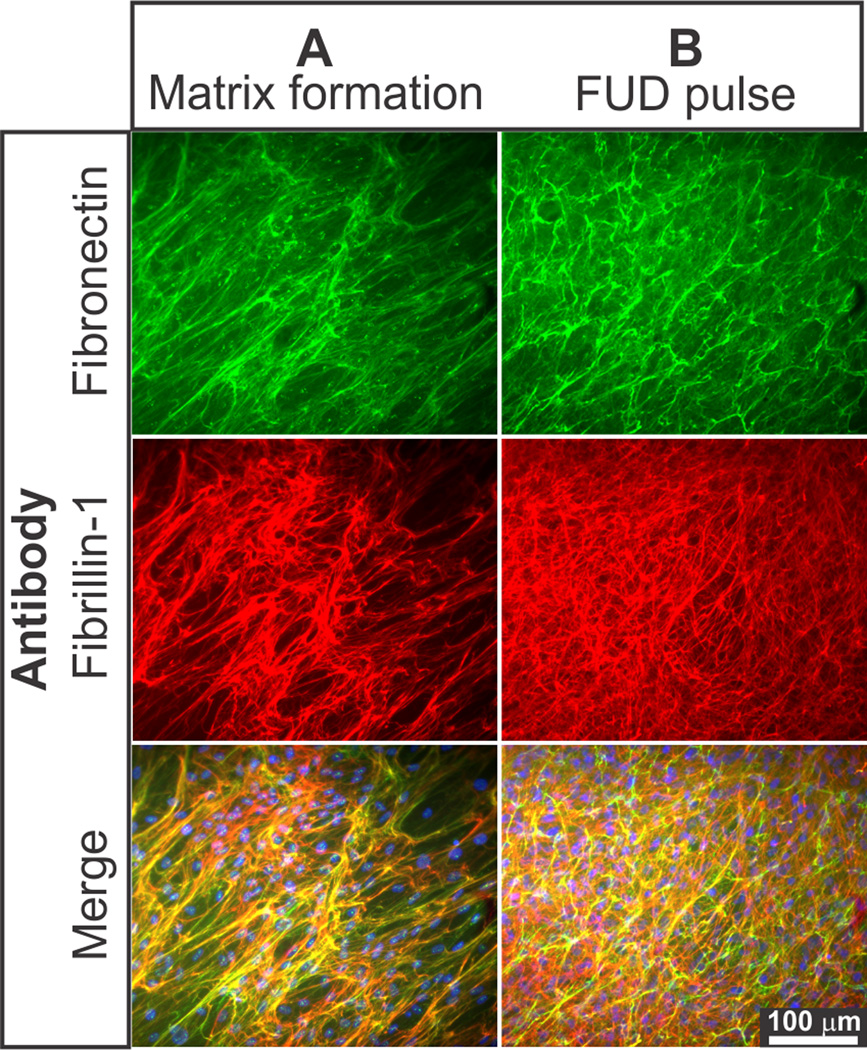
Previously, we have shown that the presence of a fibronectin network is required for the assembly of fibrillin-1 into microfibrils [36]. The functional upstream domain (FUD) peptide from the bacterial adhesin F1 protein has been shown to block fibronectin network formation [49]. As the fibronectin network is constitutively turned over [28,29], we used the FUD peptide as a tool to study the role of the fibronectin network in microfibril homeostasis. The fibronectin network and microfibrils were first allowed to be assembled by HSFs for 4 days (Fig. 4A), before the FUD peptide was added for 2 days to initiate fibronectin fiber disassembly (Fig. 4B). As expected, the fibronectin network readily disassembled after FUD treatment. Similarly, microfibrils also disassembled after the addition of FUD, suggesting that microfibrils at this early stage require the presence of a fibronectin network for homeostasis. The FUD peptide does not affect expression of fibronectin and fibrillin-1 proteins in HSFs [36]. After the FUD peptide was removed and replaced by fibronectin-depleted normal cell culture medium for 2 (Fig. 4C) and 4 (Fig. 4D) days, both networks reassembled and colocalized. These data demonstrate that fibrillin-1 homeostasis is directly coupled to fibronectin homeostasis.
Figure 4. Microfibrils require fibronectin network for homeostasis.
A: Shown is an indirect immunofluorescence of fibronectin (green) and fibrillin-1 (red) networks produced by HSFs grown for 4 days, B: pulsed for 2 days with 500 nM FUD peptide and C: chased in the absence of FUD for 2 days and D: 4 days. Note that the FUD peptide disrupted the pre-assembled fibronectin and fibrillin-1 networks. Both networks reassembled when the FUD peptide was replaced by normal medium. DAPI staining (not shown) showed similar numbers of nuclei in each image. Scale bar = 100 µm.
FUD interferes with the fibrillin-fibronectin interaction
To understand the mechanism by which the FUD peptide affects the fibrillin-1 network homeostasis, we performed inhibition experiments in solid phase assays. The FUD peptide interacts with FNI2-FNI5 domains of the fibrin-binding N-terminal fragment and FNI8 and FNI9 of the collagen/gelatin-binding domain of fibronectin [45]. Interaction of FUD with fibronectin causes significant conformational changes in fibronectin, exposing the RGD integrin binding sequence in FNIII10 [53]. The FUD peptide does not interact directly with fibrillin-1 (Fig. S2). However, FUD inhibits the interactions of the rFBN1-N and rFBN1-C with fibronectin when added simultaneously with the fibrillin fragments (Fig. 5A). FUD also partially displaces bound rFBN1-N and rFBN1-C from fibronectin, likely by competing with fibrillin for interaction with the FNI8 and FNI9 domains of the collagen/gelatin-binding region of fibronectin (Fig. 5B).
Figure 5. FUD inhibits fibrillin-1 interaction with fibronectin.
A: Shown is a representative solid phase inhibition assay. Human fibronectin was immobilized and incubated with constant concentrations (50 µg/ml) of soluble rFBN1-N or rFBN1-C in the presence of various concentrations of the FUD peptide as indicated. The signal without FUD was set to 100%. Note that FUD partially inhibited the interaction of fibrillin-1 with fibronectin. Data represent means of duplicates; standard deviations are indicated. B: Shown is a solid phase inhibition assay. Constant concentrations (50 µg/ml) of soluble rFBN1-N or rFBN1-C were first incubated for 2 h with immobilized human fibronectin. Various concentrations of the FUD peptide were then added. The signal without FUD was set to 100 % (OD = 492 nm). Data represent means of duplicates; standard deviations are indicated. Note that FUD partially displaced bound fibrillin-1 from fibronectin.
FUD does not affect fibronectin and fibrillin-1 networks in long term culture
To investigate if the fibrillin-1 network requires fibronectin for homeostasis when both networks are mature, long term cultures of HSFs were analyzed for their sensitivity to FUD treatment (Fig. 6). HSFs were grown for 3 weeks (Fig. 6A), before FUD was added for 3 days to the culture medium (Fig. 6B). After 3 weeks in culture, HSFs assembled dense fibronectin and fibrillin-1 networks. A higher concentration (2 µM) and a longer pulse of the FUD peptide (3 days) were used to compensate for the higher number of fibronectin fibers present in the long term culture compared to short term cultures. FUD peptide did not disrupt the fibronectin or fibrillin-1 networks (Fig. 6B), suggesting that both networks are more stabilized in long term cultures. Similar results were observed with cultures cultivated for 2 weeks (data not shown).
Figure 6. FUD does not affect fibronectin and fibrillin-1 networks in long term culture.
A: Shown is an indirect immunofluorescence of fibronectin (green) and fibrillin-1 (red) networks produced by HSFs cultivated for 3 weeks and B: pulsed for 3 days with the 2 µM FUD peptide. Due to the extended culture time, the fibronectin and fibrillin-1 networks are very dense. Note that the FUD peptide did not disrupt the pre-assembled fibronectin and fibrillin-1 networks. Scale bar = 100 µm.
Fibronectin is present in extracted microfibrils from cells and tissues
We previously observed that both, fibrillin-1 and fibronectin are present at the ultra-structural level in some extracellular fibers produced by HSFs [36]. To investigate if fibronectin is present in mature “beads-on-a-string” microfibrils, microfibrils were extracted by collagenase digestion and by guanidine extraction from 3 weeks old and 1 month old HSF cultures respectively and separated by gel filtration chromatography (Fig. 7A, C). Microfibrils were also extracted by collagenase and guanidine extraction from 1 month-old mouse lung, skin and aorta. Microfibrils from lung collagenase extraction (Fig. 7B) and skin guanidine extraction (Fig. 7D) are shown. Fractions from each peak were dot-blotted to determine the presence of fibrillin-1 and fibronectin. Note that the intensity of the dots for both fibrillin-1 and fibronectin do not correlate with optical density peak heights as other proteins present in those fractions also account for the optical density level. As expected based on published results, a strong signal for fibrillin-1 was detected in the peak containing the void volume (50–60 ml) for all extractions indicating the presence of high molecular weight microfibrils [51,54]. However, fractions from larger elution volumes, which were never analyzed before, also contained detectable levels of fibrillin-1 potentially representing smaller forms of microfibrils. Fibronectin was present in the microfibril fractions in all extractions (50–60 ml). It was also present in higher elution volume fractions. We observed similar results with collagenase extracted microfibrils from 1, 2 and 9 week-old HSFs cultures and from mouse skin and aorta, as well as with guanidine extracted microfibrils from mouse aorta (data not shown).
Figure 7. Fibronectin is present in various size extracted microfibrils.
Each panel shows a chromatograph of microfibril extraction separated by gel filtration. Peaks containing high molecular weight microfibrils (“MF”), intermediate (“int. MW”) or low molecular weight fibrillin assembly (“low MW”) are labeled. The black arrows indicate the column void volume at 47.52 ml. Below each chromatograph, a dot blot analysis of the main peaks (indicated as ml of elution volume) with polyclonal antibodies against fibrillin-1 (anti-rFBN1-C, (A–D) and fibronectin (anti-hFN poly IgG, (A, C) or anti-mFN poly IgG (B, D). A: Shown is a collagenase extraction from HSFs cultured for 3 weeks or from B: mouse lung tissue. C: Shown is a guanidine extraction from HSFs cultured for 4 weeks or from D: mouse skin tissue. Fibrillin-1 is strongly represented in the void volume containing mature microfibrils (50–60 ml) in all extractions, while it trails throughout the elution volume. Fibronectin is present in the mature microfibril fraction in all extractions as it co-eluted with fibrillin-1 between 50–60 ml. Fibronectin also strongly co-eluted with fibrillin-1 in the intermediate molecular weight peak (between 80–95ml for collagenase extractions, between 70–90 ml for guanidine extraction from HSFs and between 53–60 ml guanidine extraction from skin).
We investigated if fibronectin and fibrillin-1 are interacting in high molecular weight microfibrils and in the intermediate molecular weight peaks. Samples of those peaks from collagenase and guanidine extractions from HSFs were immunoprecipitated with an anti-fibronectin affinity column. To compensate for the differences in optical density at 280 nm of each peak after the affinity column, 5 µl of start material, as well as 50 µl of unbound and 200 µl of bound fractions were dot blotted with anti-fibronectin and anti-fibrillin-1 antibody (Fig. 8). Fibronectin immunoprecipitated in all the conditions tested. Fibrillin-1 was also co-immunoprecipitated in the high molecular weight microfibrils and in the intermediate molecular weights peaks regardless of the extraction method used (Fig. 8). Therefore, we conclude from these experiments that fibronectin interacts directly or indirectly with large mature microfibrils as well as with fibrillin-1 in smaller intermediate level microfibrils.
Figure 8. Fibrillin-1 co-immunoprecipitates with fibronectin from the microfibril and intermediate molecular weight gel filtration fractions.
Representative fractions of high molecular weight microfibrils (“MF”) and intermediate molecular weight (“int. MW”) peak from Figure 7 chromatographs were immunoprecipitated on a specific monoclonal anti-fibronectin (anti-FN clone 15) antibody column. Shown are dot blots of the “start”, the “unbound” and the “bound” materials from the immunoprecipitation analyzed with polyclonal antibodies against fibrillin-1 (anti-rFBN1-C) and fibronectin (anti-hFN poly IgG). To compensate for the differences in optical density at 280 nm of the different peaks, the volume of each fraction was adjusted so that 5 µl of start, 50 µl of unbound and 200 µl of bound materials were dot blotted. A: Shown are immunoprecipitation analyses of samples from collagenase extraction from HSFs cultured for 3 weeks. B: Shown are immunoprecipitation analyses of samples from guanidine extraction from HSFs cultured for 4 weeks. Note that fibrillin-1 was co-immunoprecipitated with fibronectin in “bound” materials.
Fibrillin-1 and fibronectin can colocalize to the same extracellular matrix fibers or to distinct fibers
In our previous study, we observed frequent colocalization of fibrillin-1 and fibronectin to the same extracellular fibrils produced by dermal fibroblasts through double immunogold labeling [36]. In the present study, whereas the majority of fibrillin-1 colocalizes with fibronectin in immunofluorescence, we noticed some distinct fibrillin-1 and fibronectin fibers (Fig. 4A, 6A). Considering our previous electron microscopy analysis and current immunofluorescence results, we investigated the colocalization of both proteins at the ultra-structural level in more detail. Double immunogold labeling was performed on extracellular fibrils produced by HSFs cultured for 7 days. As previously, we observed many fibers labeled with both, 12 nm gold particles representing fibronectin and 18 nm gold particles representing fibrillin-1 (Fig. 9 B–D, asterisk). However, we also noticed some fibers labeled solely for fibrillin-1 or for fibronectin (Fig. 9 A, B). These results corroborate our immunofluorescence, microfibril extraction and immunoprecipitation results where fibrillin-1 and fibronectin can be present in the same extracellular fibrils or can be localized to fibrils independent of the other protein.
Figure 9. Fibrillin-1 and fibronectin localize to the same and to different fibers.
Shown is a double-immunogold localization of extracellular fibrils produced by HSFs after 7 days in culture. 18-nm gold particles represent fibrillin-1 (black arrows) and 12-nm gold particles represent fibronectin (white arrows). The bar represents 0.2 µm for all images. Fibrillin-1 and fibronectin were sometimes present in the same fiber (asterisk, B–D). Other fibers contained either only fibrillin-1 (black arrowheads, A, B) or only fibronectin (white arrowheads, A, B).
DISCUSSION
Although a number of studies have investigated microfibril assembly, the mechanism of microfibril formation, as well as the components required to maintain their assembly, are still obscure. In the present study, we show that fibrillin interaction with fibronectin in a solid phase assay is non-ionic and sensitive to inhibition with FUD and monoclonal antibody to FNI8-9. We also demonstrate that the entire collagen/gelatin-binding domain of fibronectin, and not just FNI8-9, is required in the solid phase assay for optimal interaction with fibrillins. This latter finding is reminiscent of the multi-module interaction of type I collagen with fibronectin [55]. However, we uncovered the enigma that both FUD (Fig. 5) and gelatin [36] block the interaction between fibronectin and fibrillin in the solid phase assay whereas only FUD blocks deposition of fibrillin during short-term culture of HSFs. Using pulse treatment with FUD demonstrated that fibronectin and in turn fibrillin networks in short-term cultures are FUD-sensitive whereas these networks were unaffected in long-term cultures.
Increasing NaCl concentration did not inhibit the interaction of fibrillins with fibronectin, indicating that the dominant interaction between the proteins is not electrostatic. A covalent interaction appears unlikely as the FUD peptide (shown here) and gelatin [36] dissociate bound fibrillin-1 from fibronectin. Therefore, the data indicate that the interaction between fibrillins and fibronectin is primarily of hydrophobic nature.
All six modules of the collagen/gelatin-binding domain of fibronectin are required for full affinity interaction with gelatin [55,56]. In our study, we observed that the fibrillin C-terminal halves and the fibrillin-1 N-terminal half interact less well with smaller subfragments of the collagen/gelatin-binding domain of fibronectin. We also demonstrate that a monoclonal antibody that binds to FNI9 [45] inhibits interaction of the fibrillins to the collagen/gelatin-binding domain. Gelatin inhibits the fibrillin interaction with fibronectin in vitro [36]. Based on these results, we propose that fibrillins are like type I collagen in requiring all six modules of the collagen/gelatin-binding domain for complete interaction in vitro. However, the individual residues involved in both interactions must be different as the gelatin interaction with fibronectin was found to require essential charged residues [57], whereas we observed that the fibrillin interaction with fibronectin was insensitive to salt concentration.
Gelatin did not block assembly of fibronectin nor early assembly of fibrillin. Sottile et al. have shown that collagen type I fiber assembly requires the collagen/gelatin-binding domain of fibronectin [58]. Therefore, fibrillin assembly must be linked to fibronectin assembly via fibrillin-fibronectin interaction independent of those involving the collagen/gelatin binding region that dominate in the solid phase assay. Such interactions could be via other parts of fibronectin or unknown adapter molecules. A potential adapter could be heparan sulfate since both, fibrillin and fibronectin, interact directly with heparan sulfate and since the addition of exogenous heparin/heparan sulfate inhibits fibrillin-1 network assembly by HSFs [42,59,60]. However, several experiments support the idea of another fibrillin binding site in fibronectin. In our previous study, we observed that the N-terminal half of fibrillin-1 interacted with the FNIII1-C proteolytic fragment of fibronectin [36]. Kinsey et al. observed that two recombinant fibrillin-1 fragments, which are both contained in our rFBN1-N construct, interact in a region between FNIII12 and FNIII14 of fibronectin, as well as between FNI1 and FNI5 [35]. These authors also observed interactions of N-terminal recombinant fibrillin-1 fragments with a fibronectin region between domains FNIII7 and FNIII11. Further experiments are needed to investigate if those interactions outside the collagen/gelatin binding region are involved in early fibrillin-1 network assembly. Moreover, more experiments should define the functional significance of fibrillin interaction with the fibronectin collagen/gelatin-binding region (see also below).
In contrast to the results with gelatin, the FUD polypeptide disrupted formation of the fibronectin network and early fibrillin-1 network. FUD peptide has the potential to act through two distinct mechanisms. On the one hand, FUD prevents polymerization of fibronectin [49,61]. On the other hand, FUD inhibits the direct interaction between fibrillin-1 and fibronectin as shown in the present study by solid phase inhibition assays (Fig. 5). FUD forms a β-zipper structure with fibronectin FNI2-FNI5 modules of the fibrin-binding N-terminal domain and FNI8 and FNI9 of the collagen/gelatin-binding domain [45]. FUD binding to fibronectin disrupts fibronectin homotypic interactions and induces important conformational changes in fibronectin which extend as far as FNIII10 and expose the RGD integrin site in this domain [62]. As the FUD peptide does not interact directly with fibrillin-1 (Fig. S2), we suggest that upon binding to fibronectin, FUD induces conformational changes in fibronectin disrupting the interactions with fibrillin-1. However, as the fibronectin network disappeared after FUD treatment, FUD must act primarily through the former mechanism in our short term cell culture experiments. Regardless of which mechanism acts, we can conclude that the early fibrillin-1 network requires a stable fibronectin network for homeostasis. Remarkably, FUD did not inhibit fibronectin polymerization after pre-culturing of HSFs for three weeks (Fig. 6). We conclude that fibronectin fibers in these long-term cultures are more stable and resistant to FUD effects, likely due to more extensive cross-linking.
The finding that fibrillin-fibronectin dynamics are different after prolonged culture of HSFs prompted us to examine the localization of these proteins in vivo and in vitro in more detail. Following the extraction of microfibrils by two methods from HSF cultures and mouse tissues, we observed fibronectin co-eluting with fibrillin-1 in intermediate molecular weight fractions. Through immunoprecipitation, we demonstrated that fibronectin interacts directly or indirectly with fibrillin-1 in those fractions extracted from HSFs. We hypothesize that those fibronectin-containing fractions are intermediate or immature (nascent) fibrillin-1 fibers. In the same experiments, we demonstrated that fibronectin co-eluted in the void volume with the extractable mature microfibril peak from cell cultures as well as mouse tissues regardless of the extraction method used. Immunoprecipitations demonstrated that fibronectin interacts with these more mature microfibrils. Those results are supported by our electron microscopy data in which we observed fibrillin-1 and fibronectin frequently (but not always) colocalizing to the same extracellular fibers produced by HSFs.
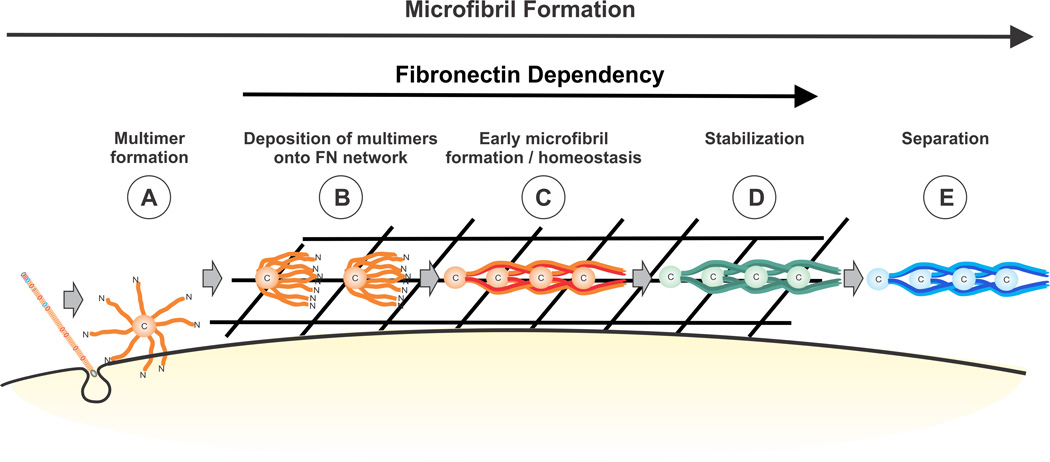
The results of this study favor the model shown in Fig. 10. We propose that fibrillins require the assistance of fibronectin for initial early assembly and for the homeostasis of immature fibers, which likely corresponds to the intermediate molecular weight fractions (Fig. 7). As microfibril maturation proceeds, the fibers are progressively larger in size, such as the microfibrils present in the void volume of the extraction column (Fig. 7) and observed by electron microscopy (Fig. 9). These maturing microfibrils lose the requirement for a fibronectin network even though fibronectin remains associated with fibrillin. One transglutaminase-mediated cross-link has been identified in fibrillin-1 from isolated microfibrils and the study suggested that many more (10–15%) of the lysine residues in fully developed microfibrils are involved in crosslinks [63]. The exact time course for this cross-linking is currently unknown. Interestingly, tissue transglutaminase interacts with fibronectin via the collagen/gelatin binding region from which it acts upon glutamine residues at the N-terminus of fibronectin [48]. It is possible that a complex between fibronectin and tissue transglutaminase serves to mediate cross-links in fibrillin-1 organized in mature microfibrils. Given the ability of fibrillin-1 to strongly interact with fibronectin’s collagen/gelatin binding region, we speculate that fibrillin-1 may be able to compete with the binding site of tissue transglutaminase on fibronectin to regulate cross-link formation in microfibrils. As maturation through cross-link formation proceeds, microfibrils become stable enough to become independent of the fibronectin network and constitute the microfibrils only labeled with fibrillin-1 (Fig. 9 A, B, Fig. 4 A and 6 A). With the current study, we provide new evidence of the complex roles of fibronectin as a “master stabilizer” of early fibrillin-1 extracellular matrix and contribute to the understanding of fibrillin matrix turnover and remodeling.
Figure 10. Hypothetical model describing the stages of microfibril assembly that depend on the presence of fibronectin (involvement of glycosaminoglycans is omitted for simplicity).
A: Fibrillin monomers become secreted and are multimerized in a cell-associated manner via their C-terminal ends. B: Fibrillin multimers associate with fibronectin fibers for concentration and alignment. C: Early microfibrils form through N-to-C-terminal interactions of the beads. D: Stabilization likely requires initial formation of cross-links. At this stage microfibrils can still be observed in association with fibronectin. E: Once when microfibrils are fully stabilized via transglutaminase and disulfide crosslinks, they become independent from fibronectin.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Jean Martin Laberge (Montreal Children’s Hospital) for providing clinical samples, and Ms. Amelie Pagliuzza for critically reading the manuscript.
FUNDING
This work was supported by the Canadian Institutes of Health Research [MOP-106494], the Natural Sciences and Engineering Research Council of Canada [RGPIN 375738-09], the Canada Foundation for Innovation, the National Institutes of Health [HL021644] and the Network for Oral and Bone Health Research (PhD student scholarship to LS).
ABBREVIATIONS USED
- FN
fibronectin
- FBN
fibrillin
- FUD
functional upstream domain
- HSF
human skin fibroblast
- int. MW
intermediate molecular weight
- low MW
low molecular weight
- LTBP
latent transforming growth factor-beta binding protein
- MF
microfibrils
- OD
optical density
- PBS-G
PBS-goat serum
- RGD
Arginine-Glycine-Aspartic acid
- TBST
TBS-Tween 20
REFERENCES
- 1.Pereira L, D'Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum. Mol. Genet. 1993;2:1762. doi: 10.1093/hmg/2.10.1762. [DOI] [PubMed] [Google Scholar]
- 2.Hubmacher D, Reinhardt DP. In: Biology of Extracellular Matrix. Mecham RP, editor. New York: Springer; 2011. pp. 233–265. [Google Scholar]
- 3.Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J. Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30:43–52. doi: 10.1016/j.matbio.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright DW, Mayne R. Vitreous humor of chicken contains two fibrillar systems: an analysis of their structure. J. Ultrastruct. Mol. Struct. Res. 1988;100:224–234. doi: 10.1016/0889-1605(88)90039-0. [DOI] [PubMed] [Google Scholar]
- 7.Keene DR, Maddox BK, Kuo HJ, Sakai LY, Glanville RW. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrilsJHistochem. Cytochem. 1991;39:441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RN, Streeten BW, Hanna RB. Rotary shadowing of elastic system microfibrils in the ocular zonule, vitreous, and ligament nuchae. Curr. Eye Res. 1991;10:99–109. doi: 10.3109/02713689109007614. [DOI] [PubMed] [Google Scholar]
- 9.Low FN. Microfibrils: fine filamentous components of the tissue space. Anat. Rec. 1962;142:131–137. doi: 10.1002/ar.1091420205. [DOI] [PubMed] [Google Scholar]
- 10.Mecham RP, Davis E. In: Extracellular Matrix Assembly and Structure. Yurchenco PD, Birk DE, Mecham RP, editors. New York: Academic Press; 1994. pp. 281–314. [Google Scholar]
- 11.Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. Invest. Ophthalmol. 1971;10:851–869. [PubMed] [Google Scholar]
- 13.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 14.Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J. Biol. Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 16.Robinson P, Arteaga-Solis E, Baldock C, Collod-Beroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, Kielty CM, Loeys B, Milewicz DM, Ney A, Ramirez F, Reinhardt DP, Tiedemann K, Whiteman P, Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J. Med. Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maslen CL, Corson GM, Maddox BK, Glanville RW, Sakai LY. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991;352:334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- 18.Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, Chillakuri CR, Macaya D, Coucke PJ, De Paepe A, Judge DP, Wigley F, Davis EC, Mardon HJ, Handford P, Keene DR, Sakai LY, Dietz HC. Mutations in Fibrillin-1 Cause Congenital Scleroderma: Stiff Skin Syndrome. Sci. Transl. Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le MM, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J. Med. Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat. Genet. 1995;11:456–458. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- 21.Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PO, Kitoh H, Krakow D, Lynch SA, Le MM, Megarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFbeta binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankov R, Yamada KM. Fibronectin at a glance. J. Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 23.Hynes R. Molecular biology of fibronectin. Annu. Rev. Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- 24.Keski-Oja J, Mosher DF, Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem. Biophys. Res. Commun. 1977;74:699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- 25.Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J. Biol. Chem. 1983;258:14539–14544. [PubMed] [Google Scholar]
- 26.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J. Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebres RA, McKeown-Longo PJ, Vincent PA, Cho E, Saba TM. Extracellular matrix incorporation of normal and NEM-alkylated fibronectin: liver and spleen deposition. Am. J. Physiol. 1995;269:G902–G912. doi: 10.1152/ajpgi.1995.269.6.G902. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 1982;92:485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman J, McDonald JA. Fibulin's organization into the extracellular matrix of fetal lung fibroblasts is dependent on fibronectin matrix assembly. Am. J. Respir. Cell Mol. Biol. 1993;8:538–545. doi: 10.1165/ajrcmb/8.5.538. [DOI] [PubMed] [Google Scholar]
- 33.Godyna S, Mann DM, Argraves WS. A quantitative analysis of the incorporation of fibulin-1 into extracellular matrix indicates that fibronectin assembly is required. Matrix Biol. 1995;14:467–477. doi: 10.1016/0945-053x(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 34.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 35.Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J. Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- 36.Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol. Biol. Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubmacher D, El-Hallous E, Nelea V, Kaartinen MT, Lee ER, Reinhardt DP. Biogenesis of extracellular microfibrils-Multimerization of the fibrillin-1 C-terminus into bead-like structures enables self-assembly. Proc. Natl. Acad. Sci. USA. 2008;105:6548–6553. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J. Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 41.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiedemann K, Bätge B, Müller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate: Implications for microfibrillar assembly. J. Biol. Chem. 2001;276:36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- 43.Tiedemann K, Sasaki T, Gustafsson E, Göhring W, Bätge B, Notbohm H, Timpl R, Wedel T, Schlötzer-Schrehardt U, Reinhardt DP. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J. Biol. Chem. 2005;280:11404–11412. doi: 10.1074/jbc.M409882200. [DOI] [PubMed] [Google Scholar]
- 44.Lin G, Tiedemann K, Vollbrandt T, Peters H, Bätge B, Brinckmann J, Reinhardt DP. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J. Biol. Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- 45.Maurer LM, Tomasini-Johansson BR, Ma W, Annis DS, Eickstaedt NL, Ensenberger MG, Satyshur KA, Mosher DF. Extended binding site on fibronectin for the functional upstream domain (FUD) of protein F1 of Streptococcus pyogenes. J. Biol. Chem. 2010;285:41087–41099. doi: 10.1074/jbc.M110.153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen SA, Reinhardt DP, Gibson MA, Weiss AS. MAGP-1, Protein interaction studies with tropoelastin and fibrillin-1. J. Biol. Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- 47.Mosher DF, Schad PE. Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J. Clin. Invest. 1979;64:781–787. doi: 10.1172/JCI109524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann BR, Annis DS, Mosher DF. Reactivity of the amino-terminal region of fibronectin to transglutaminase 2 and factor XIIIa. J. Biol. Chem. 2011;286:32220–32230. doi: 10.1074/jbc.M111.255562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomasini-Johansson BR, Kaufman NR, Ensenberger MG, Ozeri V, Hanski E, Mosher DF. A 49-residue peptide from adhesin F1 of Streptococcus pyogenes inhibits fibronectin matrix assembly. J. Biol. Chem. 2001;276:23430–23439. doi: 10.1074/jbc.M103467200. [DOI] [PubMed] [Google Scholar]
- 50.El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Bätge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adapter function to tropoelastin. J. Biol. Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- 51.Kuo CL, Isogai Z, Keene DR, Hazeki N, Ono RN, Sengle G, Bächinger HP, Sakai LY. Effects of fibrillin-1 degradation on microfibril ultrastructure. J. Biol. Chem. 2007;282:4007–4020. doi: 10.1074/jbc.M606370200. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh P, Segal R, Chen LB. Studies of fibronectin matrices in living cells with fluoresceinated gelatin. J. Cell Biol. 1980;87:14–22. doi: 10.1083/jcb.87.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ensenberger MG, Annis DS, Mosher DF. Actions of the functional upstream domain of protein F1 of Streptococcus pyogenes on the conformation of fibronectin. Biophys. Chem. 2004;112:201–207. doi: 10.1016/j.bpc.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Kielty CM, Cummings C, Whittaker SP, Shuttleworth CA, Grant ME. Isolation and ultrastructural analysis of microfibrillar structures from foetal bovine elastic tissues. J. Cell Sci. 1991;99:797–807. doi: 10.1242/jcs.99.4.797. [DOI] [PubMed] [Google Scholar]
- 55.Erat MC, Sladek B, Campbell ID, Vakonakis I. Structural analysis of collagen type I interactions with human fibronectin reveals a cooperative binding mode. J. Biol. Chem. 2013;288:17441–17450. doi: 10.1074/jbc.M113.469841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katagiri Y, Brew SA, Ingham KC. All six modules of the gelatin-binding domain of fibronectin are required for full affinity. J. Biol. Chem. 2003;278:11897–11902. doi: 10.1074/jbc.M212512200. [DOI] [PubMed] [Google Scholar]
- 57.Vuento M, Salonen E, Osterlund K, Stenman UH. Essential charged amino acids in the binding of fibronectin to gelatin. Biochem. J. 1982;201:1–8. doi: 10.1042/bj2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sottile J, Shi F, Rublyevska I, Chiang HY, Lust J, Chandler J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am. J. Physiol. Cell Physiol. 2007;293:C1934–C1946. doi: 10.1152/ajpcell.00130.2007. [DOI] [PubMed] [Google Scholar]
- 59.Vaheri A, Mosher DF. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim. Biophys. Acta. 1978;516:1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- 60.Ritty TM, Broekelmann TJ, Werneck CC, Mecham RP. Fibrillin-1 and -2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem. J. 2003;375:425–432. doi: 10.1042/BJ20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomasini-Johansson BR, Annis DS, Mosher DF. The N-terminal 70-kDa fragment of fibronectin binds to cell surface fibronectin assembly sites in the absence of intact fibronectin. Matrix Biol. 2006;25:282–293. doi: 10.1016/j.matbio.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ensenberger MG, Tomasini-Johansson BR, Sottile J, Ozeri V, Hanski E, Mosher DF. Specific interactions between F1 adhesin of Streptococcus pyogenes and N-terminal modules of fibronectin. J. Biol. Chem. 2001;276:35606–35613. doi: 10.1074/jbc.M105417200. [DOI] [PubMed] [Google Scholar]
- 63.Qian RQ, Glanville RW. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry. 1997;36:15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.