Abstract
Growing evidence supports the concept that Alzheimer’s disease (AD) is fundamentally a metabolic disease with molecular and biochemical features that correspond with diabetes mellitus and other peripheral insulin resistance disorders. Brain insulin/IGF resistance and its consequences can readily account for most of the structural and functional abnormalities in AD. However, disease pathogenesis is complicated by the fact that AD can occur as a separate disease process, or arise in association with systemic insulin resistance diseases, including diabetes, obesity, and non-alcoholic fatty liver disease. Whether primary or secondary in origin, brain insulin/IGF resistance initiates a cascade of neurodegeneration that is propagated by metabolic dysfunction, increased oxidative and ER stress, neuro-inflammation, impaired cell survival, and dysregulated lipid metabolism. These injurious processes compromise neuronal and glial functions, reduce neurotransmitter homeostasis, and cause toxic oligomeric pTau and (amyloid beta peptide of amyloid beta precursor protein) AβPP-Aβ fibrils and insoluble aggregates (neurofibrillary tangles and plaques) to accumulate in brain. AD progresses due to: (1) activation of a harmful positive feedback loop that progressively worsens the effects of insulin resistance; and (2) the formation of ROS- and RNS-related lipid, protein, and DNA adducts that permanently damage basic cellular and molecular functions. Epidemiologic data suggest that insulin resistance diseases, including AD, are exposure-related in etiology. Furthermore, experimental and lifestyle trend data suggest chronic low-level nitrosamine exposures are responsible. These concepts offer opportunities to discover and implement new treatments and devise preventive measures to conquer the AD and other insulin resistance disease epidemics.
Keywords: Alzheimer’s disease, Insulin resistance, Type 3 diabetes, Ceramides, Nitrosamines, Obesity, Metabolic syndrome, Non-alcoholic fatty liver disease, Lifestyle
1. Overview: Insulin resistance diseases and the brain
Intact insulin and insulin like growth factor (IGF) signaling have important roles in relation to brain structure and function, including myelin integrity and neuronal plasticity. Impairments in insulin and IGF signaling caused by receptor resistance or ligand deficiency disrupt energy balance and interacting networks that support vital functions such as cell survival. Mounting evidence supports the concept that cognitive impairment and neurodegeneration are associated with and probably are caused by insulin and IGF resistance. Furthermore, the sharply increased rates of AD and other insulin resistance disease states, including obesity, type 2 diabetes mellitus, non-alcoholic fatty liver disease, and metabolic syndrome within the past several decades point toward environmental or exposure factors mediating disease. However, since each of these disease processes can occur independently or overlap with one or more of the others, one concept is that their etiologies are shared but selective organ/tissue involvement is dictated by other variables such as genetics. An example of this phenomenon pertains to the varied distributions of atherosclerosis; it presence in coronary arteries leads to myocardial infarction whereas carotid deposition of plaques predisposes individuals to stroke, yet no one would argue that the underlying disease processes were different. Furthermore, having both carotid and coronary atherosclerosis would not be surprising. This review focuses on how peripheral insulin resistance contributes to cognitive impairment and neurodegeneration, and potential contributions of environmental and genetic factors in the pathogenesis of AD.
2. Brain structure and function are maintained through the actions of insulin and insulin-like growth factor
Insulin regulates glucose uptake and utilization by cells, and free fatty acid levels in peripheral blood. Free fatty acids are substrates for complex lipid biosynthesis. Insulin stimulates glucose uptake by inducing glucose transporter protein e.g. GLUT4 translocation from the Golgi to the plasma membrane [1]. Insulin-like growth factor 1 (IGF-1) regulates growth and has anabolic functions. IGF-1s actions are regulated by interactions with IGF binding proteins (IGFBPs) [2]. In the brain, insulin and IGF regulate neuronal and glial functions such as growth, survival, metabolism, gene expression, protein synthesis, cytoskeletal assembly, synapse formation, neurotransmitter function, and plasticity [3,4], and they have important roles in cognitive function. Insulin, IGF-1 and IGF-2 polypeptide and receptor genes are expressed in neurons [3] and glia [5,6], particularly in structures that are targeted in neurodegenerative diseases [3,7,8].
3. Insulin resistance and neurodegeneration
3.1. Contributions of systemic insulin resistance diseases
Systemic insulin resistance refers to the state in which high levels of blood insulin (hyperinsulinemia) are associated with hyperglycemia. However, with regard to specific organs and tissues, impairments in insulin signaling with reduced activation of pathways and the need for above-normal levels of ligand to achieve normal insulin actions also correspond to insulin resistance [1]. The overall problem is complicated by the fact that: (1) sustained high levels of insulin can cause insulin resistance [9], and worsen or broaden tissue involvement; and (2) hyperinsulinemia impairs insulin secretion from β-cells in pancreatic islets, yielding hybrid states of insulin resistance and insulin deficiency [9]. Chronic insulin resistance results in cellular energy failure (lack of fuel), elevated plasma lipids, hypertension and predisposition to develop diabetes mellitus [10], cerebrovascular and cardiovascular disease, and malignancy [11–15]. Insulin resistance is now a major public health problem because of its link to the obesity, type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), metabolic syndrome, polycystic ovarian disease, age-related macular degeneration, and Alzheimer’s disease (AD) epidemics.
3.2. Concept: AD is a metabolic disease with brain insulin/IGF resistance
Growing evidence supports the concept that insulin resistance and metabolic dysfunction are mediators of AD [16,17], and therefore, AD could be regarded as a metabolic disease mediated by brain insulin and IGF resistance [18,19]. In fact, AD shares many features in common with systemic insulin resistance diseases including, reduced insulin-stimulated growth and survival signaling, increased oxidative stress, pro-inflammatory cytokine activation, mitochondrial dysfunction, and impaired energy metabolism [8,20,21]. In the early stages, AD is marked by deficits cerebral glucose utilization [22–24], and as AD progresses, brain metabolic derangements [25,26] with impairments in insulin signaling, insulin-responsive gene expression, glucose utilization, and metabolism worsen [18,19,27].
Human postmortem studies showed that brain insulin resistance with reduced insulin receptor expression and insulin receptor binding were consistently present in AD brains and worsen with disease progression [18,19,27], and that insulin signaling impairments were associated with deficits in IGF-1 and IGF-2 networks [18,19]. Of note is that the pathways profoundly affected in AD are the ones needed to maintain neuronal viability, energy production, gene expression, and plasticity [16]. Nearly all of the major features of AD, including increased: (1) activation of kinases that aberrantly phosphorylate tau and lead to accumulation of neurofibrillary tangles, dystrophic neuritic plaques and neuropil threads; (2) the 40 or 42 amino acid amyloid beta peptide of amyloid beta precursor protein (AβPP-Aβ) accumulation; (3) oxidative and ER stress, which propagate cell death cascades; (4) mitochondrial dysfunction; and (5) cholinergic dyshomeostasis could reflect consequences of brain insulin/IGF resistance.
3.3. Is AD a brain form of insulin resistance/insulin deficiency (type 3 diabetes)?
AD-associated deficits in insulin/IGF signaling are due to the combined effects of insulin/IGF resistance and deficiency. Insulin/ IGF resistance is manifested by reduced levels of insulin/IGF receptor binding and decreased responsiveness to insulin/IGF stimulation, while the trophic factor deficiency is associated with reduced levels of insulin polypeptide and gene expression in brain and cerebrospinal fluid [17–19]. Therefore, AD can be regarded as brain diabetes that has elements of both insulin resistance (T2DM) and insulin deficiency (T1DM). To consolidate this concept, we proposed that AD be referred to as, “Type 3 diabetes” [18,19]. This hypothesis is supported by experimental data showing that intracerebroventricular injection of streptozotocin, a pro-diabetes drug, causes deficits in spatial learning and memory, along with brain insulin resistance, brain insulin deficiency, and AD-type neurodegeneration, but not diabetes mellitus [28,29]. In contrast, systemic administration of streptozotocin causes diabetes mellitus with mild hepatic steatosis and neurodegeneration [30,31]. Therefore, brain diabetes (Type 3) can occur independent of Type 1 and Type 2 diabetes. Further studies utilizing small interfering RNA molecules showed that molecular disruption of brain insulin and IGF receptors was sufficient to cause cognitive impairment and hippocampal degeneration with molecular abnormalities similar to those in AD [32]. Lastly, the neuroprotective effects of glucagon-like peptide-1 (GLP-1) [33], IGF-1 [34], and caloric restriction [35], which respectively stimulate insulin actions, slow brain aging, and reduce insulin resistance, support the notion that AD is a brain diabetes-type metabolic disease.
4. Consequences of brain insulin/IGF resistance promote AD neurodegeneration and neuropathology
4.1. Key integrated driving forces of neurodegeneration
Chronic insulin/IGF-1 resistance disrupts the functional integrity of the brain [3,36] due to impairments in neuronal survival, energy production, gene expression, and plasticity [16]. These effects are mediated by increased: (1) activation of kinases that aberrantly phosphorylate tau, compromising neuronal cytoskeletal integrity; (2) accumulation of AβPP-Aβ; (3) oxidative stress; (4) ER stress; and (5) metabolic dysfunction with attendant activation of pro-inflammatory and pro-death cascades. Consequences of brain insulin/IGF resistance include down-regulation of target genes required for cholinergic homeostasis, and compromise of systems that mediate neuronal plasticity, memory, and cognition [16–19].
4.2. Tau pathology
Neurofibrillary tangles, dystrophic neurites, and neuropil threads represent neuronal cytoskeletal lesions that correlate with dementia in AD [37]. These structural lesions contain aggregates of hyperphosphorylated, ubiquitinated, insoluble fibrillar tau. Tau becomes hyperphosphorylated due to inappropriate activation of kinases such as GSK-3β [38], cyclin-dependent kinase 5 (cdk-5), and c-Abl [39], or inhibition of protein phosphatases 1 and 2A [39,40]. Consequently, tau becomes misfold and self-aggregate, forming insoluble fibrils (paired helical filaments and straight filaments) [41] that eventually produce neurofibrillary tangles, dystrophic neurites, and neuropil threads [40]. Neuronal accumulations of fibrillar tau disrupt neuronal cytoskeletal structure and function, and impair axonal transport and synaptic integrity. In addition, pre-fibrillar tau can aggregate into neurotoxic soluble oligomers or insoluble granular deposits that promote disconnection of synapses and death of neurons [42]. Ubiquitination of hyper-phosphorylated tau [43], together with eventual dysfunction of the ubiquitin-proteasome system [44], exacerbate the accumulations of insoluble fibrillar tau. Fibrillar tau exerts its neurotoxic effects by increasing oxidative stress, ROS generation, neuronal apoptosis, mitochondrial dysfunction, and necrosis [45].
Tau gene expression [32] and phosphorylation [36] are regulated by insulin and IGF, and impairments in insulin/IGF signaling contribute to tau hyper-phosphorylation due to over-activation of specific kinases, e.g. GSK-3β [36,41] and reductions in tau gene expression [8,32,46]. Attendant failure to generate sufficient normal tau protein, vis-a-vis accumulation of hyper-phosphorylated insoluble fibrillar tau likely promotes cytoskeletal collapse, neurite retraction, and synaptic disconnection. Moreover, decreased signaling through phosphoinositol-3-kinase (PI3K), Akt [36], and Wnt/β-catenin [47], and increased activation of GSK-3β [38] correlate with brain insulin and IGF resistance. Therefore, impairments in signaling through these pathways could account for the reductions in neuronal survival, myelin maintenance, synaptic integrity, neuronal plasticity, mitochondrial function, and cellular stress management in AD.
4.3. AβPP-Aβ pathology
AD is associated with brain accumulations of AβPP-Aβ large insoluble fibrillar aggregates in the form of plaques, and soluble neurotoxic oligomeric fibrils. In familial forms of AD, increased synthesis and deposition of AβPP-Aβ is due to mutations in the amyloid beta precursor protein (AβPP), presenilin 1 (PS1), and PS2 genes, or inheritance of the Apolipoprotein E ε4 (ApoE-ε4) allele [48,49]. In sporadic AD, which accounts for 90% or more of the cases, the cause of AβPP-Aβ accumulation is still debated. However, evidence suggests that impairments in insulin/IGF signaling dysregulate AβPP expression and protein processing, leading to AβPP-Aβ accumulation [50].
Evidence suggests that AβPP-Aβ toxicity causes insulin resistance as well as inflammation [51], and that brain insulin resistance with oxidative stress and neuro-inflammation [52] promote AβPP-Aβ accumulation and toxicity. Insulin accelerates trafficking of AβPP-Aβ from the trans-Golgi network to the plasma membrane and its extracellular secretion [53], and also inhibits its intracellular degradation by insulin-degrading enzyme [54]. In hyper-insulin states, IDE can be diverted to degrade insulin, and thereby allow AβPP-Aβ to accumulate [55]. Most important, impaired insulin signaling can disrupt processing of AβPP and clearance of AβPP-Aβ [56]. At the same time, AβPP-Aβ disrupts insulin signaling by competing with insulin, or reducing the affinity of insulin for binding to its own receptor [57]. AβPP-Aβ oligomers also inhibit neuronal insulin-signaling by desensitizing and reducing surface expression of insulin receptors. Intracellular AβPP-Aβ interferes with PI3 kinase activation of Akt, leading to reduced survival signaling, increased activation of GSK-3β, and hyper-phosphorylation of tau. At the same time, increased levels of GSK-3 promote AβPP processing and AβPP-Aβ accumulation [58].
4.4. Neuro-inflammation
Neuro-inflammation remains a focus of research in AD because it occurs early in the course of disease [59], and already has been addressed in several clinical trials [60,61]. Neuro-inflammation in the context of neurodegeneration is mainly manifested by up-regulation of pro-inflammatory cytokines and microglial infiltration [62], particularly in the vicinity of plaques [63]. Neuro-inflammation contributes to AD pathology by promoting AβPP-Aβ accumulation [62], Tau hyper-phosphorylation [64], oxidative injury [65], and impairments in neuronal plasticity [66]. Furthermore, inflammation exacerbates insulin resistance and ceramide accumulation, i.e. lipotoxicity, and insulin resistance and lipotoxic injury and cell death worsen inflammation [50,67,68,21].
In a recent study, analysis of cerebrospinal fluid (CSF), ventricular fluid (VF) and postmortem brain tissue by multiplex bead-based ELISAs revealed either significantly or moderately elevated levels of at least 15 different cytokines in AD CSF and brains during early or intermediate stages of disease, but broad-based suppression rather than activation of pro-inflammatory mediators in VF and brain tissue during the late stages of AD [69]. Our finding that cytokines are activated early in AD but not late in the clinical course is consistent with previous observations [59,70]. It is particularly noteworthy that the pronounced reductions in cytokine activation overlapped with declines in the expression of trophic factor and mediators of insulin signaling/responsiveness, and increases in brain levels of AβPP-Aβ, pTau, and advanced glycation end-products [69]. Indeed, the complexity of cytokine activation profiles in AD CSF has been reported previously [71], perhaps due to time or disease duration related shifts in neuro-inflammation. Together, these findings suggest that neuro-inflammation may mediate neurodegeneration at early and possibly other selected stages of AD rather than throughout the clinical course. Correspondingly, the failure to obtain conclusive evidence that anti-inflammatory measures are neuroprotective and can halt neurodegeneration most likely reflects the complexity and non-static nature of the problem.
4.5. Oxidative and endoplasmic reticulum (ER) stress
Insulin/IGF resistance increases both oxidative and ER stress [21]. Persistent oxidative stress leads to reactive oxygen (ROS) and reactive nitrogen (RNS) species formation, as occur in AD [65]. ROS and RNS exacerbate oxidative stress by attacking organelles such as mitochondria. Their molecular attacks result in formation of stable adducts with DNA, RNA, lipids, and proteins, still further compromising neuronal integrity [72]. Oxidation of amino acids leads to formation of advanced glycation end products (AGEs) or advanced oxidation protein products, and protein unfolding, rendering them inactive and vulnerable to cleavage. Oxidation of aliphatic side-chains yields peroxides and carbonyls (aldehydes and ketone) that can attack other molecules and generate radicals, as well as AGE accumulation. Consequences include progressive cellular dysfunction [73,74]. Therefore, elevated levels of AGE in AβPP-Aβ plaques and neurofibrillary tangles [75–77] can contribute to the progressive neuronal loss that occurs with neurodegeneration [72,75,77].
Oxidative stress and its responses can: (1) activate pro-inflammatory networks that exacerbate organelle dysfunction and pro-apoptosis mechanisms; (2) stimulate AβPP gene expression [78] and AβPP cleavage, resulting in increased formation of AβPP-Aβ neurotoxic fibrils [79]; and (3) activate or dis-inhibit GSK-3β, which promotes tau phosphorylation. Therefore, oxidative stress stemming from brain insulin/IGF resistance and metabolic dysfunction contribute to neuronal loss, AβPP-Aβ toxicity, tau cytoskeletal pathology, and neuro-inflammation in AD [3,18,80].
ER functions including protein synthesis, modification, and folding, calcium signaling, and lipid biosynthesis are regulated by glucose metabolism. Insulin resistance-associated impairments in glucose uptake and utilization are associated with increased ER stress [81–83]. Chronically high levels of ER stress dysregulate lipid metabolism, causing accumulation of toxic lipids, e.g. ceramides, and activation of pro-inflammatory and pro-apoptosis cascades [7,84,85]. ER stress and dysregulated lipid metabolism in the brain worsen with severity of AD and brain insulin/IGF resistance [21]. Correspondingly, treatment with Liraglutide, a GLP-1 analogue, protects against high glucose-induced ER stress [86].
4.6. Metabolic deficits
Insulin and IGF signaling regulate glucose utilization and ATP production in brain. In AD, deficits in cerebral glucose utilization and metabolism occur early and prior to significant cognitive decline [87]. Insulin resistance leads to deficiencies in energy metabolism and increased oxidative stress [88–90]. These and other consequences help drive pro-apoptosis, pro-inflammatory, and pro-AβPP-Aβ cascades, which worsen DNA damage, mitochondrial dysfunction, oxidative stress, and ROS generation [3,8,18,19,28]. Therefore, impairments in brain insulin signaling are likely pivotal to AD pathogenesis [19]. Correspondingly, experimental brain insulin/IGF resistance produces cognitive impairment and AD-type neurodegeneration [28,91]. Oxidative stress and ROS damage mitochondrial membranes, mitochondrial DNA, and electron transport systems, reducing capacity to generate ATP and worsening ROS.
4.7. Cerebral microvascular disease
Cerebral microvascular disease is a consistent feature of AD and probable mediator of cognitive impairment. Chronic cerebral microvascular injury is characterized by proliferation of vascular endothelial cells, thickening of the intima, fibrosis of the media, and narrowing of lumens. Mural scarring reduces vascular compliance and compromises blood flow and nutrient delivery, particularly in periods of high metabolic demand. Moreover, blood vessel walls are rendered leaky and therefore permeable to toxins due to their structural weakness [92,93]. Restricted blood flow and delivery of oxygen/nutrients exacerbate the adverse effects of insulin/IGF resistance by further increasing oxidative stress, leading to activation of signaling mechanisms that promote aberrant tau phosphorylation, AβPP cleavage, AβPP-Aβ deposition, and mitochondrial dysfunction [78]. The main microvascular disease-associated lesions in AD include multifocal small infarcts and leukoaraiosis, i.e. extensive white matter fiber attrition with pallor or myelin staining [94]. Since T2DM and hypertension also cause brain microvascular disease, they most likely contribute to neurodegeneration and cognitive impairment in AD [95]. Mechanistically, hyper-insulinemia causes progressive injury to micro-vessels, with attendant chronic cerebral hypoperfusion.
5. Underlying causes of brain insulin resistance
5.1. Aging
Insulin and IGF resistance increase with aging, while longevity is associated with preservation of insulin/IGF responsiveness [55,96,97]. However, cumulative challenges and stresses over a lifespan can damage cells and tissues due to excessive signaling through insulin/IGF-1 receptors [98,99]. Therefore, chronic overuse of insulin/IGF signaling networks, as occurs with hyper-insulinemia and insulin resistance, may be harmful and accelerate aging.
Declines in growth hormone levels and metabolism could also potentiate aging due to the co-occurrence of anabolic deficiencies that accelerate metabolic dysfunction and mortality [100]. Since growth hormone deficiency promotes obesity [101], and obesity promotes insulin resistance and hyperinsulinemia, aging-associated declines in growth hormone could mediate their effects by causing insulin resistance [102]. Attendant impairments in energy balance increase oxidative stress, activate pro-inflammatory pathways, and generate ROS. End results include increased mitochondrial DNA adducts, DNA damage, mitochondrial dysfunction, and cell death.
It is doubtful that insulin resistance, cognitive impairment, and AD are just inevitable consequences of aging [103] since one of the key factors in the equation is that the chronic low-grade inflammation, which accompanies aging [104,105], drives insulin resistance [105,106]. In addition, evidence suggests that underlying, possibly genetic factors may dictate consequences of aging because: (1) the rates and characteristics of aging vary widely among individuals; (2) the nature of and target organs diseased by insulin resistance are heterogeneous; and (3) there is no clear reason why aging per se should result in chronic inflammation, insulin and IGF resistance, or growth hormone deficiency. To account for individual variability in aging and insulin resistance, we hypothesize that underlying genetic or epigenetic host factors dictate organ-system susceptibility to insulin resistance diseases. Genetic factors could be the inheritance of AD-associated genes. Epigenetic factors could be wear and tear effects of poor lifestyle choices, diet, or toxic exposures.
5.2. Lifestyles promoting systemic insulin resistance
Insulin resistance diseases, including AD, obesity, T2DM, non-alcoholic steatohepatitis (NASH), and metabolic syndrome are now pandemic [107–109] and the major cause of sky-rocketing healthcare costs, disability rates, and premature death. The causes are directly linked to increased consumption of highly processed, starch-, sugar- and fat-laden, calorically dense foods that are rendered “tasty” by commercial enterprises. Highly effective marketing continues to lure people to “convenience” foods and lifestyles. Within the past 40–50 years, initially the USA and now the world has witnessed rapid increases in insulin resistance-related disease prevalence among young and middle-aged individuals, including adolescents and children. Type 2 diabetes, non-alcoholic fatty liver disease, metabolic syndrome, cognitive impairment, and cardiovascular diseases are epidemic and occur earlier than in prior years [103]. These trends are linked to the increased prevalence of obesity and sedentary lifestyles. Since the nature and consequences of insulin resistance diseases in younger groups are nearly the same as in older individuals, it could be argued that certain lifestyles, habits, and behaviors cause disease by accelerating aging. The corollary is that lifestyle modifications should slow aging and prevent aging-associated insulin resistance diseases.
5.2.1. Obesity
Obesity is linked to insulin resistance and substantially increases risk for T2DM, NAFLD, NASH, metabolic syndrome, and cognitive impairment [110–113]. With regard to the brain, epidemiological and clinical studies showed that glucose intolerance, deficits in insulin secretion, and insulin resistance diseases (T2DM, obesity/dyslipidemic disorders, or NASH) all increase risk for developing mild cognitive impairment (MCI) or AD-type dementia [8,114–116]. Furthermore, obese individuals have higher rates of executive function impairment [116,117], and have at least double the risk of developing AD than the general population [118]. Correspondingly, experimental diet-induced obesity and T2DM cause cognitive declines [35] with deficits in spatial learning and memory [119], and brain atrophy with insulin resistance, inflammation, oxidative stress, and cholinergic dysfunction [110,120]. In humans, weight loss sufficient to reduce peripheral insulin resistance improves cognitive performance [121,122] and enhances neuropsychiatric function [123], and reductions in metabolic indices through Mediterranean diet adherence lower the risk for AD [124].
5.2.2. Type 2 diabetes mellitus (T2DM)
The molecular and biochemical abnormalities in AD brains mimic the effects of T2DM or NASH on skeletal muscle, adipose tissue, and liver, further suggesting that AD is a brain insulin resistance-related disease. Insulin resistance diseases often overlap in the same individuals. Correspondingly, longitudinal studies showed that T2DM [125] and obesity/dyslipidemic disorders [126] correlate with subsequent development of MCI, dementia, or AD [125,127]. However, postmortem studies suggest that peripheral insulin resistance states contribute to cognitive impairment and AD progression, but do not independently cause AD [128,129]. Similarly, although experimental diet-induced obesity with T2DM causes cognitive impairment with deficits in spatial learning and memory [119], brain atrophy, brain insulin resistance, neuro-inflammation, oxidative stress, and deficits in cholinergic function are relatively mild relative to AD [110,130].
5.2.3. Non-alcoholic fatty liver disease (NAFLD)
The fact that obesity per se, is not an independent risk factor for MCI and neurodegeneration suggests that factors associated with obese states govern these propensities [131]. Independent studies have shown that cognitive impairment and neuropsychiatric dysfunction occur with steatohepatitis and hepatic insulin resistance of various etiologies, including obesity, alcohol abuse, chronic Hepatitis C virus infection, Reyes syndrome, and nitrosamine exposures [130,132–134]. Mechanistically, inflammation in the setting of hepatic steatosis, increases ER stress, oxidative damage, mitochondrial dysfunction, and lipid peroxidation, which together drive hepatic insulin resistance [113]. Insulin resistance dysregulates lipid metabolism and promotes lipolysis [135], which increases production of toxic lipids, including ceramides, which further impair insulin signaling, mitochondrial function, and cell viability [113,136,137]. Liver disease worsens because ER stress and mitochondrial dysfunction exacerbate insulin resistance [112], lipolysis, and ceramide accumulation [81–83].
NAFLD with T2DM and visceral obesity is associated with brain atrophy, neurodegeneration, and cognitive impairment [80,110,120,130,134]. In humans with NASH, neuropsychiatric disease, including depression and anxiety [138], and risks for developing cognitive impairment [139] are increased. In fact, cognitive impairment and neuropsychiatric dysfunction correlate more with steatohepatitis and insulin resistance than with obesity or T2DM [140,141]. Therefore, it is important to consider the potential roles of hepatic insulin resistance and steatohepatitis as mediators of neurodegeneration. To this end, we hypothesized a novel mechanism by which increased levels of cytotoxic ceramides generated in liver could cause neurodegeneration [80,120,130,134]. However, visceral fat is yet another potential source of cytotoxic ceramides.
With steatohepatitis, irrespective of cause, ceramide-related gene expression and ceramide levels are increased [7,8,110,142–145]. Furthermore, cultured CNS neurons exposed to short-chain cytotoxic ceramides develop AD-type molecular and biochemical abnormalities [146,147], and in vivo treatment with short-chain toxic ceramides causes cognitive-motor deficits, brain insulin resistance, oxidative stress, metabolic dysfunction, and neurodegeneration [145]. In addition, brain slice cultures exposed to long-chain ceramide-containing plasma lipids from diet-induced obese rats with steatohepatitis, or purified synthetic long-chain ceramides, produced neurotoxic responses with impairments in culture viability and mitochondrial function [142]. Therefore, toxic lipids generated in liver can cause neurodegeneration.
5.2.4. Metabolic syndrome
Metabolic syndrome is a cluster of disease processes centered around insulin resistance, visceral obesity, hypertension, and dyslipidemia [148]. Metabolic syndrome increases risk for coronary artery disease, atherosclerosis, and T2DM, and is frequently associated with NAFLD/NASH, pro-inflammatory and pro-thrombotic states, and sleep apnea [148]. Studies have linked peripheral insulin resistance [149], visceral obesity [150], and metabolic syndrome [151–153] to brain atrophy, cognitive impairment, and impaired executive function [154]. The aggregate findings in humans and experimental models suggest that peripheral/systemic insulin resistance disease states serve as cofactors in the pathogenesis and progression of neurodegeneration. Therefore, measures that strategically address systemic insulin resistance should help reduce progression and severity of neurodegeneration [155].
6. Environmental and dietary exposure factors as mediators of insulin resistance and neurodegeneraton
6.1. Nitrosamine-mediated cellular and molecular injury
The prevalence rates of insulin resistance disease, including AD have increased rapidly over the past several decades [156–160]. These dramatic shifts in morbidity and mortality occurred across a broad range of age-groups such that the effects are more consistent with exposure-related rather than genetic etiologies. The striking increases in AD mortality rates corrected for age followed sharp increases consumption of processed foods, use of preservatives, and demand for nitrogen-containing fertilizers [109]. The common thread is that these lifestyle changes have inadvertently increased chronic exposures to nitrosamines (R1N(–R2)–N=O) and related compounds.
Nitrosamines form by chemical reactions between nitrites and secondary amines (proteins). Nitrosamines exert their toxic and mutagenic effects by alkylating N-7 of guanine, leading to increased DNA damage [161] and ROS production, followed by lipid peroxidation, protein adduct formation, and pro-inflammatory cytokine activation [162,84]. Curiously, these very same molecular and biochemical pathogenic cascades occur in insulin-resistance diseases, including T2DM, NASH, and AD [90,125,163–167]. The concept that chronic alkylating agent-injury could cause malignancy and/or tissue degeneration is important and not exactly foreign, given the facts that: (1) chronic exposures to tobacco nitrosamines cause lung cancer and emphysema; and (2) streptozotocin (STZ), a nitrosamine-related compound, causes hepatocellular or pancreatic carcinoma, T2DM, AD-type neurodegeneration, and steatohepatitis, depending on dose and route of administration [28,30,80,87,168–172]. Therefore, although nitrosamine-related research has largely focused on mutagenesis, at this junction it seems warranted to investigate the degenerative effects as well.
6.2. Nitrosamines and neurodegeneration: Role of streptozotocin (STZ)
STZ, like other N-nitroso compounds, causes tissue injury and disease because if functions as an alkylating agent [30], an inducer of DNA adducts that lead to increased apoptosis [173], an inducer of single-strand DNA breaks and stimulus for nitric oxide (NO) formation [169]; and an enhancer of the xanthine oxidase system, increasing production of superoxide anion, H2O2, and OH− radicals [174]. In addition, STZ mediates neural injury by inducing pro-inflammatory responses [29,175,176]. Progressive cellular injury, DNA damage, and oxidative stress cause mitochondrial dysfunction [169], ATP deficiency [177], poly-ADP ribosylation, and finally apoptosis. The findings of brain insulin deficiency and insulin resistance, deficits in cholinergic function, impairments in spatial learning and memory, and AD-type histopathologic lesions in rats that were given intracerebral injections of streptozotocin [28,84,91,178–181], raised questions about nitrosamine toxin exposures as mediators of AD in humans. This concept was reinforced by data showing that STZ also causes T2DM and NAFLD [182–184], and that STZ’s degenerative effects are mediated by impairments in insulin signaling and metabolism, and increased oxidative stress, mitochondrial dysfunction, and cell death [28,80,170,178,180,185].
6.3. Dietary nitrosamines as potential mediators of AD neurodegeneration
The structural similarities between STZ and nitrosamines, including N-nitrosodiethylamine (NDEA) and N-nitrosodimethylamine (NDMA) [186], together with experimental evidence that high doses of STZ cause cancer while lower doses cause diabetes or AD-type neurodegeneration with cognitive impairment [28,169,172], led to the following hypothesis. High exposure levels of environmental and consumed nitrosamines cause cancer, whereas lower, sub-mutagenic doses produce insulin-resistance mediated degenerative diseases, including T2DM, NASH, metabolic syndrome, visceral obesity, and AD. STZ has little relevance to human diseases due to minimal or absent exposures. In contrast, humans are frequently exposed to NDEA and NDMA through the diet; NDEA and NDMA are structurally related to STZ. In addition, human exposures to tobacco nitrosamines also increased steadily until public health and policy measures blunted tobacco consumption. Correspondingly, meta-analysis studies have disclosed links between cigarette smoking and AD [187].
The above hypothesis was tested by treating rats with low and limited sub-mutagenic doses of NDEA. The rats developed systemic insulin resistance with hepatic steatosis, visceral obesity, T2DM, and neurodegeneration. Coupling the NDEA exposures with chronic high fat diet feeding additively worsened the outcomes with respect to insulin resistance diseases [84,143]. These findings support our hypothesis that the relatively recent epidemics of sporadic AD, T2DM, and NASH/metabolic syndrome are mediated by chronic environmental or dietary nitrosamine exposures [109].
7. Reverberating loop of insulin/metabolic malsignaling in AD
7.1. Insulin resistance cascade: Ceramides and lipotoxocity
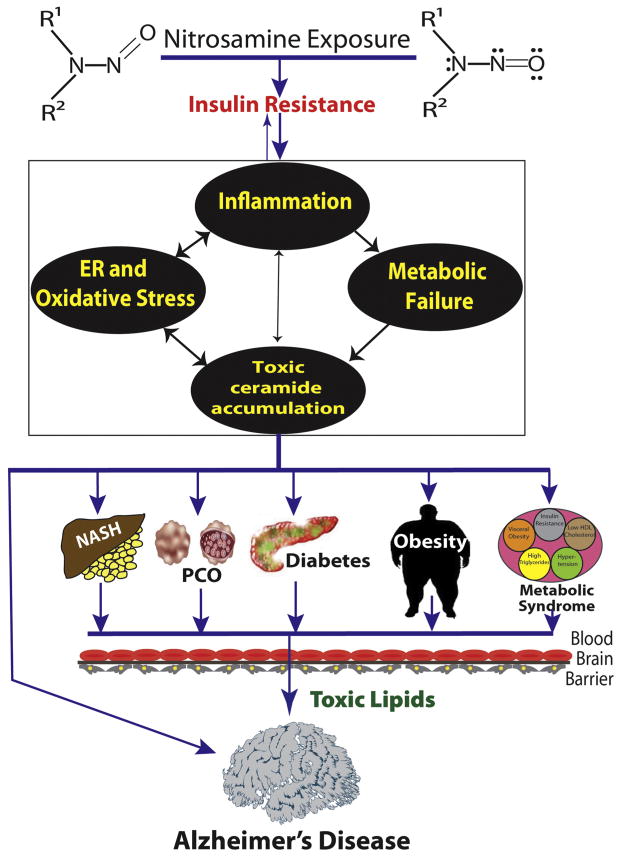
Chronic obesity, T2DM, NASH, and AD share in common, insulin resistance which is associated with inflammation and lipid dyshomeostasis. Chronic inflammation is mediated by activation of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [67,188,189]. Lipid dyshomeostasis results in increased ceramide generation in adipose tissue and liver [146,190–192]. Insulin resistance, inflammation, and ceramide accumulation promote oxidative and ER stress, which impair mitochondrial function, energy balance, and membrane integrity, and worsen insulin resistance, inflammation, and ceramide generation [81,82,193–196]. Unchecked, the rates of injury eventually exceed those of repair. Resulting states of chronic insulin resistance initiate a harmful positive feedback mal-signaling loop that mediates progressive organ-system degeneration (Fig. 1).
Fig. 1.
Primary and secondary mediators of neurodegeneration are linked to harmful positive feedback loops driven by insulin resistance. Alzheimer’s disease, non-alcoholic steatohepatitis (NASH), polycystic ovarian syndrome (PCO), Type 2 diabetes mellitus, obesity, and metabolic syndrome are all associated with insulin resistance. Once established, insulin resistance drives a positive feedback cycle of inflammation, endoplasmic reticulum (ER) stress, dysregulated lipid metabolism with increased ceramide generation, and metabolic dysfunction in the affected organs and tissues. The consequences are increased cell death, impaired function, and organ/tissue degeneration. With regard to the brain, insulin resistance can occur as a primary disease process leading to selective neurodegeneration (Alzheimer’s disease). Alternatively, neurodegeneration could be consequential to insulin resistance in other organs, and arise in association with NASH, PCO, diabetes, obesity, and metabolic syndrome. We propose that toxic lipids (ceramides) released from injured and dying cells exert neurotoxic effects and cause insulin resistance. In primary AD, toxic ceramides are generated primarily in the brain. With regard to systemic diseases, ceramides made in peripheral organs injure the brain after crossing the blood–brain barrier. A self-reinforcing mal-signaling loop leads to progressive neurodegeneration. However, we are still left with the question of underlying etiologies. Epidemiologic and experimental data point toward chronic low-level nitrosamine exposures through dietary, agricultural, and smoking sources as upstream causes of insulin resistance diseases. Our over-arching hypothesis is that while high levels of nitrosamine exposures cause cancer, chronic low, sub-mutagenic doses cause insulin resistance-associated degenerative diseases. Host factors including aging and lifestyle measures may dictate propensity for different subtypes of insulin resistance diseases, including Alzheimer’s.
7.2. Extrinsic factors mediating neurodegeneration
Although cognitive impairment, brain insulin/IGF resistance, brain atrophy, and neurodegeneration frequently develop in peripheral insulin resistance disease states associated with chronic obesity, T2DM, NASH, and metabolic syndrome, the common variables are steatohepatitis and/or visceral obesity with increased ceramide accumulation [67,136,193,197]. Ceramides are lipid signaling molecules [191,198] that regulate positive (growth, motility, adhesion, differentiation) and negative (senescence, apoptosis, insulin resistance) cellular functions. Ceramides accumulate in cells due to disturbances in sphingolipid metabolism [67,199–201] and upregulation of pro-ceramide genes [110,202]. Altered sphingolipid metabolism aberrantly increases intracellular ceramide levels and insulin resistance [113,136,199,200,203–205] in obesity, T2DM, NASH, and AD [81,82,146,190–196].
Since none of the experimental models of peripheral insulin resistance were associated with substantial alterations ceramide-related gene expression or enzymatic activity in brain, if toxic ceramides were to mediate disease, they must originate from sources outside of the CNS. Studies showing that systemically administered toxic ceramides can cross the blood–brain barrier and cause neurodegeneration, insulin resistance, and neurobehavioral abnormalities [144], together with the finding that ceramides present in peripheral blood of rats with steatohepatitis cause neurotoxic injury in vitro [206], led us to the liver–brain axis hypothesis. The basic concept is that cytotoxic ceramides generated in liver and probably also visceral fat, leak into peripheral blood following injury or cell death caused by local tissue inflammation. Cytotoxic ceramides then traffic through the circulation, and due to their lipid soluble nature, cross the blood–brain barrier and exert neurotoxic and neurodegenerative effects by impairing insulin signaling [7,144,207] and activating pro-inflammatory cytokines [67,208]. This scheme explains how brain insulin resistance, which is an early and important feature of AD, could be mediated by peripheral insulin resistance diseases that are associated with hepatic or visceral fat accumulation, inflammation, dysregulated lipid metabolism, ER/oxidative stress, mitochondrial dysfunction, and activation of pro-death signaling networks [142,144,207].
7.3. Intrinsic pathway to type 3 diabetes
Although T2DM, obesity, NASH, and metabolic syndrome are major driving forces in the cognitive impairment and AD epidemics, it is important to bear in mind that most cases of AD are not associated with obesity or significant peripheral insulin resistance diseases. Yet, AD is clearly a metabolic degenerative disease with brain insulin/IGF resistance and deficiency. In addition, the brain-restricted insulin/IGF resistance is associated with dysregulated lipid metabolism, long-chain ceramide accumulation, inflammation, ER and oxidative stress, and mitochondrial dysfunction [21,142]. Although the causes of primary brain insulin/IGF resistance and deficiency in sporadic AD are not known, experimental evidence suggests roles for nitrosamine exposures. This concept fits with data indicating widespread and abundant exposures to nitrosamines and their precursors in our diets and resulting from lifestyle trends over the past 50 years. Experiments showed that low-level nitrosamine exposures cause the full spectrum of insulin resistance diseases, including T2DM, visceral obesity, NASH, metabolic syndrome, and AD-type neurodegeneration. These findings led to the concept of an intrinsic pathway for neurodegeneration. We propose that AD and probably other neurodegenerative diseases are mediated by chronic, low-level exposures to nitrosamines, through diet, lifestyle choices, and possibly tobacco. The nitrosamine toxins exert their degenerative effects by causing insulin resistance and oxidative stress in various organs, including brain. In addition, nitrosamine exposures exacerbate the effects of obesity and aging-associated insulin resistance, and thereby serve to initiate, propagate, and exacerbate the AD neurodegeneration cascade.
8. Conclusions
AD is fundamentally a metabolic disease of the brain that is driven by insulin and IGF resistance and deficiency, and mimics the effects and consequences of diabetes mellitus. The molecular, biochemical, and degenerative features of AD correspond with the abnormalities that occur within the spectrum of systemic insulin resistance diseases.
Brain insulin/IGF resistance, whether primary or secondary, initiates a cascade driven by increased oxidative stress, neuro-inflammation, impaired cell survival, mitochondrial dysfunction, dysregulated lipid metabolism, and ER stress. These processes compromise neuronal and glial functions, reducing neurotransmitter homeostasis, disrupting neuronal cytoskeletal and amyloid-beta precursor protein (AβPP) functions, and causing toxic oligomeric fibrils and insoluble aggregates (neurofibrillary tangles and AβPP-Aβ plaques) to accumulate.
AD progresses due to: (1) activation of a harmful, self-reinforcing, positive feedback loop that worsens the effects of insulin resistance; and (2) the formation of ROS- and RNS-related lipid, protein, and DNA adducts that permanently damage basic cellular and molecular functions.
Since the underlying cellular, molecular, and biochemical abnormalities in various insulin/IGF resistance diseases are nearly identical, the underlying mechanisms are likely to be shared. Obvious shifts in disease prevalence and lifestyles over the past 50 years point toward exposure factors as causal agents. We propose that chronic low-level nitrosamine exposures through diet, smoking, and agriculture, plus excessive caloric intake of fats and simple sugars, are responsible for the insulin resistance diseases epidemic. This hypothesis is supported by experimental data.
Our concept regarding the pathogenesis of AD broadens opportunities for prevention, and the discovery of treatments that may be effective across the full spectrum of metabolic-insulin/IGF resistance diseases.
Acknowledgments
Funding sources are Grants AA-11431 and AA-12908 from the National Institutes of Health.
References
- 1.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance – a mini-review. Gerontology. 2009;55:379–86. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 2.Kuemmerle JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am. 2012;41(vii):409–23. doi: 10.1016/j.ecl.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 4.D’Ercole AJ, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–62. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Curr Alzheimer Res. 2009;6:213–23. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- 6.Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR. The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health. 2009;6:2055–75. doi: 10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–8. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 10.Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32:1464–6. doi: 10.2337/dc09-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia RG, Rincon MY, Arenas WD, Silva SY, Reyes LM, Ruiz SL, et al. Hyperinsulinemia is a predictor of new cardiovascular events in Colombian patients with a first myocardial infarction. Int J Cardiol. 2011;148:85–90. doi: 10.1016/j.ijcard.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Kasai T, Miyauchi K, Kajimoto K, Kubota N, Dohi T, Kurata T, et al. The adverse prognostic significance of the metabolic syndrome with and without hypertension in patients who underwent complete coronary revascularization. J Hypertens. 2009;27:1017–24. doi: 10.1097/HJH.0b013e32832961cf. [DOI] [PubMed] [Google Scholar]
- 13.Agnoli C, Berrino F, Abagnato CA, Muti P, Panico S, Crosignani P, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metabol Cardiovasc Dis. 2010;20:41–8. doi: 10.1016/j.numecd.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24:58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 15.Colonna SV, Douglas Case L, Lawrence JA. A retrospective review of the metabolic syndrome in women diagnosed with breast cancer and correlation with estrogen receptor. Breast Cancer Res Treat. 2012;131:325–31. doi: 10.1007/s10549-011-1790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–38. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–25. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 19.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this type 3 diabetes. J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 20.de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci. 2012;E4:1582–605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Monte SM, Re E, Longato L, Tong M. Dysfunctional pro-ceramide, ER stress, and insulin/IGF signaling networks with progression of Alzheimer’s disease. J Alzheimers Dis. 2012;30:S217–29. doi: 10.3233/JAD-2012-111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65:1231–6. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 23.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–95. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langbaum JB, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67:462–8. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer S, Nitsch R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J Neural Transm. 1989;75:227–32. doi: 10.1007/BF01258634. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3:1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 27.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 29.Weinstock M, Shoham S. Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J Neural Transm. 2004;111:347–66. doi: 10.1007/s00702-003-0058-y. [DOI] [PubMed] [Google Scholar]
- 30.Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 31.Koulmanda M, Qipo A, Chebrolu S, O’Neil J, Auchincloss H, Smith RN. The effect of low versus high dose of streptozotocin in cynomolgus monkeys (Macaca fascilularis) Am J Transplant. 2003;3:267–72. doi: 10.1034/j.1600-6143.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 32.de la Monte SM, Tong M, Bowling N, Moskal P. si-RNA inhibition of brain insulin or insulin-like growth factor receptors causes developmental cerebellar abnormalities: relevance to fetal alcohol spectrum disorder. Mol Brain. 2011;4:13. doi: 10.1186/1756-6606-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–99. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–9. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP. The impact of dietary energy intake on cognitive aging. Front Aging Neurosci. 2010;2:5. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–5. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 38.Fraser PE, Yu G, Levesque L, Nishimura M, Yang DS, Mount HT, et al. Presenilin function: connections to Alzheimer’s disease and signal transduction. Biochem Soc Symp. 2001;67:89–100. doi: 10.1042/bss0670089. [DOI] [PubMed] [Google Scholar]
- 39.Morales I, Farias G, Maccioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17:202–4. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal K, Liu F, Gong CX, del Alonso AC, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhat R, Xue Y, Berg S, Hellberg S, Ormo M, Nilsson Y, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278:45937–45. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 42.Takashima A. Drug development for tauopathy and Alzheimer’s disease. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010;30:177–80. [PubMed] [Google Scholar]
- 43.Arnaud L, Robakis NK, Figueiredo-Pereira ME. It may take inflammation, phosphorylation and ubiquitination to ‘tangle’ in Alzheimer’s disease. Neurodegener Dis. 2006;3:313–9. doi: 10.1159/000095638. [DOI] [PubMed] [Google Scholar]
- 44.Oddo S. The ubiquitin-proteasome system in Alzheimer’s disease. J Cell Mol Med. 2008;12:363–73. doi: 10.1111/j.1582-4934.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–85. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 46.de la Monte SM, Chen GJ, Rivera E, Wands JR. Neuronal thread protein regulation and interaction with microtubule-associated proteins in SH-Sy5y neuronal cells. Cell Mol Life Sci. 2003;60:2679–91. doi: 10.1007/s00018-003-3305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robakis NK. Mechanisms of AD neurodegeneration may be independent of Abeta and its derivatives. Neurobiol Aging. 2011;32:372–9. doi: 10.1016/j.neurobiolaging.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu L, Rosa-Neto P, Hsiung GY, Sadovnick AD, Masellis M, Black SE, et al. Early-onset familial Alzheimer’s disease (EOFAD) Can J Neurol Sci. 2012;39:436–45. doi: 10.1017/s0317167100013949. [DOI] [PubMed] [Google Scholar]
- 50.Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J Cell Mol Med. 2011;15:1807–21. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emmerling MR, Spiegel K, Watson MD. Inhibiting the formation of classical C3-convertase on the Alzheimer’s beta-amyloid peptide. Immunopharmacology. 1997;38:101–9. doi: 10.1016/s0162-3109(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 52.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–76. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 53.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 54.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease. Trends Pharmacol Sci. 2002;23:288–93. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 55.Schuh AF, Rieder CM, Rizzi L, Chaves M, Roriz-Cruz M. Mechanisms of brain aging regulation by insulin: implications for neurodegeneration in late-onset Alzheimer’s disease. ISRN Neurol. 2011;2011:306905. doi: 10.5402/2011/306905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 2005;12:311–28. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–9. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 59.Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA. Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 60.Blasko I, Jungwirth S, Jellinger K, Kemmler G, Krampla W, Weissgram S, et al. Effects of medications on plasma amyloid beta (Abeta) 42: longitudinal data from the VITA cohort. J Psychiatr Res. 2008;42:946–55. doi: 10.1016/j.jpsychires.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–9. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- 62.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Eikelenboom P, van Gool WA. Neuroinflammatory perspectives on the two faces of Alzheimer’s disease. J Neural Transm. 2004;111:281–94. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- 64.Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117:919–47. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 65.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–46. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology. 2010;58:561–8. doi: 10.1016/j.neuropharm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 68.de la Monte SM. Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30:S231–49. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S, Tong M, Hang S, Deochand C, de la Monte SM. CSF and brain indices of insulin resistance, oxidative stress and neuro-inflammation in early versus late Alzheimer’s disease. J Alzheimers Dis Parkinsonism. 2013;3:128. doi: 10.4172/2161-0460.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galimberti D, Fenoglio C, Scarpini E. Inflammation in neurodegenerative disorders: friend or foe. Curr Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 71.Olson L, Humpel C. Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer’s disease and mild cognitive impairment. Exp Gerontol. 2010;45:41–6. doi: 10.1016/j.exger.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:763–74. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greilberger J, Fuchs D, Leblhuber F, Greilberger M, Wintersteiger R, Tafeit E. Carbonyl proteins as a clinical marker in Alzheimer’s disease and its relation to tryptophan degradation and immune activation. Clin Lab. 2010;56:441–8. [PubMed] [Google Scholar]
- 74.Gu F, Zhu M, Shi J, Hu Y, Zhao Z. Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice. Neurosci Lett. 2008;440:44–8. doi: 10.1016/j.neulet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 75.Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adhes Migrat. 2009;3:88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahmadi A, Steiner N, Munch G. Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer’s disease. Clin Chem Lab Med. 2011;49:385–91. doi: 10.1515/CCLM.2011.079. [DOI] [PubMed] [Google Scholar]
- 77.Krautwald M, Munch G. Advanced glycation end products as biomarkers and gerontotoxins – a basis to explore methylglyoxal-lowering agents for Alzheimer’s disease. Exp Gerontol. 2010;45:744–51. doi: 10.1016/j.exger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Chen GJ, Xu J, Lahousse SA, Caggiano NL, de la Monte SM. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5:209–28. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 79.Tsukamoto E, Hashimoto Y, Kanekura K, Niikura T, Aiso S, Nishimoto I. Characterization of the toxic mechanism triggered by Alzheimer’s amyloid-beta peptides via p75 neurotrophin receptor in neuronal hybrid cells. J Neurosci Res. 2003;73:627–36. doi: 10.1002/jnr.10703. [DOI] [PubMed] [Google Scholar]
- 80.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 81.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–77. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 82.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–9. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sundar-Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125:411–24. [PubMed] [Google Scholar]
- 84.de la Monte SM, Tong M. Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2009;17:817–25. doi: 10.3233/JAD-2009-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21(Suppl 3):S7–9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- 86.Schisano B, Harte AL, Lois K, Saravanan P, Al-Daghri N, Al-Attas O, et al. GLP-1 analogue, Liraglutide protects human umbilical vein endothelial cells against high glucose induced endoplasmic reticulum stress. Regul Pept. 2012;174:46–52. doi: 10.1016/j.regpep.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–52. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- 88.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 89.Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–9. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S20–7. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 91.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–70. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 92.Kincaid-Smith P. Hypothesis: obesity and the insulin resistance syndrome play a major role in end-stage renal failure attributed to hypertension and labelled ‘hypertensive nephrosclerosis’. J Hypertens. 2004;22:1051–5. doi: 10.1097/00004872-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto H, Nakao T, Okada T, Nagaoka Y, Iwasawa H, Tomaru R, et al. Insulin resistance contributes to obesity-related proteinuria. Intern Med. 2005;44:548–53. doi: 10.2169/internalmedicine.44.548. [DOI] [PubMed] [Google Scholar]
- 94.Etiene D, Kraft J, Ganju N, Gomez-Isla T, Gemelli B, Hyman BT, et al. Cerebrovascular pathology contributes to the heterogeneity of Alzheimer’s disease. J Alzheimers Dis. 1998;1:119–34. doi: 10.3233/jad-1998-1205. [DOI] [PubMed] [Google Scholar]
- 95.Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia aging study. Diabetes Care. 2006;29:2268–74. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- 96.Sato N, Takeda S, Uchio-Yamada K, Ueda H, Fujisawa T, Rakugi H, et al. Role of insulin signaling in the interaction between Alzheimer disease and diabetes mellitus: a missing link to therapeutic potential. Curr Aging Sci. 2011;4:118–27. doi: 10.2174/1874609811104020118. [DOI] [PubMed] [Google Scholar]
- 97.Holzenberger M. Igf-I signaling and effects on longevity. Nestle Nutr Workshop Ser Pediatr Prog. 2011;68:237–45. doi: 10.1159/000325914. discussion 46–9. [DOI] [PubMed] [Google Scholar]
- 98.Valentini S, Cabreiro F, Ackerman D, Alam MM, Kunze MB, Kay CW, et al. Manipulation of in vivo iron levels can alter resistance to oxidative stress without affecting ageing in the nematode C. elegans. Mech Ageing Dev. 2012;133:282–90. doi: 10.1016/j.mad.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zemva J, Udelhoven M, Moll L, Freude S, Stohr O, Bronneke HS, et al. Neuronal overexpression of insulin receptor substrate 2 leads to increased fat mass, insulin resistance, and glucose intolerance during aging. Age (Dordr) 2012;35:1881–97. doi: 10.1007/s11357-012-9491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castilla-Cortazar I, Garcia-Fernandez M, Delgado G, Puche JE, Sierra I, Barhoum R, et al. Hepatoprotection and neuroprotection induced by low doses of IGF-II in aging rats. J Transl Med. 2011;9:103. doi: 10.1186/1479-5876-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luque RM, Lin Q, Cordoba-Chacon J, Subbaiah PV, Buch T, Waisman A, et al. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PloS One. 2011;6:e15767. doi: 10.1371/journal.pone.0015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Srikanth V, Westcott B, Forbes J, Phan TG, Beare R, Venn A, et al. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci. 2012;68:68–73. doi: 10.1093/gerona/gls100. [DOI] [PubMed] [Google Scholar]
- 103.Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem. Biochem Pharmacol. 2012;84:737–45. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 104.Oxenkrug G. Interferon-gamma – inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging Dis. 2011;2:474–86. [PMC free article] [PubMed] [Google Scholar]
- 105.Horrillo D, Sierra J, Arribas C, Garcia-San Frutos M, Carrascosa JM, Lauzurica N, et al. Age-associated development of inflammation in Wistar rats: effects of caloric restriction. Arch Physiol Biochem. 2011;117:140–50. doi: 10.3109/13813455.2011.577435. [DOI] [PubMed] [Google Scholar]
- 106.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY) 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G697–702. doi: 10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 109.de la Monte SM, Neusner A, Chu J, Lawton M. Epidemiological trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;17:519–29. doi: 10.3233/JAD-2009-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, et al. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715–29. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes: evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–13. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Capeau J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008;34:649–57. doi: 10.1016/S1262-3636(08)74600-7. [DOI] [PubMed] [Google Scholar]
- 113.Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–41. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- 114.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–5. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 115.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 116.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–52. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007;4:123–6. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- 119.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–9. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and Type 2 diabetes mellitus. J Alzheimers Dis. 2008;15:29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–79. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bryan J, Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001;36:147–56. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- 124.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22:483–92. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–14. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- 126.Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipo-protein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 127.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7:373–80. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 128.Nelson PT, Smith CD, Abner EA, Schmitt FA, Scheff SW, Davis GJ, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–69. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–81. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 130.Tong M, Longato L, de la Monte SM. Early limited nitrosamine exposures exacerbate high fat diet-mediated type2 diabetes and neurodegeneration. BMC Endocr Dis. 2010;10:4. doi: 10.1186/1472-6823-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 132.Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Dig Dis Sci. 2008;53:307–21. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- 133.Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006;3:176–81. doi: 10.1007/s11904-006-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J Alzheimers Dis. 2009;17:827–44. [PMC free article] [PubMed] [Google Scholar]
- 135.Kao Y, Youson JH, Holmes JA, Al-Mahrouki A, Sheridan MA. Effects of insulin on lipid metabolism of larvae and metamorphosing landlocked sea lamprey, Petromyzon marinus. Gen Comp Endocrinol. 1999;114:405–14. doi: 10.1006/gcen.1999.7265. [DOI] [PubMed] [Google Scholar]
- 136.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Langeveld M, Aerts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res. 2009;48:196–205. doi: 10.1016/j.plipres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68:563–9. doi: 10.1097/01.psy.0000221276.17823.df. [DOI] [PubMed] [Google Scholar]
- 139.Felipo V, Urios A, Montesinos E, Molina I, Garcia-Torres ML, Civera M, et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab Brain Dis. 2011;27:51–8. doi: 10.1007/s11011-011-9269-3. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, et al. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20:286–91. doi: 10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- 141.Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148–54. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- 142.de la Monte SM. Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30:S231–S49. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de la Monte SM, Tong M, Lawton M, Longato L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener. 2009;4:54. doi: 10.1186/1750-1326-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J Alzheimers Dis. 2010;21:967–84. doi: 10.3233/JAD-2010-091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tong M, de la Monte SM. Mechanisms of ceramide-mediated neurodegeneration. J Alzheimers Dis. 2009;16:705–14. doi: 10.3233/JAD-2009-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem Soc Trans. 2004;32:144–6. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- 147.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–68. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham offspring study. Diabetes Care. 2011;34:1766–70. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–44. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dement Geriatr Cogn Disord. 2010;29:356–62. doi: 10.1159/000296071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frisardi V, Solfrizzi V, Capurso C, Imbimbo BP, Vendemiale G, Seripa D, et al. Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? J Alzheimers Dis. 2010;21:57–63. doi: 10.3233/JAD-2010-100015. [DOI] [PubMed] [Google Scholar]
- 153.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–7. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burns JM, Honea RA, Vidoni ED, Hutfles LJ, Brooks WM, Swerdlow RH. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta. 2012;1822:333–9. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20:723–36. doi: 10.3233/JAD-2010-091687. [DOI] [PubMed] [Google Scholar]
- 156.Delgado JS. Evolving trends in nonalcoholic fatty liver disease. Eur J Intern Med. 2008;19:75–82. doi: 10.1016/j.ejim.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 157.Launer LJ. Next steps in Alzheimer’s disease research: interaction between epidemiology and basic science. Curr Alzheimer Res. 2007;4:141–3. doi: 10.2174/156720507780362155. [DOI] [PubMed] [Google Scholar]
- 158.Nugent C, Younossi ZM. Evaluation and management of obesity-related nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:432–41. doi: 10.1038/ncpgasthep0879. [DOI] [PubMed] [Google Scholar]
- 159.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152–6. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 160.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185–92. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Swann PF, Magee PN. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968;110:39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schook LB, Lockwood JF, Yang SD, Myers MJ. Dimethylnitrosamine (DMN)-induced IL-1 beta, TNF-alpha, and IL-6 inflammatory cytokine expression. Toxicol Appl Pharmacol. 1992;116:110–6. doi: 10.1016/0041-008x(92)90151-h. [DOI] [PubMed] [Google Scholar]
- 163.Marchesini G, Marzocchi R. Metabolic syndrome and NASH. Clin Liver Dis. 2007;11(ix):105–17. doi: 10.1016/j.cld.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 164.Nicolls MR. The clinical and biological relationship between Type II diabetes mellitus and Alzheimer’s disease. Curr Alzheimer Res. 2004;1:47–54. doi: 10.2174/1567205043480555. [DOI] [PubMed] [Google Scholar]
- 165.Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409–15. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 166.Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193–9. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837–47. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 168.Doi K. Studies on the mechanism of the diabetogenic activity of streptozotocin and on the ability of compounds to block the diabetogenic activity of streptozotocin (author’s transl) Nippon Naibunpi Gakkai Zasshi. 1975;51:129–47. doi: 10.1507/endocrine1927.51.3_129. [DOI] [PubMed] [Google Scholar]
- 169.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 170.Hoyer S, Lannert H, Noldner M, Chatterjee SS. Damaged neuronal energy metabolism and behavior are improved by Ginkgo biloba extract (EGb 761) J Neural Transm. 1999;106:1171–88. doi: 10.1007/s007020050232. [DOI] [PubMed] [Google Scholar]
- 171.Iwai S, Murai T, Makino S, Min W, Morimura K, Mori S, et al. High sensitivity of fatty liver Shionogi (FLS) mice to diethylnitrosamine hepatocarcinogenesis: comparison to C3H and C57 mice. Cancer Lett. 2007;246:115–21. doi: 10.1016/j.canlet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 172.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977;74:2485–9. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murata M, Takahashi A, Saito I, Kawanishi S. Site-specific DNA methylation and apoptosis: induction by diabetogenic streptozotocin. Biochem Pharmacol. 1999;57:881–7. doi: 10.1016/s0006-2952(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 174.Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J. Enhancement by streptozotocin of O2-radical generation by the xanthine oxidase system of pancreatic beta-cells. FEBS Lett. 1988;239:295–8. doi: 10.1016/0014-5793(88)80938-4. [DOI] [PubMed] [Google Scholar]
- 175.Cai Z, Zhao Y, Yao S, Bin Zhao B. Increases in beta-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-kappaB pathway activation. Pharmacol Rep. 2011;63:381–91. doi: 10.1016/s1734-1140(11)70504-7. [DOI] [PubMed] [Google Scholar]
- 176.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol. 2011;31:1796–804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 178.Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasinski A, et al. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease. Acta Neurochir Suppl. 2010;106:177–81. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- 179.Wang S, Kamat A, Pergola P, Swamy A, Tio F, Cusi K. Metabolic factors in the development of hepatic steatosis and altered mitochondrial gene expression in vivo. Metabolism. 2011;60:1090–9. doi: 10.1016/j.metabol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 180.Hoyer S, Muller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl. 1994;44:259–68. doi: 10.1007/978-3-7091-9350-1_20. [DOI] [PubMed] [Google Scholar]
- 181.Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 182.Shi FH, Wu Y, Dai DZ, Cong XD, Zhang YM, Dai Y. Hepatosteatosis and hepatic insulin resistance are blunted by argirein, an anti-inflammatory agent, through normalizing endoplasmic reticulum stress and apoptosis in diabetic liver. J Pharm Pharmacol. 2013;65:916–27. doi: 10.1111/jphp.12051. [DOI] [PubMed] [Google Scholar]
- 183.Gandhi GR, Stalin A, Balakrishna K, Ignacimuthu S, Paulraj MG, Vishal R. Insulin sensitization via partial agonism of PPARgamma and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim Biophys Acta. 2013;1830:2243–55. doi: 10.1016/j.bbagen.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 184.Moreno P, Nuche-Berenguer B, Gutierrez-Rojas I, Acitores A, Sancho V, Valverde I, et al. Normalizing action of exendin-4 and GLP-1 in the glucose metabolism of extrapancreatic tissues in insulin-resistant and type 2 diabetic states. J Mol Endocrinol. 2012;48:37–47. doi: 10.1530/JME-11-0127. [DOI] [PubMed] [Google Scholar]
- 185.Duelli R, Schrock H, Kuschinsky W, Hoyer S. Intracerebroventricular injection of streptozotocin induces discrete local changes in cerebral glucose utilization in rats. Int J Dev Neurosci. 1994;12:737–43. doi: 10.1016/0736-5748(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 186.Robbiano L, Mereto E, Corbu C, Brambilla G. DNA damage induced by seven N-nitroso compounds in primary cultures of human and rat kidney cells. Mutat Res. 1996;368:41–7. doi: 10.1016/s0165-1218(96)90038-5. [DOI] [PubMed] [Google Scholar]
- 187.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19:465–80. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–14. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 189.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–76. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 190.Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease. Neurochem Res. 2007;32:845–56. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 191.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–35. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 192.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 193.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 194.Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596–607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- 195.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–87. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Dis. 2011;12:163–72. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- 197.Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61:47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 199.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–8. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 200.Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997;8:311–22. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- 201.Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983–90. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 202.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–8. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 203.Arboleda G, Huang TJ, Waters C, Verkhratsky A, Fernyhough P, Gibson RM. Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur J Neurosci. 2007;25:3030–8. doi: 10.1111/j.1460-9568.2007.05557.x. [DOI] [PubMed] [Google Scholar]
- 204.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–53. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 205.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–17. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 206.de la Monte SM, Lyn-Cook L, Lawton M, Tong M, Silberman E, Longato L, et al. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. Handbook of animal models in Alzheimer’s disease. 2011;1:179–99. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–66. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]