Abstract
Introduction
Ischemic stroke has been associated with stunned myocardium and neurogenic pulmonary edema (NPE). We studied a population of patients with large vessel brainstem ischemic stroke to see if there was an increased risk of pulmonary edema associated with strokes in this region independent of myocardial stunning.
Hypothesis
Large vessel ischemic strokes of the brainstem are associated with neurogenic pulmonary edema and occur independently of myocardial stunning.
Methods
This is a retrospective case control study of 1,278 patient admissions. Two hundred ten patients were identified with large vessel ischemic stroke or transient ischemic attack (mean age 65 years, 55% female, 50% black). Infarction locations included: brainstem (N=22), right middle cerebral artery involving the insula (N=38), left middle cerebral artery involving the insula (N=37), and transient ischemic attack (N=113). Multivariate logistic regression models for presence of echocardiographic wall motion abnormalities, QTc-interval prolongation, elevated serum troponin, and pulmonary edema were developed to examine the relative contribution of stroke location and markers of cardiopulmonary dysfunction to each respective outcome, controlling for patient characteristics.
Results
Large vessel brainstem stroke was associated with pulmonary edema (adjusted OR 29.23, 95% CI 1.90–449.51) but not cardiac abnormalities. Large vessel left middle cerebral artery stroke was also associated with pulmonary edema (76.44, 6.93–843.54) as well as QTc-interval prolongation (4.55, 10.77–19.24). Large vessel right middle cerebral artery stroke was associated with pulmonary edema (10.88, 1.02–116.70) as well as elevated serum troponin (10.51, 1.71–64.82).
Conclusion
In a retrospective case control study, large vessel brainstem stroke was associated with the development of pulmonary edema independent of cardiac abnormalities associated with myocardial stunning, suggesting a separate brainstem pathophysiologic mechanism which directly affects the lungs but not the heart.
Keywords: Ischemic stroke, Pulmonary edema, Myocardial stunning
INTRODUCTION
Over 790,000 strokes occur annually in the United States, making it the fourth leading cause of death and the leading cause of disability in people over the age of 65 [1]. Pulmonary and cardiac complications after stroke are common. The association of ischemic stroke with electrocardiographic change and elevated cardiac enzymes has been known for decades [2–4]. The classic triads of findings for neurogenic myocardial stunning are transient left ventricular wall motion abnormalities, electrocardiographic abnormalities and elevation in myocardial enzymes in the serum in the absence of coronary artery disease [5]. The physiological mechanisms underlying these associations are not fully understood. They are thought to involve sympathetic hyperactivity and possibly anatomic inhibition due to insular cortical injury [6–8]. Localization of ischemic stroke to the insula and the parietal lobe has been associated with fatal arrhythmias in animal models and human studies [7–9].
Respiratory failure occurs in 5–10% of patients with acute ischemic stroke, most often secondary to aspiration and decreased airway protection. Pulmonary parenchymal disease has been observed as a direct consequence of centrally mediated injury due to neurogenic pulmonary edema (NPE) [9,10]. NPE has been noted as a complication of a variety of neurological syndromes, including basilar artery thrombosis and intracerebral hemorrhage [9–11]. The anterior and posterior cerebrovascular distributions have both been implicated in NPE [9,11,12].
It is unknown how often acute pulmonary edema noted in the setting of ischemic stroke occurs independently or as a result of cardiac dysfunction. It is also unknown if there is a relationship of particular cerebrovascular distributions to particular patterns of cardiac and/or pulmonary dysfunction in ischemic stroke. Here we describe a retrospective review of patients treated at a tertiary stroke center for large vessel ischemic stroke, to explore the association of the cerebrovascular distribution of infarction to pulmonary edema and cardiac function derangements as measured by electrocardiography, elevated cardiac enzymes and echocardiography. We hypothesized that we would find an association between large vessel ischemia in the brainstem and pulmonary edema occurring independently of cardiac abnormalities associated with myocardial stunning.
PATIENTS AND METHODS
A retrospective review was performed on all patients admitted or transferred to the cerebrovascular neurology service at Johns Hopkins Hospital from June 2009 through June 2011. Clinical data obtained included: age; gender; race; and medical history of diabetes, hypertension, hypercholesterolemia, smoking, arrhythmia, atrial fibrillation, coronary artery disease, heart failure, diabetes and prior stroke or transient ischemic attack (TIA). Past medical history was recorded as per documentation in the medical record at time of admission.
All patients >17 years of age admitted to the Johns Hopkins Hospital cerebrovascular service were included, whether or not they died while admitted. Patients were included if they had a discharge diagnosis of TIA (control group) [13]; ischemic stroke of the right or left middle cerebral artery (RMCA or LMCA) ; or basilar artery ischemic stroke, stenosis or occlusion. All other patients were excluded, including those who had ischemic strokes in multiple cerebrovascular distributions. To ensure inclusion of patients with large vessel middle cerebral artery infarction, of those patients with discharge diagnosis of ischemic stroke of the RMCA or LMCA, only those patients with radiographic findings consistent with infarction of the insular cortex were included. To ensure inclusion of patients with large vessel brainstem infarctions, of those patients with discharge diagnosis of basilar artery ischemic stroke, stenosis or occlusion, only those with radiographic findings consistent with infarction within the midbrain, pons and/or medulla in the setting of basilar artery stenosis or occlusion were included.
Imaging and laboratory data included: echocardiogram reports; electrocardiogram (EKG) reports upon admission and 48 hours after admission; initial and peak serum troponin levels; chest radiographs during admission. Heart failure was defined as an ejection fraction of ≤ 35% as measured by echocardiogram [14]. Cardiac wall motion abnormalities were documented by cardiologist-based interpretation of echocardiogram. Troponinemia was defined as >0.06 ng/mL, per institutional clinical laboratory designation. Nonspecific ST segment abnormalities and arrhythmias were determined from the final cardiologist report of EKG. Corrected QT (QTc) interval prolongation was defined as >460 ms per EKG report. The presence or absence of pulmonary edema was determined by review of chest radiographs within 96 hours of admission, when available. These were reviewed by physician reviewers blinded to patient diagnosis and study hypothesis (T. C. and D. V.) using previously established criteria [15].
STATISTICAL ANALYSIS
For continuous variables, medians with interquartile range (IQR) and means with standard deviations (SD) were calculated. For categorical variables, frequencies were measured. Group differences for continuous variables were tested by one-way analysis of variance with Bonferroni’s adjustment for multiple comparisons. The χ2 test for independence or Fisher’s exact test was used to examine group differences for categorical variables. Univariate analyses were performed to assess for significant risk factors for development of pulmonary edema. Multivariate logistic regression analyses were performed with outcomes of interest (i.e. pulmonary edema, echocardiographic wall motion abnormalities, presence of elevated serum troponin, and QTc-interval prolongation) serving as the dependent variables. When clinical data was missing, the documented diagnosis was used based on clinical examination or report of clinical data at time of admission.
For each multivariate logistic regression model, patient characteristics including age ≥ 57 years [16], history of atrial fibrillation, history of coronary artery disease, history of diabetes mellitus, history of heart failure, history of hypertension, history of smoking, and stroke location were included as independent variables and controlled for. In addition, diagnostic results indicative of cardiopulmonary dysfunction including presence of wall motion abnormalities on echocardiogram, arrhythmia on admission EKG, QTc prolongation on admission EKG, presence of pulmonary edema, and elevated serum troponin were included and controlled for as independent variables except when the dependent variable of interest for a respective multiple logistic regression model. Collinearity diagnostics were performed to assess for intercorrelations among independent variables. The amount of variation in the dependent variable explained by each respective model was assessed using Cox & Snell R Square and the Nagelkerke R Square tests. Goodness-of-fit of each multivariate logistic regression model was assessed by Hosmer and Lemeshow’s test (H-L). Significance of regression coefficients to respective logistic regression models were assessed using Wald’s test. Two-tailed statistical significance was assessed at the p<0.05 level. All statistical analyses were performed using the SPSS (version 22, IBM, Armonk, NY) statistical package while figures were made using the Prism (version 5, GraphPad, San Diego, CA) statistical package.
RESULTS
Clinical characteristics
We completed a review of 1,278 patient records. There were 210 patients with large vessel ischemic stroke or TIA identified and included in our analysis. Of these patients, 55% were female, 50% were black, 75% had a history of hypertension and their average age was 63.5 years (SD 15.9). Twenty-two patients had brainstem stroke in the setting of basilar occlusion or stenosis, 38 patients had large vessel right middle cerebral artery (RMCA) strokes involving the insula, 37 patients had large vessel left middle cerebral artery (LMCA) strokes involving the insula, and 113 TIA control patients were identified. The large vessel brainstem stroke group included two patients with isolated medullary infarct, ten patients with isolated pontine infarct, and ten with infarction of multiple brainstem structures. With the exception of history of atrial fibrillation (χ2 (3,n=208) =14.75, p=0.002) and history of heart failure (χ2 (3,n=208) =10.72, p=0.01), patient groups were similar in terms of age; gender; race; history of prior stroke or TIA, diabetes, smoking and hypercholesterolemia (Table 1).
Table 1. Patient Characteristics.
Means (SD) are presented for the continuous variable “age”, and N(%) presented for all other categorical variables. Group differences for continuous variables were tested by one-way analysis of variance with Bonferroni’s adjustment for multiple comparisons, with p-value presented. The χ2 test for independence was used to examine group differences for categorical variables with p-value presented.
| TIA (N=113) | R MCA (N=38) | L MCA (N=37) | Brainstem (N=22) | Test Result | |
|---|---|---|---|---|---|
| Age | 63 (23) | 71 (24) | 65 (33) | 65 (12) | p=0.87 |
| Female Sex | 70 (62%) | 21 (55%) | 15 (41%) | 10 (46%) | p=0.11 |
| African American | 57 (50%) | 18 (47%) | 16 (43%) | 13 (59%) | p=0.68 |
| History of Stroke or TIA | 46 (41%) | 12 (32%) | 9 (25%) | 7 (32%) | p=0.34 |
| History Atrial Fibrillation | 6 (5%) | 10 (27%) | 7 (19%) | 2 (9%) | p=0.002 |
| History of Heart Failure | 8 (7%) | 9 (24%) | 8 (22%) | 2 (9%) | p=0.01 |
| History of Diabetes | 30 (27%) | 8 (22%) | 9 (25%) | 10 (46%) | p=0.22 |
| History of Hypertension | 84 (74%) | 28 (76%) | 26 (70%) | 20 (91%) | p=0.33 |
| History of Smoking | 47 (42%) | 18 (47%) | 15 (41%) | 11 (50%) | p=0.82 |
| History of Hypercholesterolemia | 47 (42%) | 13 (35%) | 11 (31%) | 12 (55%) | p=0.29 |
Pulmonary evaluation
Chest radiographs were available for review for 65 TIA, 28 RMCA, 31 LMCA, 19 brainstem stroke patients. The presence of pulmonary edema was determined based on review of available chest radiographs within 96 hours of admission [15]. For all other patients, pulmonary edema assessment was based on pulmonary examinations and available chest radiograph reports at time of admission.
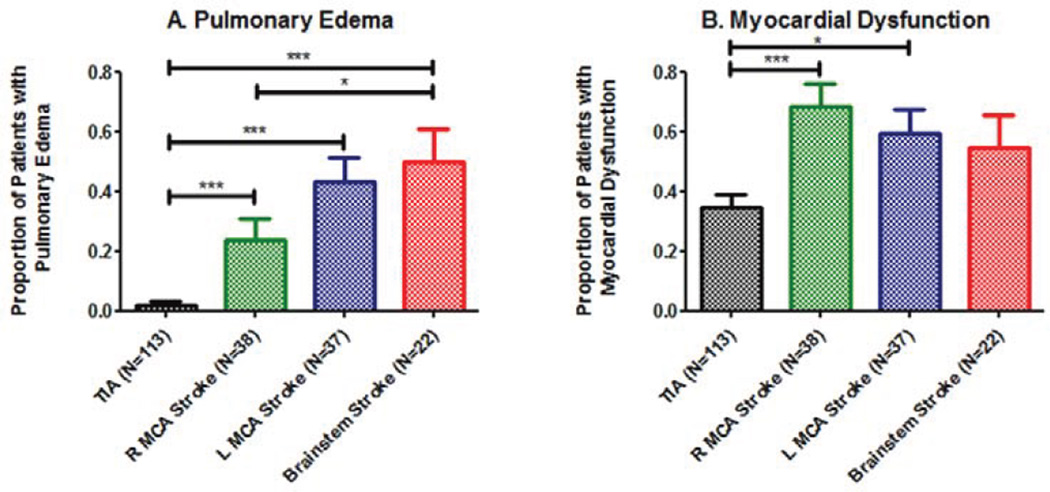
The proportion of patients with pulmonary edema varied across the four groups (χ2 (3, N=210) =52.02, p<0.0001, Figure. 1A). A greater proportion of brainstem (0.50) and LMCA (0.43) stroke patients had pulmonary edema compared to both the TIA control (0.02) and RMCA stroke (0.24) groups.
Figure 1. Cardiopulmonary Response by Ischemic Stroke Location.
A) Proportion of patients with pulmonary edema varied across stroke groups and was greatest for patients with large vessel brainstem stroke. B) Myocardial dysfunction was defined as a composite outcome of wall motion abnormalities on echocardiogram, elevated serum troponin, arrhythmia on admission EKG, and/or QTc-interval prolongation on admission EKG. Myocardial dysfunction also varied in proportion across stroke groups, and was greatest among patients with large vessel left middle cerebral artery or right cerebral artery strokes involving the insula relative to control and large vessel brainstem stroke patients. * designates p<0.05 and *** designates p<0.001 by Fisher’s exact test.
Of the potential predictors for presence of pulmonary edema after large vessel ischemic stroke, only patient age ≥57 years, patient history of atrial fibrillation, patient history of heart failure, stroke location and elevated serum troponin were predictive (Table 2). The multivariate logistic regression model for presence of pulmonary edema explained between 36.7% and 53.5% of the variance in development of pulmonary edema, correctly classifying 81.4% of cases compared to baseline prediction rate of 73.5%, and fit the observed data well (H-L χ2 (8) =5.50,p=0.70) without evidence of intercorrelations among the independent variables. As shown in Table 3, having a LMCA stroke (adjusted OR 76.44, 95% CI 6.93–843.54), a RMCA stroke (10.88, 1.02–116.70) or a brainstem stroke (29.23, 1.90–449.51) was predictive of development of pulmonary edema.
Table 2. Univariate Analyses of Potential Predictors for Presence of Pulmonary Edema.
The χ2 test for independence or Fisher’s exact test was used to examine group differences for categorical variables, with p-value presented.
| Pulmonary Edema Present | Pulmonary Edema Not Present | Test Result | |
|---|---|---|---|
| Patient Factors | |||
| Age≥57 (N=143) | 31 (21.7%) | 112 (78.3%) | p=0.05 |
| History Atrial Fibrillation (N=25) | 10 (40.0%) | 15 (60.0%) | p=0.004* |
| History of Coronary Artery Disease (N=41) | 8 (19.5%) | 33 (80.5%) | p=0.75 |
| Prior History of Heart Failure (N=27) | 11 (40.7%) | 16 (59.3%) | p=0.002* |
| History of Diabetes (N=57) | 11 (19.3%) | 46 (80.7%) | p=0.73 |
| History of Hypertension (N=158) | 30 (19.0%) | 128 (81.0%) | p=0.39 |
| History of Smoking (N=91) | 15 (16.5%) | 76 (83.5%) | p=0.60 |
| Stroke Location (TIA, RMCA, LMCA, Basilar) | p<0.001 | ||
| TIA (N=113) | 2 (1.8%) | 111 (98.2%) | |
| RMCA (N=38) | 9 (23.7%) | 29 (76.3%) | |
| LMCA (N=37) | 16 (43.2%) | 21 (56.8%) | |
| Basilar (n=22) | 11 (50.0%) | 11 (50.0%) | |
| Echocardiogram during Admission | |||
| Wall Motion Abnormality (N=32) | 9 (28.1%) | 23 (71.9%) | p=0.22 |
| Elevated Serum Troponin during admission (>0.06 ng/mL; N=27) | 14 (51.9%) | 13 (48.1%) | p<0.001** |
| EKG on Admission | |||
| Arrhythmia (N=26) | 8 (30.8%) | 18 (69.2%) | p=0.11* |
| QTc Prolongation (>460ms; N=62) | 16 (25.8%) | 46 (74.2%) | p=0.10 |
designates those factors examined by Fisher’s exact test. History of hypercholesterolemia (p=0.65), ejection fraction of ≤ 35% on echocardiogram (p=0.26), new or worsened heart failure (p=0.29*), PR interval prolongation on initial electrocardiogram (0.33*), ST segment abnormality on initial electrocardiogram (p=0.77), and delayed R wave progression on initial electrocardiogram (p=0.57*) were not significant predictors for the presence on pulmonary edema.
designates that significant difference in proportions noted in performing χ2 test reflects difference in proportions of patients with pulmonary edema (N=17, 16.2%) and without pulmonary edema (N=88, 83.8%) in the setting of normal serum troponin (N= 105) compared to as seen in the setting of elevated serum troponin (N=27).
Table 3. Multivariate Logistic Regression Model for Prediction of Pulmonary Edema.
Stroke location, with TIA serving as the reference category, contributed significantly to the multivariate logistic regression model predictive of development of the presence or absence of pulmonary edema. Age≥57 years (adjusted OR 1.40; 95% CI 0.25–7.68), history of atrial fibrillation (1.81, 0.28–11.60), history of coronary artery disease (0.93, 0.18–4.87), history of diabetes mellitus (0.41, 0.09–1.76), history of heart failure (5.22, 0.94–28.96), history of hypertension (1.78, 0.34–9.24), history of smoking (0.43, 0.10–1.86), wall motion abnormality noted on echocardiogram during admission (0.50, 0.11–2.33), troponinemia (3.42, 0.79–14.71), presence of arrhythmia on admission EKG (0.39, 0.05–2.77) and QTc prolongation (>460ms) on admission EKG (0.75, 0.18–3.17) did not contribute significantly to this multivariate logistic regression model.
| Adjusted Odds Ratio | 95% Confidence Interval | P | |
|---|---|---|---|
| Stroke Location | |||
| RMCA | 10.88 | 1.02–116.70 | 0.049 |
| LMCA | 76.44 | 6.93–843.54 | <0.001 |
| Basilar | 29.23 | 1.90–449.51 | 0.02 |
Echocardiography
Echocardiograms were performed on 83 TIA, 35 RMCA, 31 LMCA and 14 brainstem stroke patients. There was no difference in proportion of patients with an ejection fraction ≤ 35% (χ2 (3, N=167) =3.93, p=0.27). Similarly, there was no difference in proportion of patients with new diagnosis of heart failure or decreased ejection fraction from baseline across groups (χ2 (3, N=167) =5.15, p=0.16). However, the proportion of patients with cardiac wall motion abnormalities on echocardiogram by cardiologist interpretation varied across groups (χ2 (3, N=163) =9.04, p=0.03) with a greater proportion of patients with RMCA stroke (0.34) than TIA (0.13) and brainstem stroke (0.07). There was no difference in proportions between the RMCA and LMCA (0.26) stroke groups.
The multivariate model for echocardiographic wall motion abnormalities explained between 31.4% and 45.8% of the variance in having cardiac wall motion abnormalities on echocardiogram, correctly classifying 83.3% of cases, compared to baseline prediction rate of 73.5%, and fit the observed data well (H-L χ2 (8) =9.20, p=0.33) without evidence of intercorrelations among the independent variables. Stroke location made no significant contribution to the model. Only patient history of heart failure (adjusted OR 9.63, 95% CI 2.17–42.78) and patient history of coronary artery disease (8.44, 2.04–34.93) were predictive of the presence of wall motion abnormalities on echocardiogram during admission.
Serum troponin
Serum troponin levels were measured for 65 TIA control patients, 27 RMCA, 28 LMCA and 12 brainstem stroke patients and varied significantly across groups (χ2 (3, N=210) =20.43, p<0.0001). A smaller proportion of TIA patients (0.05) had elevated serum troponin levels than RMCA (0.41), LMCA (0.32) and brainstem (0.33) stroke patients.
The multivariate logistic regression model for presence of elevated serum troponin explained between 26.8% and 40.8% of the variance in having an elevated serum troponin, correctly classifying 84.3% of cases compared to baseline prediction rate of 77.5%, while fitting the data well (H-L χ2 (8) =4.71,p=0.79) without evidence of intercorrelations among the independent variables. Only having a RMCA stroke (adjusted OR 10.51, 95% CI 1.71–64.82) was predictive of having an elevated serum troponin level.
Electrocardiography
Electrocardiograms were performed on 106 TIA control, 37 RMCA, 36 LMCA and 22 brainstem stroke patients. The proportion of patients with prolonged QTc-intervals (>460ms) was different across groups (χ2 (3, N=201) =14.92, p=0.002), with the proportion within both the RMCA (0.43) and LMCA (0.50) stroke groups being greater than for the TIA (0.20) and brainstem stroke (0.32) groups. The proportion of patients with prolonged PR-intervals (>200ms; χ2 (3, N=178) =7.62, p=0.06), arrhythmia (χ2 (3, N=201) =6.46, p=0.09), abnormal intraventricular conduction (χ2 (3, N=201) =2.58, p=0.46), or any ST-segment abnormality (χ2 (3, N=201) =0.91, p=0.82) did not vary across groups.
The multivariate logistic regression model for presence of a prolonged QTc-interval on admission EKG explained between 26.3% and 36.0% of the variance in having a prolonged QTc-interval, correctly classifying 73.5% of cases compared to baseline prediction rate of 63.7%, while fitting the data well (H-L χ2 (8) =8.09,p=0.43) without evidence of intercorrelations among the independent variables. Only having a LMCA stroke (adjusted OR 4.55, 95% CI 10.77–19.24) was predictive of having QTc-interval prolongation.
Myocardial dysfunction
The proportion of patients with either wall motion abnormalities on echocardiogram, elevated serum troponin, arrhythmia on admission EKG and/or prolonged QTc-interval on admission EKG varied across groups (χ2 (3, N=210) =16.87, p=0.001). Both RMCA (0.68) and LMCA (0.60) stroke groups had proportions greater than brainstem stroke (0.55) and TIA (0.35) groups (Figure 1B).
DISCUSSION
In a limited retrospective case control study of patients treated at a single tertiary medical center, large vessel ischemic stroke of the midbrain, pons, and/or medulla was associated with the development of pulmonary edema independent of evidence of cardiac abnormalities typically associated with myocardial stunning. In contrast, both large vessel RMCA and LMCA strokes with involvement of respective insular cortex were associated with pulmonary edema as well as cardiac abnormalities associated with myocardial stunning [5]. This suggests separate brainstem pathophysiological mechanisms for pulmonary edema with effects on the lungs but not the heart.
Neurogenic pulmonary edema and cardiac derangements, including stunned myocardium, have long been associated with increased mortality in a variety of neurological diseases, from seizures and trauma to hemorrhagic and ischemic stroke [9,17]. Catecholamine surge in the setting of injury to the diencephalon and brainstem is thought to cause activation of both α- and β-adrenergic receptors within both the pulmonary venous bed and myocardium, and neurogenic pulmonary edema independent of myocardial dysfunction has been felt to be a relatively rare phenomenon [9,10]. Specifically, sympathetic activation has been suggested to play a primary role in precipitating raised hydrostatic pressure and elevated vascular permeability in the pulmonary venous bed, similar to that seen in acute respiratory distress syndrome (ARDS) [11,18–21]. Here we present findings which suggest that in ischemic stroke of the brainstem, pulmonary edema can occur in the absence of cardiac derangement and stunned myocardium.
The relationship of stroke location (i.e. whether of the anterior or posterior circulation, laterality) to the development of cardiac derangements and neurogenic pulmonary edema following stroke is an open question. Cortical involvement in the development of cardiac derangements (e.g. dysrhythmia, QTc prolongation, heart failure) following ischemic stroke has been proposed to rely on connectivity of cortical regions, such as the medial prefrontal cortex and insular cortex, with the hypothalamus, midbrain, pons and medulla [22]. The laterality of infarction, whether right hemispheric or left, has been of unclear significance to the development of cardiac derangements and sudden death [23–25]. Similarly, direct injury to the hypothalamus, nucleus tractus solitarius and area postrema and their respective sympathetic projections to the hypothalamus and spinal cord have been implicated in the generation of isolated neurogenic pulmonary edema [9]. In animal model experiments, severe hypertension and neurogenic pulmonary edema have been induced by lesions to the hypothalamus as well as by irritation of the tractus solitarius nuclei of the medulla while increased cardiac output, peripheral vascular resistance and hypertension have been induced by unilateral stimulation of the area postrema of the medulla [9,26,27]. In animal models using phentolamine, an alpha-adrenergic antagonist active at the postganglionic adrenergic receptors, pulmonary edema has been prevented [26,27]. Hexamethonium, a preganlionic nicotinic acetylcholine receptors blocker caused reduction of serum catecholamine levels, reversal of hypertension, preservation of cardiac function, and prevention of hemorrhagic pulmonary edema after pharmacological ablation of the nucleus tractus solitarius [28].
There are isolated case reports of treatment responsive neurogenic pulmonary edema treated with phentolamine to interrupt cyclical hemodynamic instability and hypoxic respiratory failure. This has been reported in the setting of progressively declining urine catecholamine levels [29]. Fulminant neurogenic pulmonary edema has also been treated with prone positioning in ventilated patients [11].
These experimental and clinical observations suggest cardiopulmonary compromise in the setting of central nervous system injury. This study suggests neurogenic pulmonary edema in ischemic stroke can occur in the setting of large vessel infarction of the brainstem. This occurs independently of concomitant cardiac involvement as indicated by elevated serum troponin levels, EKG changes, and altered wall motion on echocardiography. This pattern of pulmonary edema independent of cardiac involvement associated with large vessel brainstem infarcts was markedly different from patterns observed in large vessel cortical strokes involving the RMCA and LMCA which were also associated with objective signs of stunned myocardium.
This study is limited by its retrospective case-control design of data from a single tertiary facility with inherent potential for selection and recall bias including incomplete clinical data available for review. This also includes the effect of lead-time bias for the onset of pulmonary edema and variability in treatment across cases [9,30] Ischemic stroke size may have varied between groups and was not controlled for in this study. The large confidence intervals for multivariate analyses likely reflect the small sample size of this study, warranting larger, prospective study. Prospective multi-center studies of the pulmonary and cardiac derangements associated with ischemic stroke would help to clarify the temporal relationship of infarction with cardiopulmonary derangements.
CONCLUSION
In patients with large vessel brainstem infarction, unlike in patients with large vessel RMCA and LMCA strokes involving the insula, pulmonary edema can occur independent of myocardial stunning as analyzed by separate and composite cardiac endpoints including cardiac dysfunction, elevations of troponin and abnormalities of electrical conduction. This suggests that neurogenic pulmonary edema can be caused by ischemia of brainstem structures independent of cardiac dysfunction. Confirmation of these findings in a prospective analysis assessing the region of cerebrovascular involvement as well as brainstem structural involvement and the risk of neurogenic and cardiogenic pulmonary edema are required.
ACKNOWLEDGEMENT
This work was supported through grant RO1NS062059-01A1 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), Rockville, Maryland, USA.
Dr. Nyquist reports no conflicts of interest and discloses support through grant RO1NS062059-01A1 from the National Institute of Neurological Disorders and Stroke (National Institutes of Health, Rockville, Maryland).
Footnotes
CONFLICTS OF INTEREST
Dr. Probasco, Dr. Chang, and Dr. Victor have neither conflicts of interest nor disclosures to report.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977;8:448–455. doi: 10.1161/01.str.8.4.448. [DOI] [PubMed] [Google Scholar]
- 3.Butcher KS, Parsons MW. Cardiac enzyme elevations after stroke: the importance of specificity. Stroke. 2002;33:1944–1945. doi: 10.1161/01.str.0000023346.80463.a4. [DOI] [PubMed] [Google Scholar]
- 4.Ay H, Arsava EM, SaribaÅÿ O. Creatine kinase-MB elevation after stroke is not cardiac in origin: comparison with troponin T levels. Stroke. 2002;33:286–289. doi: 10.1161/hs0102.101544. [DOI] [PubMed] [Google Scholar]
- 5.Kida K, Akashi YJ, Fazio G, Novo S. Takotsubo cardiomyopathy. Curr Pharm Des. 2010;16:2910–2917. doi: 10.2174/138161210793176509. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- 7.White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation. 1995;92:2183–2189. doi: 10.1161/01.cir.92.8.2183. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheimer S. Cerebrogenic cardiac arrhythmias: Cortical lateralization and clinical significance. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16:212. doi: 10.1186/cc11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JD, Green DM, Metivier K, DeFusco C. Intensive care management of acute ischemic stroke. Emerg Med Clin North Am. 2012;30:713–744. doi: 10.1016/j.emc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Marshall SA, Nyquist P. A change of position for neurogenic pulmonary edema. Neurocrit Care. 2009;10:213–217. doi: 10.1007/s12028-008-9164-x. [DOI] [PubMed] [Google Scholar]
- 12.Tan CK, Lai CC. Neurogenic pulmonary edema. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2007;177:249–250. doi: 10.1503/cmaj.061584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 15.Aberle DR, Wiener-Kronish JP, Webb WR, Matthay MA. Hydrostatic versus increased permeability pulmonary edema: Diagnosis based on radiographic criteria in critically ill patients. Radiology. 1988;168:73–79. doi: 10.1148/radiology.168.1.3380985. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Nyquist P. Wet lungs and a battered brain stem: can we stop one if we cannot stop the other? Crit Care Med. 2013;41:1373–1374. doi: 10.1097/CCM.0b013e31827bf734. [DOI] [PubMed] [Google Scholar]
- 18.Hoff JT, Nishimura M, Garcia-Uria J, Miranda S. Experimental neurogenic pulmonary edema. Part 1: The role of systemic hypertension. J Neurosurg. 1981;54:627–631. doi: 10.3171/jns.1981.54.5.0627. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski ML, Didier A, Kaliner MA. Neurogenic inflammation in the airways. I. Neurogenic stimulation induces plasma protein extravasation into the rat airway lumen. Am Rev Respir Dis. 1989;140:101–109. doi: 10.1164/ajrccm/140.1.101. [DOI] [PubMed] [Google Scholar]
- 20.Minnear FL, Kite C, Hill LA, van der Zee H. Endothelial injury and pulmonary congestion characterize neurogenic pulmonary edema in rabbits. J Appl Physiol (1985) 1987;63:335–341. doi: 10.1152/jappl.1987.63.1.335. [DOI] [PubMed] [Google Scholar]
- 21.Smith WS, Matthay MA. Evidence for a hydrostatic mechanism in human neurogenic pulmonary edema. Chest. 1997;111:1326–1333. doi: 10.1378/chest.111.5.1326. [DOI] [PubMed] [Google Scholar]
- 22.Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- 23.Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35:2094–2098. doi: 10.1161/01.STR.0000138452.81003.4c. [DOI] [PubMed] [Google Scholar]
- 24.Algra A, Gates PC, Fox AJ, Hachinski V, Barnett HJ North American Symptomatic Carotid Endarterectomy Trial Group. Side of brain infarction and long-term risk of sudden death in patients with symptomatic carotid disease. Stroke. 2003;34:2871–2875. doi: 10.1161/01.STR.0000099964.34430.2D. [DOI] [PubMed] [Google Scholar]
- 25.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–483. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 26.Brown RH, Jr, Beyerl BD, Iseke R, Lavyne MH. Medulla oblongata edema associated with neurogenic pulmonary edema. Case report. J Neurosurg. 1986;64:494–500. doi: 10.3171/jns.1986.64.3.0494. [DOI] [PubMed] [Google Scholar]
- 27.Nathan MA, Reis DJ. Fulminating arterial hypertension with pulmonary edema from release of adrenomedullary catecholamines after lesions of the anterior hypothalamus in the rat. Circ Res. 1975;37:226–235. doi: 10.1161/01.res.37.2.226. [DOI] [PubMed] [Google Scholar]
- 28.Lu WH, Hsieh KS, Lu PJ, Wu YS, Ho WY, Lai CC, et al. Hexamethonium reverses the lethal cardiopulmonary damages in a rat model of brainstem lesions mimicking fatal enterovirus 71 encephalitis. Crit Care Med. 2013;41:1276–1285. doi: 10.1097/CCM.0b013e3182771364. [DOI] [PubMed] [Google Scholar]
- 29.Davison DL, Chawla LS, Selassie L, Tevar R, Junker C, Seneff MG. Neurogenic pulmonary edema: successful treatment with IV phentolamine. Chest. 2012;141:793–795. doi: 10.1378/chest.11-0789. [DOI] [PubMed] [Google Scholar]
- 30.Colice GL. Neurogenic pulmonary edema. Clin Chest Med. 1985;6:473–489. [PubMed] [Google Scholar]