Abstract
Obesity, type 2 diabetes mellitus (T2DM), and non-alcoholic steatohepatitis (NASH) can be associated with cognitive impairment or early neurodegeneration. Previously, we showed that diet-induced obesity with T2DM and NASH results in mild neurodegeneration with some features of AD, including brain insulin resistance. In a companion study, we correlated obesity/T2DM/NASH-associated central nervous system (CNS) abnormalities with increased pro-ceramide gene expression in liver. Since ceramides are neurotoxic and cause insulin resistance, we directly investigated the role of ceramides as mediators of neurodegeneration using an in vitro culture model. We treated PNET2 human CNS neuronal cells with D-erythro-Ceramide analogs (C2Cer:N-acetylsphinganine and C6Cer: N-hexanoylsphinganine), or the inactive dihydroceramide analog (C2DCer) for 48 h, and probed for changes in genes and proteins that are critical to insulin/IGF signaling, and associated with neurodegeneration. Exposure to C6Cer > C2Cer impaired energy metabolism, viability, and insulin and insulin-like growth factor signaling mechanisms, and resulted in increased levels of AβPP-Aβ and pTau, whereas C2D had no significant effect on these parameters. CNS exposure to neurotoxic ceramides from exogenous sources, including liver, can cause neurodegeneration with impairments in insulin and IGF signaling mechanisms, similar to the findings in experimental models of obesity/T2DM, and NASH.
Keywords: Alzheimer’s disease, central nervous system, ceramide, diabetes mellitus, insulin resistance, neurodegeneration, neurons, non-alcoholic steatohepatitis
INTRODUCTION
Obesity, type 2 diabetes mellitus (T2DM), non-alcoholic steatohepatitis (NASH), Alzheimer’s disease (AD), and experimental AD-type neurodegeneration produced by intracerebral streptozotocin treatment are all associated with insulin resistance, oxidative stress, mitochondrial dysfunction, and pro-inflammatory cytokine activation [1–7]. Growing evidence suggests that ceramides play a critical role in the pathogenesis of obesity, T2DM and NASH [8–11] because ceramides cause insulin resistance [8,12–16]. Moreover, ceramide synthesis is stimulated by pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [8], and pro-inflammatory cytokines are highly activated in obesity, T2DM, NASH, and AD [17–24]. We suspect that ceramides also mediate at least some components of the brain insulin resistance associated with AD or T2DM/obesity mediated neurodegeneration because ceramides: 1) can be generated in brain [8,9, 25,26]; 2) are increased in various dementia-associated diseases, including AD [9,10,27,28]; and 3) are lipid soluble and therefore likely to readily cross the blood-brain barrier, providing a mechanism by which obesity, T2DM, or NASH could lead to brain insulin resistance.
Ceramides comprise a family of lipids generated from fatty acid and sphingosine [8,29,30]. Ceramides are distributed in cell membranes, and in addition to their structural functions, they regulate intracellular signaling pathways that mediate growth, proliferation, motility, adhesion, differentiation, senescence, and apoptosis. Ceramides are generated by de novo biosynthesis through the actions of ceramide synthases and serine palmitoyltransferase [25,26,31]. In addition, ceramides can be generated by sphingolipid catabolism through activation of neutral or acidic sphingomyelinases [25,30], or through degradation of complex sphingolipids and glycosphingolipids localized in late endosomes and lysosomes [29]. Ceramides are metabolized by ceramidases to form sphingosine, which is converted to sphinogsine 1-phosphate by spingosine kinase. Ceramide, sphingosine, and sphingosine-1-phosphate have all been implicated in the pathogenesis of obesity and insulin resistance [25]. Correspondingly, inhibition of ceramide synthesis or its accumulation prevents obesity-mediated insulin resistance [12,15].
Complex sphingolipids including gangliosides [32], and long-chain naturally occurring ceramides, i.e., up to 24 carbon atoms in length [33], positively influence cell growth and functions, whereas sphingosine-containing lipids, including shorter ceramides, have inhibitory effects of cells resulting in increased apoptosis, cytotoxicity, or impaired growth [32,34,35]. Sphingomyelinases are activated by pro-inflammatory cytokines, e.g., TNF-α [8], and pro-apoptotic stimuli including Fas, trophic factor withdrawal, and ionizing radiation [29, 30]. Ceramides adversely alter cellular function and cause apoptosis by modulating phosphorylation states of proteins, including those that regulate insulin signaling [36], activating enzymes such as interleukin-1β converting enzyme (ICE)-like proteases, which promote apoptosis [29], or inhibiting Akt phosphorylation and kinase activity [37] through activation of protein phosphatase 2A [38].
In obesity, adipose tissue, skeletal muscle, and liver exhibit major abnormalities in sphingolipid metabolism that result in increased ceramide production, inflammation, and activation of pro-inflammatory cytokines, and impairments in glucose homeostasis and insulin responsiveness [8,14,25,39]. In both humans with NASH [40], and the C57BL/6 mouse model of diet-induced obesity with T2DM and NASH [41], ceramide levels in adipose tissue are elevated due to increased activation of sphingomyelin transferase, and acidic and neutral sphingomyelinases [29]. However, in liver, ceramide synthase and sphingomyelin transferase mRNA levels are increased during the early stages of hepatic steatosis, but with the emergence of NASH and neurodegeneration, those mRNA transcripts decline while sphingomyelinase gene expression increases [42]. Since the obesity/T2DM/NASH-associated neurodegeneration in this model was not associated with increased CNS expression of pro-ceramide genes, we extended the analysis by directly investigating the role of exogenous ceramide exposure in the pathogenesis of neurodegeneration and neuronal insulin resistance using an in vitro model.
MATERIALS AND METHODS
Ceramide exposure model
Human primitive neuroectodermal tumor 2 (PNET2), CNS-derived neuronal cells [43,44] were maintained as previously described [45]. PNET2 cells were used because they respond appropriately to insulin stimulation and express all of the genes required for transmitting insulin and insulin-like growth factor (IGF) signals, and they express all of the known genes and proteins relevant to AD pathogenesis [46–48]. In addition, PNET2 cells express neurotrophins, neurotrophin receptors, and both cholinergic and dopaminergic neurotransmitter associated genes [49]. PNET2 cells were treated with 0.25–125.8 µM of ceramide analogs, D-erythro-N-Acetyl-Sphingosine (C2 Cer), D-erythro-N-Hexanoyl-Sphingosine (C6 Cer), or Derythro-Dihydro-N-Acetyl-Sphingosine (C2 Dihydroceramide; C2D). C2D is a structurally similar, inactive analog of C2 Cer, and was used as a negative control. Ceramide reagents were dissolved in ethanol, but cells were exposed to less than 0.5% ethanol with the ceramide treatments. Vehicle-treated control cells were simultaneously exposed to 0.5% ethanol. After 48 h of treatment, cells grown in 96-well cultures were analyzed for viability and ATP content using the CyQuant (Molecular Probes, Eugene, OR) and ATP-Lite (Perkin-Elmer, Boston, MA) assays according to the manufacturers’ protocols. Cells grown in 6-well cultures were used to measure gene expression by qRTPCR or by direct binding enzyme-linked immunosorbant assays (ELISAs).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assay of gene expression
We used qRT-PCR to measure mRNA levels of insulin, IGF types 1 and 2, their corresponding receptors, insulin receptor substrates (IRS) types 1, 2, and 4, Tau, amyloid-β protein precursor (AβPP), ChAT, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as previously described [4]. In brief, total RNA isolated from cultured cells was reverse transcribed using random oligodeoxynucleotide primers. The resulting cDNA templates were used in qPCR amplification reactions with previously published gene specific primer pairs [4]. PCR amplifications were performed in 20 µl reactions containing cDNA generated from 2.5 ng of original RNA template for mRNA analysis or 0.5 ng of template for 18S rRNA analysis, 300 nM each of gene specific forward and reverse primer, and 10 µl of 2× QuantiTect SYBR Green PCR Mix (Qiagen Inc, Valencia, CA). The amplified signals from triplicate reactions were detected and analyzed using the Mastercycler ep realplex instrument and software (Eppendorf AG, Hamburg, Germany).
The amplification protocol used was as follows: initial 15-minutes denaturation and enzyme activation at 95°C, 40 cycles of 95°C × 15 sec, 55–60°C × 30 sec, and 72°C × 30 sec. Annealing temperatures were optimized using the temperature gradient program provided with the software. PCR amplification efficiencies were all greater than 95%. The mRNA levels were determined using the equations of regression lines generated with serial 10-fold dilutions of 20 ng of recombinant plasmid DNA containing the target sequences studied. Relative mRNA abundance was determined from the ng ratios of specific mRNA to 18S rRNA measured in the same samples [50,51]. Inter-group statistical comparisons were made using the calculated mRNA/18S ratios. 18S rRNA values were used as denominators because the large abundance of 18S provides an excellent loading control that is virtually invariant with disease state or experimental condition. Moreover, expression levels of traditional housekeeping genes, e.g., β-actin, glyceraldehyde 3 phosphate dehydrogenase(GAPDH), and cyclophilin shift unpredictably with disease and experimental treatments, particularly under conditions of increased oxidative stress, rendering them unreliable for normalizing data with respect to specific genes of interest when comparing experimental disease states.
Enzyme-Linked Immunosorbant Assay (ELISA)
We used direct ELISAs to measure tau, phospho-tau, 4-hydroxy-2-nonenol (4-HNE), ubiquitin, β-actin, and choline acetyltransferase (ChAT), GAPDH, and acetylcholinesterase (AChE) as previously described [52]. In brief, cells were lysed in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors [4]. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Immunoreactivity was detected with horseradish peroxidase (HRP)-conjugated secondary antibody and Amplex Red soluble fluorophore [52]. Fluorescence was measured (Ex 579/Em 595) in a SpectraMax M5 microplate reader (Molecular Devices Corp., Sunnyvale, CA). Parallel negative control assays included incubations in which the primary, secondary, or both antibodies were omitted.
Source of reagents
QuantiTect SYBR Green PCR Mix was obtained from (Qiagen Inc, Valencia, CA). Rabbit or goat generated monoclonal or polyclonal antibodies to ubiquitin, tau, phospho-tau, 4-HNE, choline ChAT, and β-actin were purchased from Chemicon (Tecumsula, CA), Cal-Biochem (Carlsbad, CA) or Molecular Probes (Eugene, OR). Secondary antibodies were purchased from Pierce Chemical Co. (Rockford, IL). Amplex Red reagent was obtained from Molecular Probes (Eugene,OR). All other fine chemicals were purchased from either Cal-Biochem (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO).
Statistical analysis
Data depicted in the graphs represent the means ± S.E.M.’s for each group. Inter-group comparisons were made using two-way Analysis of Variance (ANOVA) with either the Bonferroni (repeated measures ANOVA), or one-way ANOVA with the Dunn’s multiple comparison test. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA). The computer software generated significant P-values are indicated within the graph panels.
RESULTS
Ceramide impairs neuronal viability and energy metabolism
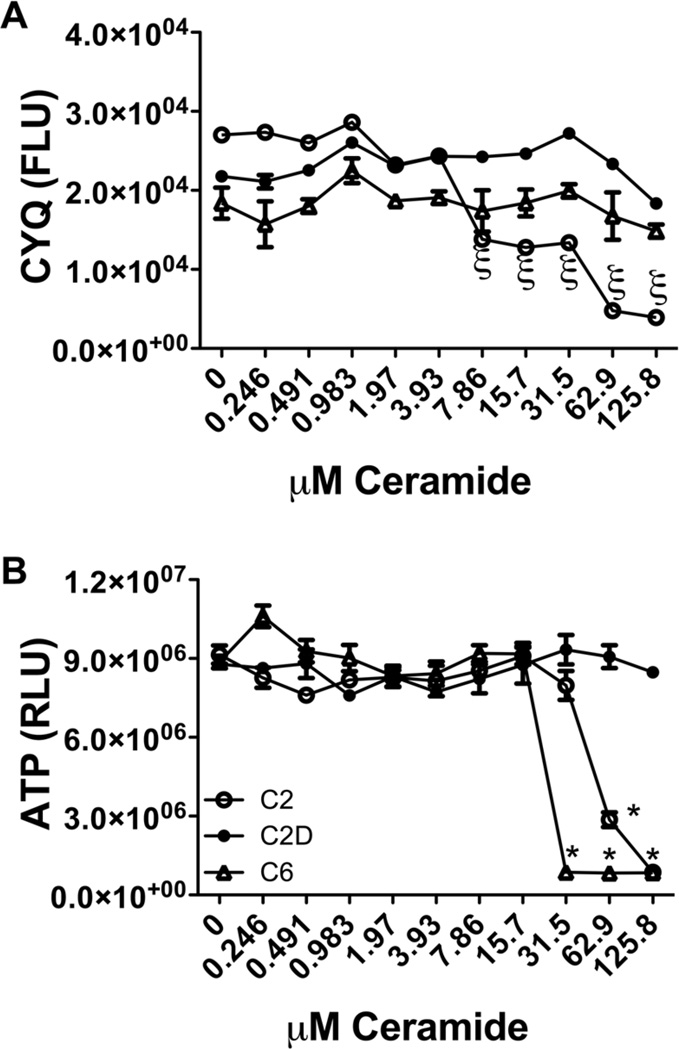
PNET2 human neuronal cells were treated with vehicle, or 0.25–125.8 µM C2 ceramide, C6 ceramide or C2D inactive ceramide for 48 h (8–12 replicate cultures/assay point). The CyQuant assay was used to measure neuronal viability, and the ATPLite assay was used to measure ATP content as an index of energy metabolism. 7.9 µM or higher concentrations of C2 ceramide proved to be highly toxic, resulting in 50% cell loss relative to C6 and C2D ceramide treatments (Fig. 1A). In contrast, cell viability remained relative stable in cultures treated with up to 125.8 µM C6 ceramide. Mean ATP content was similar to control in cultures treated with 0.25–15.7 µM ceramide, but at higher concentrations, ATP content declined sharply in both C2 and C6 relative to C2D ceramide treated cultures (Fig. 1B). Further studies were performed using 15.7 µMceramide treatments for 48 h. Two-way ANOVA tests demonstrated significant relative reductions in viability (CyQyant fluorescence) in cells treated with moderate to high concentrations of C2 ceramide relative to the fluctuations observed following C2D or C6 ceramide treatment (ξ P < 0.01 or better), and significantly lower mean level of ATP content in cells treated with 31.5 µMC6 or 62.9 µMC2 relative to C2D (*P < 0.0001). It is noteworthy that ATP content, i.e., energy metabolism, was sharply reduced in the absence of corresponding cell death in C6 ceramide treated cells.
Fig. 1.
Ceramide is neurotoxic and impairs energy metabolism. PNET2 human CNS-derived neuronal cells seeded into 96-well plates were treated with graded concentrations (0.246–125.8 µM) of C6 Ceramide (Cer), C2 Cer, or C2D (Inactive ceramide), or vehicle (0 dose) for 48 h, and then analyzed for (A) viability using the CyQuant fluorescence based assay (fluorescence light units-FLU), and (B) ATP content, reflecting energy metabolism, using the ATP luminescence-based assay (relative light units-RLU). Graphs depict the mean ± S.E.M. of results from 8 or 12 replicate assays and all experiments were performed 3 times. Inter-group statistical comparisons were performed using one-way ANOVA with the Bonferroni post hoc significance tests. (*P < 0.0001 relative to C2D treatment).
Ceramide impairs neuronal insulin, IGF and IRS signaling mechanisms
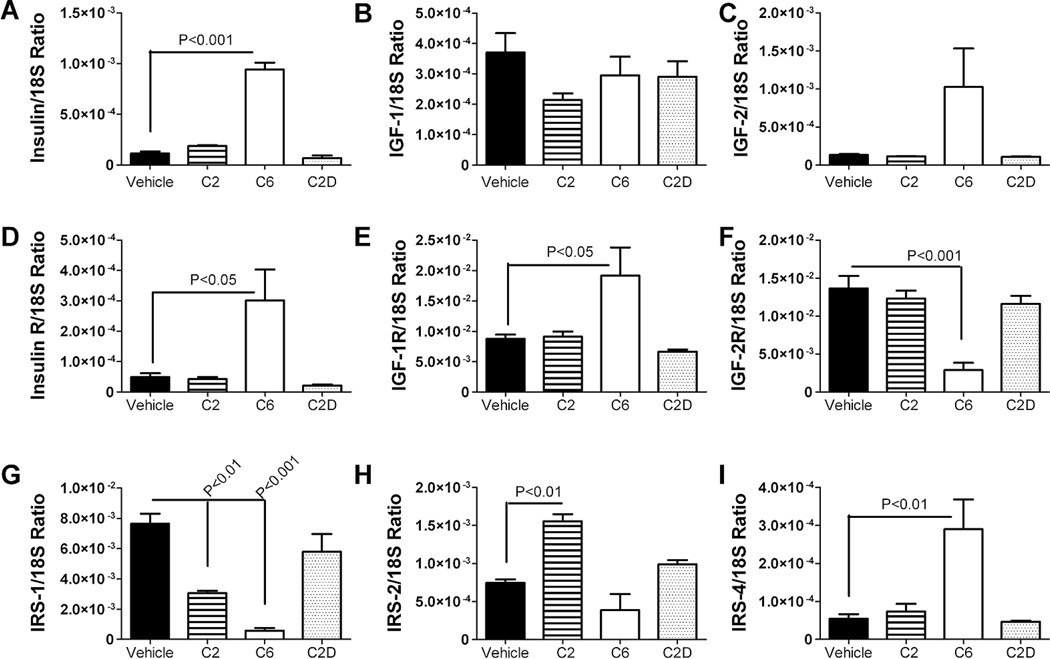
Previously, we demonstrated that chronic high fat diet (HFD) feeding caused mild AD-type neurodegeneration with brain insulin resistance. In the present study, we investigated whether neuronal insulin/IGF resistance could be mediated by ceramide treatment. C6 ceramide exposure significantly increased the mean levels of insulin, insulin receptor, IGF-1 receptor, and IRS-4, and decreased the mean levels of IGF-2 receptor and IRS-1 mRNA as demonstrated by qRT-PCR analysis and one-way ANOVA tests (Fig. 2). Although IGF-2 mRNA levels were also increased in C6 ceramide treated cells, the difference from control and C2D did not reach statistical significance (P = 0.08). The effects of C2 ceramide treatment were modest relative to C6, with the major effects being reduced IRS-1 and increased IRS-2 mRNA expression relative to vehicle-treated control cells. Treatment with C2D inactive ceramide had no significant effects on the expression of insulin, IGF, or IRS genes relative to vehicle-treated controls (Fig. 2).
Fig. 2.
Ceramide treatment alters neuronal insulin/IGF signaling mechanisms. Total RNA extracted from PNET2 cells treated with vehicle or 15.7 µM C6, C2, or C2D (inactive) ceramide for 48 h, was reverse transcribed using random oligodeoxynucleotide primers, and the cDNA templates were used in qRT-PCR assays to measure expression of (A) insulin, (B) IGF-I, (C) IGF-II, (D) insulin receptor (R), (E) IGF-I R, (F) IGF-II R, (G) IRS-1, (H) IRS-2, and (I) IRS-4, with results normalized to 18S rRNA. Graphs depict the mean ± S.E.M. levels of gene expression in each group (N=6 samples per group). Inter-group comparisons were made using one-way ANOVA with post-hoc Dunn’s multiple comparison test of significance. Significant P-values are indicated within the panels. Experiments were repeated twice.
Ceramide induces AD-type molecular indices of neurodegeneration
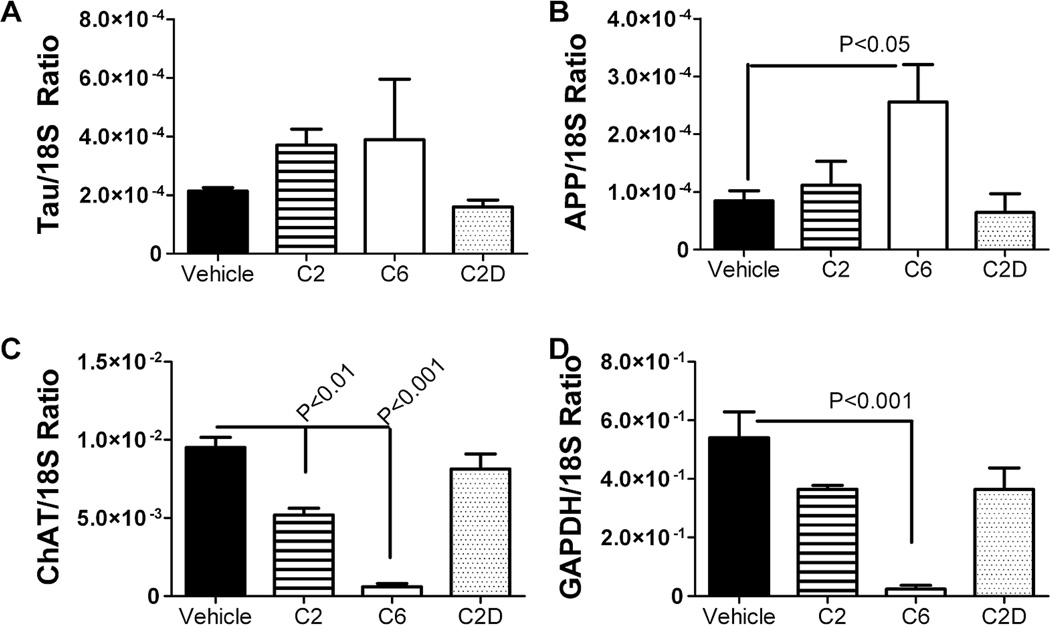
Further studies were used to determine if ceramide-induced alterations in insulin/IGF signaling mechanisms were associated with AD-type abnormalities in gene expression. qRT-PCR analysis revealed significantly increased mRNA levels of AβPP, and reduced levels of ChAT and GAPDH (insulin-responsive gene) in C6 ceramide treated relative to control cells (Fig. 3). C2 ceramide treatment also significantly inhibited ChAT expression relative to vehicle-treated control cells, but it did not increase AβPP or reduce GAPDH expression. Tau mRNA was not significantly altered by C2 or C6 ceramide, and C2D inactive ceramide had no significant effects on tau, AβPP, ChAT, or GAPDH relative to vehicle-treatment.
Fig. 3.
Ceramide treatment alters neuronal insulin/IGF signaling mechanisms. Total RNA extracted from PNET2 cells treated with vehicle or 15.7 µM C6, C2, or C2D ceramide for 48 h, was reverse transcribed using random oligodeoxynucleotide primers, and the cDNA templates were used in qRT-PCR assays to measure expression of AD-associated genes including, (A) tau, B) amyloid-β protein precursor-AβPP, C) choline acetyltransferase-ChAT, and D) glyceraldehydes-3-phosphate dehydrogenase-GAPDH (which is insulin responsive). Results were normalized to 18S rRNA measured in parallel reactions. Graphs depict the mean ± S.E.M. levels of gene expression in each group (N=6 samples/group). Inter-group comparisons were made using one-way ANOVA with post-hoc Dunn’s multiple comparison test of significance relative to vehicle-treated controls. Significant P-values are indicated within the panels. Experiments were repeated twice.
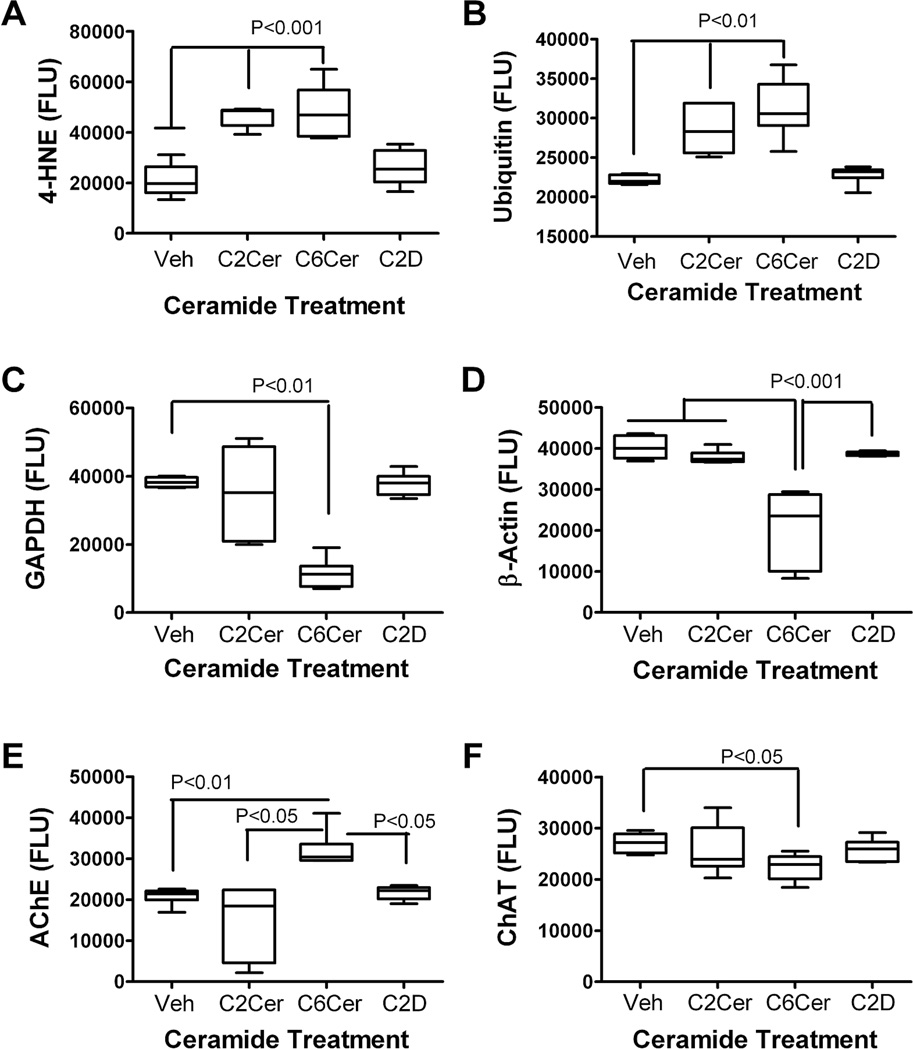
We used ELISAs to examine the effects of C6 ceramide on neuronal indices of oxidative stress, acetylcholine homeostasis, cytoskeletal integrity, and insulin-responsive gene expression. The effects of C2 ceramide were not investigated here due to its relatively modest effects compared with C6 in all other studies. Posthoc statistical comparisons were made with respect to the vehicle-treated control group. C6 ceramide exposure significantly increased the mean levels of 4-HNE, ubiquitin and AChE, and reduced the mean levels of GAPDH, β-actin and ChAT (Fig. 4). In contrast, treatment with C2D inactive ceramide had minimal effects on the expression levels of these proteins, except for modestly increased levels of AChE.
Fig. 4.
Ceramide treatment modulates neuronal indices of AD-type neurodegeneration. PNET2 cells were treated with vehicle or 15.7 µM C6, or C2D ceramide for 48 h. Protein homogenates were used to measure (A) 4-hydroxynonenol-4-HNE; B) ubiquitin; (C) GAPDH; (D) β-actin; (E) acetylcholinesterase (AChE); and (F) choline acetyltransferase (ChAT) by direct ELISA (see Materials and Methods). Immunoreactivity was detected with HRP-conjugated secondary antibody and Amplex Red soluble fluorophor. Fluorescence light units (FLU) were measured (Ex 579 nm/Em 595 nm) in a Spectromax M5. Graphs depict mean ± S.E.M of results (N = 6 samples per group). Inter-group comparisons were made using one-way ANOVA with the post-hoc Dunn’s multiple comparison test of significance relative to vehicle-treated controls. Significant P-values are indicated within the panels.
DISCUSSION
In previous [53] and companion [42] studies, we demonstrated that chronic HFD feeding results in obesity, T2DM, NASH, and mild neurodegeneration that shares some molecular and biochemical features in common with AD, including brain insulin resistance. However, the effects of these disease did not adequately replicate the spectrum and severity of abnormalities observed in AD. We also showed that neurodegeneration in the HFD/obesity/T2DM/NASH model was associated with significantly increased expression of pro-ceramide genes in liver, but not brain [42]. Since ceramides are lipid soluble and therefore likely to readily cross the blood-brain barrier, we proposed that increased hepatic and possibly adipocyte production of neurotoxic ceramides in obesity, T2DM, or NASH accounts for the co-occurrences of cognitive impairment and neurodegeneration in these disease states. This line of investigation is important because it could lead to the discovery of biomarkers for detecting individuals at risk for developing cognitive impairment or progressing from MCI to AD in the context of obesity, T2DM, or NASH. In this regard, it is noteworthy that: 1) peripheral ceramide production is increased in adipose tissue in NASH; 2) ceramides, as well as long-chain fatty acids, mediate insulin resistance [8,15,16]; and 3) ceramides can be neurotoxic, and their levels are increased in AD [9,10,27,28,37,54].
The in vitro experiments helped clarify the potential role of ceramides as mediators of neurodegeneration. Short-term (48 h) exposure to C6 ceramide impaired energy metabolism and altered the expression of several genes that are critical to the insulin and IGF signaling pathways, including insulin, insulin, IGF-1, and IGF-2 receptors, IRS-1, and IRS-4. The ceramide-induced increases in insulin and IGF-1 receptors most likely reflect insulin/IGF-1 resistance. This effect could represent impaired ligand-receptor binding, as previously observed in mice chronically fed with the HFD [53]. Alternatively, the result could reflect compensatory responses to decreased levels of IRS-1,which is one of the critical molecules responsible for transmitting insulin and IGF-1 stimulated signals [55,56]. The modest increases in IGF-2 may also reflect an adaptive response to decreased levels of the corresponding receptor.
The in vitro C6 ceramide treatments also significantly increased AβPP and decreased ChAT and GAPDH mRNA expression, corresponding with the findings in AD [5,6]. Overexpression of the AβPP gene results in increased AβPP-Aβ deposition in Down syndrome [57, 58] and transgenic mouse models [59], and similarly, the higher levels of AβPP expression in AD correspond with the generally increased AβPP-Aβ burden in AD brains [5–7]. In a previous study, C6 ceramide was demonstrated to increase AβPP-Aβ biogenesis by stabilizing β-secretase BACE1 activity [60], indicating a post-translational mechanism by which increased levels of ceramide could contribute to neurodegeneration. In addition, TNF-α or AβPP-Aβ exposure was experimentally shown to increase CNS ceramide levels [9], suggesting the potential for establishing a reverberating loop of ever-increasing AβPP-Aβ and ceramide levels in brain after an initial oxidative stress insult. The ceramide-induced reductions in ChAT and GAPDH also correspond with findings in AD, but reflect insulin and IGF resistance as both genes are modulated by insulin and IGF stimulation [61,62]. Reduced ChAT expression reflects a neuronal cholinergic deficit, which one of the pivotal features correlating with dementia in AD as well as other neurodegenerative diseases [63, 64].
GAPDH is a key enzyme in the glycolytic pathway needed for energy metabolism and principally regulated by insulin [65]. Reduced GAPDH expression correlated with the ceramide-mediated impairments in ATP production illustrated in Fig. 1. The ceramide-induced sharp reductions in ATP appeared disproportionate with the degrees of impaired insulin/IGF signaling mechanisms. This phenomenon could be explained by the finding that, in addition to reduced insulin/IGF signaling, ceramides activate pro-inflammatory cytokines and increase oxidative stress, which together hamper mitochondrial function and energy metabolism [16]. The ELISA analyses confirmed the mRNA studies and further demonstrated that C6 and/or C2 ceramide exposure significantly increased neuronal level of 4-HNE and ubiquitin, i.e., indices of lipid peroxidation and oxidative stress, caused cytoskeletal collapse (reduced β-actin levels), and impaired acetylcholine homeostasis, i.e., increased AChE and decreased ChAT expression. Therefore, exogenous exposure to ceramide could lead to neuronal insulin resistance with increased oxidative stress, deficits in energy metabolism, and impairments in cholinergic function, similar to the findings in AD.
Altogether, the neurodegenerative effects of C6 ceramide treatment resembled many of the abnormalities caused by chronic HFD feeding, in that both resulted in increased insulin and IRS-4 expression, decreased ChAT and GAPDH expression, and increased 4-HNE and ubiquitin immunoreactivity [53]. However, their effects differed in that C6 ceramide treatment also increased expression of insulin, IGF-1 receptor, AChE, and AβPP, and decreased expression of IRS-1, whereas chronic HFD feeding did not produce these responses [53]. Overall, our results are consistent with a previous report demonstrating ceramide-inhibition of IGF-1 stimulated energy metabolism and viability in neurons, i.e., neuronal IGF-1 resistance [37], but provide new information about other adverse effects of ceramide on insulin signaling and indices of neurodegeneration. It is noteworthy that ceramide and TNF-α both increase the levels of AβPP-Aβ [60], and that AβPP-Aβ promotes ceramide and sphingomyelin accumulation by increasing sphingomyelinase activity [9,66]. Therefore, obesity with T2DM or NASH has the potential to establish a reverberating loop in brain centered on ever-increasing levels of ceramide, TNF-α, and AβPP-Aβ, resulting in progressive insulin and IGF resistance with attendant disturbances in energy metabolism and acetylcholine homeostasis.
The present work provides additional support for the concept that extra-CNS sources of ceramide, such as liver or adipose tissue, can contribute to the pathogenesis of neurodegeneration. It is noteworthy that the effects of in vitro ceramide treatment were not identical to those of chronic HFD feeding [42,53], yet they overlapped with many features of insulin and IGF resistance observed in early and intermediate stages of AD in humans [5]. The fact that the effects of ceramide treatment were not identical to that which actually happens in AD, lends support to our hypothesis that T2DM, NASH, and obesity do not cause AD, and instead they probably serve as pathogenic co-factors. This phenomenon could account for both the absence of complete overlap and the two- or three-fold increased risk of developing MCI or AD among individuals with T2DM [67–69]. Improved ability to detect increased levels of toxic ceramides and related lipids in peripheral blood and cerebrospinal fluid may help identify individuals at risk for developing cognitive impairment in the context of obesity, T2DM, and/or NASH.
ACKNOWLEDGMENTS
Research supported by AA02666, AA02169, AA11431, AA12908, and AA16126 from the National Institutes of Health.
References
- 1.Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xirouchakis E, Sigalas A, Manousou P, Calvaruso V, Pleguezuelo M, Corbani A, Maimone S, Patch D, Burroughs AK. Models for non-alcoholic fatty liver disease: a link with vascular risk. Curr Pharm Des. 2008;14:378–384. doi: 10.2174/138161208783497705. [DOI] [PubMed] [Google Scholar]
- 4.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 5.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 6.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 7.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 8.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem Soc Trans. 2004;32:144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- 10.Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 11.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 13.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 15.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–241. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- 17.Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 18.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 19.Yalniz M, Bahcecioglu IH, Ataseven H, Ustundag B, Ilhan F, Poyrazoglu OK, Erensoy A. Serum adipokine and ghrelin levels in nonalcoholic steatohepatitis. Mediators Inflamm. 2006;2006:34295. doi: 10.1155/MI/2006/34295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–1952. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- 21.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 23.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 27.Nakane M, Kubota M, Nakagomi T, Tamura A, Hisaki H, Shimasaki H, Ueta N. Lethal forebrain ischemia stimulates sphingomyelin hydrolysis and ceramide generation in the gerbil hippocampus. Neurosci Lett. 2000;296:89–92. doi: 10.1016/s0304-3940(00)01655-4. [DOI] [PubMed] [Google Scholar]
- 28.Adibhatla RM, Hatcher JF. Altered Lipid Metabolism in Brain Injury and Disorders. Subcell Biochem. 2008;48 doi: 10.1007/978-1-4020-8831-5_9. nihpa41041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997;8:311–322. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Munoz A, Frago LM, Alvarez L, Varela-Nieto I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem J. 1997;325(Pt 2):435–440. doi: 10.1042/bj3250435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Brocklyn JR. Sphingolipid signaling pathways as potential therapeutic targets in gliomas. Mini Rev Med Chem. 2007;7:984–990. doi: 10.2174/138955707782110123. [DOI] [PubMed] [Google Scholar]
- 36.Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217:1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- 37.Arboleda G, Huang TJ, Waters C, Verkhratsky A, Fernyhough P, Gibson RM. Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur J Neurosci. 2007;25:3030–3038. doi: 10.1111/j.1460-9568.2007.05557.x. [DOI] [PubMed] [Google Scholar]
- 38.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 39.Assimacopoulos-Jeannet F. Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S53–S57. doi: 10.1038/sj.ijo.0802857. [DOI] [PubMed] [Google Scholar]
- 40.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 41.Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983–990. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Lyn-Cook LE, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide mediates brain insulin resistance and neurodegeneration in obesity with type 2 diabetes mellitus and Non-alcoholoic steatohepatitis. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-0984. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu YY, Bhavani K, Wands JR, de la Monte SM. Insulininduced differentiation and modulation of neuronal thread protein expression in primitive neuroectodermal tumor cells is linked to phosphorylation of insulin receptor substrate-1. J Mol Neurosci. 1995;6:91–108. doi: 10.1007/BF02736769. [DOI] [PubMed] [Google Scholar]
- 44.Xu YY, Bhavani K, Wands JR, de la Monte SM. Ethanol inhibits insulin receptor substrate-1 tyrosine phosphorylation and insulin-stimulated neuronal thread protein gene expression. Biochem J. 1995;310(Pt 1):125–132. doi: 10.1042/bj3100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter JJ, Tong M, Silbermann E, Lahousse SA, Ding FF, Longato L, Roper N, Wands JR, de la Monte SM. Ethanol impaired neuronal migration is associated with reduced aspartyl-asparaginyl-beta-hydroxylase expression. Acta Neuropathol. 2008;116:303–315. doi: 10.1007/s00401-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de la Monte SM, Chiche J, von dem Bussche A, Sanyal S, Lahousse SA, Janssens SP, Bloch KD. Nitric oxide synthase-3 overexpression causes apoptosis and impairs neuronal mitochondrial function: relevance to Alzheimer’s-type neurodegeneration. Lab Invest. 2003;83:287–298. doi: 10.1097/01.lab.0000056995.07053.c0. [DOI] [PubMed] [Google Scholar]
- 47.de la Monte SM, Ganju N, Feroz N, Luong T, Banerjee K, Cannon J, Wands JR. Oxygen free radical injury is sufficient to cause some Alzheimer-type molecular abnormalities in human CNS neuronal cells. J Alzheimers Dis. 2000;2:261–281. doi: 10.3233/jad-2000-23-406. [DOI] [PubMed] [Google Scholar]
- 48.de la Monte SM, Wands JR. Alzheimer-associated neuronal thread protein mediated cell death is linked to impaired insulin signaling. J Alzheimers Dis. 2004;6:231–242. doi: 10.3233/jad-2004-6304. [DOI] [PubMed] [Google Scholar]
- 49.Tong M, Dong M, de la Monte SM. Brain insulinlike growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-0995. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, Eun Yeon J, Chang H, Tison G, Jun Chen G, Wands JR, De La Monte SM. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: Role of PTEN phosphatase. J Biol Chem. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- 51.Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- 52.Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 53.Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and Type 2 diabetes mellitus. J Alzheimers Dis. 2008;15:29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G, Silva J, Dasgupta S, Bieberich E. Long-chain ceramide is elevated in presenilin 1 (PS1M146V) mouse brain and induces apoptosis in PS1 astrocytes. Glia. 2008;56:449–456. doi: 10.1002/glia.20626. [DOI] [PubMed] [Google Scholar]
- 55.Fritsche L, Weigert C, Haring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver–implications for health and disease. Curr Med Chem. 2008;15:1316–1329. doi: 10.2174/092986708784534956. [DOI] [PubMed] [Google Scholar]
- 56.Youngren JF. Regulation of insulin receptor function. Cell Mol Life Sci. 2007;64:873–891. doi: 10.1007/s00018-007-6359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Monte SM. Molecular abnormalities of the brain in Down syndrome: relevance to Alzheimer’s neurodegeneration. J Neural Transm Suppl. 1999;57:1–19. doi: 10.1007/978-3-7091-6380-1_1. [DOI] [PubMed] [Google Scholar]
- 58.Head E, Lott IT. Down syndrome and beta-amyloid deposition. Curr Opin Neurol. 2004;17:95–100. doi: 10.1097/00019052-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 61.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 62.Soscia SJ, Tong M, Xu XJ, Cohen AC, Chu J, Wands JR, de la Monte SM. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–2056. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuello AC, Bruno MA, Bell KF. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- 64.Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl) 2008;198:1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- 65.Alexander BM, Dugast I, Ercolani L, Kong XF, Giere L, Nasrin N. Multiple insulin-responsive elements regulate transcription of the GAPDH gene. Adv Enzyme Regul. 1992;32:149–159. doi: 10.1016/0065-2571(92)90014-q. [DOI] [PubMed] [Google Scholar]
- 66.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- 68.Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Hennerici M, O’Brien J, Pantoni L, Salvadori E, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78:1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]