Abstract
Ceramides are lipid signaling molecules that cause cytotoxicity and cell death mediated by insulin resistance, inflammation, and endoplasmic reticulum (ER) stress. However, insulin resistance dysregulates lipid metabolism, which promotes ceramide accumulation with attendant inflammation and ER stress. Herein, we discuss two major pathways, extrinsic and intrinsic, that converge and often overlap in propagating AD-type neurodegeneration via a triangulated mal-signaling network. First, we review evidence that systemic insulin resistance diseases linked to obesity, type 2 diabetes, and non-alcoholic steatohepatitis promote neurodegeneration. Mechanistically, we propose that toxic ceramides generated in extra-CNS tissues (e.g., liver) get released into peripheral blood, and subsequently transit across the blood-brain barrier into the brain where they induce brain insulin resistance, inflammation, and cell death (extrinsic pathway). Then we discuss the role of the intrinsic pathway of neurodegeneration which is mediated by endogenous or primary brain insulin/IGF resistance, and impairs neuronal and oligodendrocyte survival, energy metabolism, membrane integrity, cytoskeletal function, and AβPP-Aβ secretion. The end result is increased ER stress and ceramide generation, which exacerbate brain insulin resistance, cell death, myelin degeneration, and neuroinflammation. Altogether, the data suggest that the triangulated mal-signaling network mediated by toxic ceramides, ER stress, and insulin resistance should be targeted to disrupt positive feedback loops that drive the AD neurodegeneration cascade.
Keywords: Central nervous system, ceramide, diabetes mellitus, insulin resistance, myelin, neurodegeneration, neurons, non-alcoholic steatohepatitis, oligodendrocytes
INTRODUCTION
Insulin resistance diseases—crisis of the Western lifestyle
Insulin resistance disease states, including Alzheimer's disease (AD), obesity, type 2 diabetes mellitus (T2DM), non-alcoholic steatohepatitis (NASH), and metabolic syndrome are among the most prevalent and costly causes of morbidity, disability, and mortality in the United States [1–5]. Furthermore, the rapid transfer of Western “high-tech” diets and lifestyles has helped insulin resistance diseases spread throughout the world and bear their tolls on global health. In the central nervous system (CNS), insulin and insulin-like growth factor (IGF) signaling networks maintain a broad array of functions including cell growth and survival, energy metabolism, gene expression, protein synthesis, cytoskeletal assembly, synapse formation, neurotransmitter function, and plasticity [6–8]. Correspondingly, impaired signaling through insulin and IGF receptors, i.e., insulin and IGF resistance, has dire consequences with respect to the structural and functional integrity of the CNS [7, 9–11].
Insulin resistance, irrespective of cause, results in cellular and tissue injury because it promotes oxidative stress, formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), mitochondrial dysfunction, and pro-inflammatory cytokine activation [7, 12–19]. Increased levels of ROS and RNS contribute to nucleic acid and protein oxidative damage in AD and diabetes mellitus [20]. Oxidation of amino acid residues results in the formation of advanced glycation end products (AGEs) or advanced oxidation protein products. Increased protein oxidation drives protein unfolding and inactivitation, and it enhances protein susceptibility to cleavage. Oxidation of aliphatic side-chains leads to formation of peroxides, alcohols, and carbonyls (aldehydes and ketone). Carbonyls are toxic and cause stress-induced AGE accumulation, which contributes to progressive impairment of cellular functions in aging, diabetes, AD, experimental models of AD, and many other degenerative diseases [21–24]. In AD, elevated levels of AGEs in amyloid plaques and neurofibrillary tangles [25–27] may promote cell death and neurodegeneration [20, 25, 27].
The consequences of insulin resistance, particularly the increased stress responses themselves, promote insulin resistance. If the factors mediating insulin resistance persist unabated such that the rates of injury exceed those of repair, then disease will progress and organ-system degeneration will ensue. Besides unmitigated injury and insulin/IGF resistance, candidate triggers of organ-system degeneration include, impaired energy balance, lipid dyshomeostasis, loss of membrane integrity, and endoplasmic reticulum (ER) stress, because these factors all contribute to increased ceramide generation [28–34]. For example, in chronic obesity, T2DM, and NASH, lipid dyshomeostasis results in increased ceramide generation in adipose tissue and/or liver [30, 35–37].
Ceramide pathobiology-probable role in neurodegeneration
Ceramides are lipid signaling molecules that can be cytotoxic, cause insulin resistance [38–42], and activate pro-inflammatory cytokines. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [43], are highly activated in obesity, T2DM, NASH, and AD [44–50]. Inflammation and insulin resistance increase ceramide production, which promotes oxidative and ER stress. The end result of these interconnecting pathophysiological processes is the inducement of positive feedback mal-signaling that establishes a cascade of progressive organ-system degeneration.
AD has several features in common with systemic insulin resistance disease states, including inhibition of insulin-stimulated pro-cell growth and survival pathways, increased oxidative stress, pro-inflammatory cytokine activation, mitochondrial dysfunction, and impaired energy metabolism [12, 51]. In addition, there is some evidence that ceramide levels are increased in brains with AD [37, 52–55], and that the AD neurodegeneration cascade may contribute to ceramide generation and accumulation in the brain [35, 56]. Therefore, AD neurodegeneration could be mediated by similar or even the same cascades that promote organ-system degeneration in non-CNS insulin resistance diseases. However, clinical and experimental data suggest that CNS insulin resistance in AD can be mediated by exogenous or systemic diseases such as T2DM or NASH, or primary or endogenous disease processes in the absence of peripheral insulin resistance [6, 12, 51, 57–59]. To reconcile these seemingly discordant observations, we hypothesize that, with respect to systemic diseases, brain insulin resistance is mediated by CNS trafficking of toxic ceramides produced in diseased (insulin resistant) peripheral organs, e.g., livers with steatohepatitis, while primary brain insulin resistance in AD results in increased CNS production of neurotoxic ceramides due to lipid dyshomeostasis and sphingomyelin degradation. Our interests are focused on ceramides because ceramides: 1) can be generated in brain [35, 43, 60, 61]; 2) are increased in various dementia-associated diseases, including AD [35, 37, 56]; and 3) are lipid soluble and therefore may readily cross the blood-brain barrier, providing a mechanism by which obesity, T2DM, or NASH could lead to brain insulin resistance [12, 62, 63]. The present work reviews ceramide biology and the mechanisms by which ceramides contribute to AD neurodegeneration via extrinsic or intrinsic pathways.
CERAMIDES- REVIEWED
Lipid composition of biological membranes
The lipid component of biological membranes contains glycerophospholipids such as phosphatidyl-choline, phosphatidyl-ethanolamine, phosphatidyl-serine, phosphatidyl-inositol and phosphatidyl-glycerol, sphingolipids (sphingomyelin and sometimes glycosphingolipids), and sterols (mainly cholesterol). The relative abundance of each lipid class varies with cell type and organelle, although glycerophospholipids and sphingolipids are most abundant in eukaryotic membranes. Membrane lipids characteristically have amphipathic structures in which the hydrophobic (distributed centrally) and hydrophilic (distributed peripherally) regions are well-separated to maintain an energetically favorable lipid bilayer structure [64, 65].
Glycerophospholipids are the most abundant class of biological membrane lipids. Their glycerol backbones have fatty acids linked through an ester oxygen to Carbons 1 and 2. Carbon 3 of the backbone has a phosphate ester-link, as well as other moieties such as amino acids. Glycerophospholipid structure varies with the nature of the fatty acids attached to Carbons 1 and 2; variations in membrane lipid composition contribute to differences in cellular structure and function [66].
Sphingolipids are the next most abundant class of lipids in biological membranes in which a fatty acid is attached to the amino group of sphingosine (18-carbon amine alcohol), and either a simple or complex sugar is linked to the alcohol on Carbon 1. However, sphingomyelin is an important exception as it contains a phosphoryl-choline group instead of a sugar moiety, making it an analog of phosphatidyl-choline. Among the sphingolipids, sphingomyelin is dominant in biological membranes. The physical properties of sphingolipids are mainly dictated by the moiety attached to Carbon 1, and secondarily by the fatty acid component. For example, with regard to glycosphingolipids, the sugar attached to Carbon 1 of spingosine is located on the outer membrane surface of where it functions as a receptor, antigen, or signaling molecule [67–70].
Finally, cholesterol is the main sterol present in animal cell membranes. Cholesterol has a four fused-ring steroid nucleus and a hydroxyl group on the A ring. Sterols differ based on the number and positions of carbon-carbon double bonds and the hydrocarbon side chain attached to the D ring. Cholesterol plays a key role in regulating membrane fluidity because its presence and abundance add rigidity and strength to the liquid-crystalline phospholipid bilayer [68, 71].
Ceramide biosynthesis
Ceramides comprise a family of relatively simple sphingolipids and are generated from fatty acid and sphingosine [30, 70, 72–76]. In contrast to glycerophospholipids and sphingomyelin, ceramides are present at low levels within biological membranes. Ceramides contribute to cell membrane structure by participating in lipid raft formation, and they exert diverse regulatory effects on cell signaling pathways that mediate growth, proliferation, motility, adhesion, differentiation, senescence, and apoptosis. For the most part, ceramides are formed as relatively transient intermediates in the biosynthesis of complex sphingolipids, including sphingomyelin and glycosylceramides. Ceramides differ according to the length and nature of the di- and trihydroxy long-chain bases linked to long-chain fatty acids (up to C24 or greater), and the saturated and monoenoic components. More than 200 structurally distinct ceramide species have been identified in mammalian cells.
Ceramides can be formed via biosynthetic, catabolic, or salvage pathway mechanisms. Ceramide synthesis is regulated by 28 or more distinct enzymes, including 6 ceramide synthases and 5 sphingomyelinases, each of which is expressed by a different gene. Ceramide biosynthesis is achieved through ceramide synthase and serine palmitoyltransferase activities [60, 72, 74, 77, 78]. Ceramide synthases couple sphinganine to a long-chain fatty acid, yielding dihydroceramide. Multiple isoforms of ceramide synthase exist, and each one may utilize specific fatty acids (different lengths) for ceramide biosynthesis, and thereby regulate distinct cellular functions.
Ceramides can also be generated by catabolism of complex sphingolipids through activation of sphingomyelinases (basic, neutral, or acidic) or phospholipase C [72, 79–83], or through a relatively inefficient mechanism involving glycosidase hydrolysis of glycosphingolipids localized in late endosomes and lysosomes [81]. Reversal of the sphingomyelin synthesis reaction can also generate ceramides. Sphingomyelinases, which increase ceramide levels by stimulating sphingomyelin hydrolysis, are activated by various drugs, physical agents, and natural compounds, including chemotherapeutic agents, pro-inflammatory cytokines, e.g., TNF-α, endotoxins, trophic factor withdrawal, heat, ionizing radiation, Fas, and 25-dihydroxy-vitamin D3 [41, 43, 56, 72, 81, 84, 85].
The third mechanism of ceramide synthesis is via the salvage pathway in which sphingoid bases released by acid ceramidase activity escape from the lysosomes and get re-utilized for ceramide biosynthesis through the action of a ceramide synthase [72, 74, 86]. The salvage pathway may be responsible for between 50% and 90% of sphingolipid biosynthesis. In addition, ceramides generated via the salvage pathway may be spatially and functionally distinct from those synthesized via the de novo pathway. Moreover, sphingoid bases released from lysosomes may possess unique or independent biological functions. Finally, sphingosine-1-phosphate is an important biologically active metabolite that can only be generated from free sphingosine [85]. Therefore, the regulation of ceramide synthesis, levels, and actions is central to many cellular and biological processes.
Ceramide degradation
As mentioned, ceramides are used in the synthesis of complex sphingolipids. For the most part, ceramides needed to generate complex sphingolipids are synthesized on the cytoplasmic leaflet of the ER, and subsequently processed in the Golgi [74, 87]. Ceramides used to generate sphingomyelin are extracted from lipid bilayer membranes in the ER by the CERT transporter protein [88, 89]. Short peptide motifs in CERT recognize a specific protein in the ER. CERT protein extracts ceramides from lipid bilayer membranes with limited specificity for those containing C14 to C20 fatty acids [87, 90–92]. The pool of ceramides used to synthesize glycosylceramide differs from that used to generate sphingomyelin [93, 94]. The process requires ceramides to be transported between the ER and Golgi by a separate transport mechanism that is still under investigation.
Ceramidases hydrolyze ceramides to sphingoid bases and free fatty acids. Five ceramidase enzymes have been identified in humans; 2 have acidic, and 3 have alkaline pH optima for enzymatic function [95]. In addition, ceramidase differ with respect to their sub-cellular localizations and fatty acid specificities. Acidic ceramidases are located in lysosomes, and they efficiently hydrolyze ceramides with medium chain fatty acid components [96]. Neutral ceramidases are located in the plasma membrane and use long-chain to very-long-chain fatty acid components (C16 to >C24) as substrates [97, 98]. Alkaline ceramidases 1 and 2 (ACER1 and ACER2) are localized in the ER and Golgi, and they also utilize very-long-chain acyl groups as substrates. ACER3 is localized in both the ER and Golgi, but has specificity for ceramides, dihydroceramides, and phytoceramides linked to unsaturated long-chain fatty acids (18 : 1, 20 : 1 or 20 : 4) [99].
Ceramide functions
The rapid turnover and short half-life of ceramides are critical to their functions as second messengers for intracellular signaling. In addition, the levels of specific ceramide species are regulated in order to target particular proteins. The de novo pathway of ceramide biosynthesis may have a more important role in physiological maintenance of ceramides, while catabolic pathways that utilize sphingomyelinases may allow for rapid production of ceramides under stress conditions. As noted, the range of ceramides produced differs among cellular compartments, and the profiles of ceramides formed and accumulated in different organelles and cell types can shift, either as adaptive or pathological responses.
Ceramides are physically concentrated in ceramide-rich platforms or rafts, i.e., lateral liquid-ordered microdomains [100]. Acid sphingomyelinase mediates ceramide production within rafts, and with their accumulation, ceramides displace cholesterol from sphingomyelin-enriched rafts [101]. The accumulation of ceramides causes small rafts to merge into larger units, resulting in modification of membrane structure and enabling specific proteins, such as receptors, to oligomerize or cluster and thereby potentiate positive signals or minimize negative signals [102–106]. Positive signaling can produce positive or negative effects. For example, the formation of ceramide-rich platforms could enhance trophic factor signaling with attendant cell growth, or stress-related signaling with resultant inflammation and cell death.
Ceramides regulate a broad range of both positive and negative cellular functions, including growth, proliferation, motility, adhesion, differentiation, senescence, and apoptosis [28, 31, 32, 43, 53, 74, 76, 82, 85, 107, 108]. Appreciation of the large number of variations in ceramide structure and subcellular localization has led to the recogniztion that ceramide signaling effects might be cataloged according to their molecular structures. Thus far, emphasis has been placed on fatty acid chain length and glycosylation or phosphorylation of the sphingolipid [109–111]. Earlier studies demonstrated that complex sphingolipids including gangliosides [112], and long-chain naturally occurring ceramides, i.e., up to 24 carbon atoms in length [113] function by stimulating positive cellular responses such as growth, while sphingosine-containing lipids, including shorter-chain ceramides, have inhibitory effects that result in increased apoptosis, cytotoxicity, or impaired growth [112, 114, 115]. In addition, C16 ceramide mediates apoptosis, while C18 ceramide causes growth-arrest and may function as an adjuvant by helping to trigger apoptosis in cancer cells exposed to chemotherapeutic drugs [116, 117]. Finally, N-acetylsphingosine, the simplest ceramide molecule (C2-ceramide), has distinct signaling functions relative to more complex ceramides and mediates mitochondrial cytochrome c release and apoptosis [118]. Since different ceramide synthase isoforms enable synthesis of specific chain-lengths of the base and fatty acid, shifts in the expression profiles of ceramide synthases may reflect changes in the types of ceramides expressed in cells and different organelles, and functional responses to different environmental or intracellular stimuli.
While the rapid and dynamic turnover of ceramides is critical for modulating responses to changing intracellular and extracellular cues and stresses, the actions of ceramides are modulated by opposing effects of two of their own metabolites, sphingosine-1-phosphate and ceramide-1-phosphate [85, 119, 120]. In contrast to ceramides, sphingosine-1-phosphate and ceramide-1-phosphate have inhibitory effects on apoptosis [119, 121–123]. In addition, ceramide-1-phosphate modulates inflammation by activating phospholipase A2 [124]. Therefore, the action of synthesizing ceramides to promote apoptosis can be rapidly halted by activating ceramidases to generate metabolites that oppose the effects of ceramide signaling. Future therapeutic compounds could be developed to manipulate the balance away from ceramide degradation in the case of cancer treatment, or in favor of ceramide metabolite accumulation to rescue neurodegenerative, ischemic, or autoimmune diseases.
CERAMIDES, INSULIN RESISTANCE, ENDOPLASMIC RETICULUM STRESS
Insulin resistance dysregulates lipid metabolism and promotes ceramide synthesis and accumulation
Insulin stimulates lipogenesis, which results in increased triglyceride storage in the liver and adipose tissue [125, 126]. Although this process is generally benign and important for maintaining energy balance, chronic high caloric intake leads to obesity, disrupting metabolic homeostasis and resulting in insulin resistance [42, 57, 125, 127]. In liver, simple steatosis can progress to steatohepatitis, which is associated with hepatic insulin resistance. Besides impairments in transducing insulin stimulated signals, the accompanying inflammation, pro-inflammatory cytokine activation, oxidative stress, and increased cell death via mitochondrial or apoptotic mechanisms, all contribute to the progression of liver disease in NASH.
One additional factor contributing to the progression of liver disease in NASH is that insulin resistance promotes lipolysis [128], which leads to increased generation of toxic lipids, i.e., ceramides, that further impair insulin signaling, mitochondrial function, and cell viability [41, 42, 129]. Importantly, ER stress and mitochondrial dysfunction can also initiate or potentiate insulin resistance and lipolysis leading to increased ceramide production [33, 34, 130, 131]. Ceramides cause insulin resistance by activating proinflammatory cytokines and inhibiting transmission of signals through phosphatidyl-inositol-3 kinase (PI3K) and Akt [104, 132–134]. Therefore, insulin resistance leads to increased generation of ceramides that exacerbate insulin resistance, inflammation, and tissue injury, and they also promote ER stress and mitochondrial dysfunction, which worsen inflammation and insulin resistance. This thickly inter-connected cycle corresponds to a harmful positive feedback loop that mediates disease progression and tissue degeneration.
Endoplasmic reticulum stress
The ER mediates wide-ranging functions, including protein synthesis, folding, maturation, and trafficking, i.e., post-translational protein processing and transport [135]. The ER is also critical for Ca2+ homeostasis and triglyceride synthesis. ER stress is caused by disruption of homeostatic mechanisms that cause unfolded proteins to accumulate, and ROS and RNS species to form [135]. Normally, the ER adapts to stress by activating the unfolded protein response (UPR) [136, 137], which quickly increases the levels and activities of ER stress sensor proteins including: inositol-requiring enzyme 1 (IRE1), PKR-like ER-localized eIF2α kinase (PERK), and the activating transcription factor 6α (ATF-6α; ER membrane-anchored transcription factor). PERK and IRE1 activate ER stress signaling networks by transmitting stress signals in response to protein misfolding or unfolding. In the unstressed state, the luminal domains of PERK and IRE1 are stably complexed with the ER chaperone BiP. ER stress induced by the UPR dissociates BiP from the luminal domains of PERK and IRE1. BiP's translocation to the cytosol correlates with the activation of PERK or IRE1 [136–138]. In addition, with ER stress, Bim, a proapoptotic member of the Bcl-2 family which is normally sequestered by Bcl-xL to prevent apoptosis, dissociates from Bcl-xL, translocates to the ER, and activates a Capase-12-mediated pro-death cascade [139]. Insulin resistance contributes to ER stress because vital ER functions such as protein synthesis, modification, and folding, calcium signaling, and lipid biosynthesis utilize glucose as the main source of energy to drive these processes, and insulin resistance impairs glucose uptake and metabolism. Therefore, with chronic steatohepatitis and insulin resistance, hepatocellular injury and death can be promoted by activation of ER stress pathways [33, 34, 131, 140].
Increased ER stress marks lipid dyshomeostasis and may reflect activation of pro-ceramide and proinflammatory pathways with increased generation of toxic lipids, e.g., ceramides [141–143]. Correspondingly, ceramide immunoreactivity and ER stress gene expression were found to be increased in livers with steatohepatitis [62]. ER stress leads to activation of PERK, and the growth arresting and DNA damaging, GADD34/PP1 phosphatase complex, which dephosphorylates eIF2α, promoting apoptosis. Correspondingly, recent studies showed that ceramide immunoreactivity and ER stress genes are significantly upregulated in livers with chronic steatohepatitis [144, 145].
PERIPHERAL INSULIN RESISTANCE, CERAMIDES, AND DISEASE
Ceramide-mediated tissue injury and degeneration
Ceramides can have positive or negative effects on cells by modulating a broad array of functions including, differentiation, proliferation, transformation, inflammation, autophagy, and apoptosis. Pro-growth and pro-differentiation effects of ceramides are examples of positive regulation. Ceramide activation of autophagy could also represent positive responses needed to maintain cellular homeostasis. On the other hand, ceramide inducement of inflammation reflects negative signaling that results in tissue injury by various mechanisms, including the activation of proinflamatory cytokines such as tumor necrosis factor-α, or the recruitment of cytotoxic responses to the amyloid-β (Aβ) peptide, which contributes to AD.
Ceramides participate in intracellular signaling by targeting ceramide-activated protein phosphatase and kinases, causing net changes in glycogen synthesis, insulin responsiveness, cell survival, and membrane permeability. Cytotoxic and degenerative effects of ceramides are mediated by: 1) altering phosphorylation states of various proteins, including those that regulate insulin signaling [146]; 2) activating enzymes such as interleukin-1β converting enzyme (ICE)-like proteases, which promote apoptosis [81]; or 3) inhibiting Akt activity [147] by activating protein phosphatase 2A [117] and glycogen synthase kinase 3β (GSK-3β) [148, 149] recruiting phosphatase and tensin homologue deleted on chromosome 10 (PTEN) [150]. Therefore, ceramide homeostasis is needed to enable positive cell signaling while minimizing cell injury. This balancing act is achieved through rapid conversion of ceramide intermediates to complex sphingolipids, and ceramidase metabolism of ceramides to sphingosine-1-phosphate and ceramide-1-phosphate. Pathophysiological increases in the levels of ceramides relative to sphingosine and sphingosine-1-phosphate have been implicated in the pathogenesis of obesity and insulin resistance [61]. Correspondingly, inhibition of ceramide synthesis or its accumulation was shown to prevent obesity-associated insulin resistance [32, 38]. Beyond this, ceramides have been implicated in the pathophysiology of aging and infertility due to activation of anti-proliferation and inhibition of pro-survival responses.
Endogenous versus exogenous ceramides as mediators of steatohepatitis and hepatic insulin resistance
Chronic steatohepatitis caused by various mechanisms and exposures, including obesity, alcohol, and nitrosamines, activate pro-ceramide genes and enzymes, resulting in increased ceramide levels in liver [12, 13, 62, 144, 151]. This response is accompanied by increased inflammation and activation of stress pathways that promote further injury, and exacerbate hepatic insulin resistance. Therefore, in the context of chronic steatohepatitis, a positive feedback loop can become established whereby hepatic insulin resistance promotes the generation of cytotoxic ceramides that render hepatic insulin resistance persistent and progressive (endogenous mechanism of liver injury).
Besides local injury caused by ceramide accumulation and insulin resistance, evidence suggests that cytotoxic ceramides generated in one tissue can injure cells and tissues at distant sites. For example, with obesity, enhanced ceramide production in adipocytes can adversely affect the liver and promote hepatic insulin resistance [32, 43, 152–155]. In vitro experiments utilizing cell lines or liver precision cut slice cultures (L-PCSC) demonstrated that exposures to synthetic cytotoxic ceramides (C2 or C6) cause significant tissue injury with oxidative stress, impaired insulin signaling, reduced insulin-responsive gene expression, and mitochondrial dysfunction [156]. Moreover, in vivo experiments demonstrated that intraperitoneal administration of C2 or C6 cytotoxic ceramides causes hepatic steatosis with inflammation and insulin resistance [63]. Together, these results indicate that cytotoxic ceramides from exogenous sources can cause hepatic injury with insulin resistance.
Recently, we showed that treatment with peroxi-some proliferator-activated receptor (PPAR) agonists which function as insulin sensitizer agents as well as anti-inflammatory compounds, increase insulin responsiveness and decrease pro-ceramide gene expression and ceramide levels in livers with chronic steatohepatitis [157]. On the other hand, ceramide enzyme inhibitor treatment of PCSCs generated from models of steatohepatitis, reduced cytotoxicity and increased insulin responsiveness. These results suggest that insulin resistance and ceramide accumulation/toxicity are highly inter-related and contribute to one another's pathogenesis. Therefore, targeting these two disease mechanisms may halt or reverse tissue degeneration in the context of steatohepatitis. This point is of particular interest with regard to potential secondary neurodegenerative effects of chronic steatohepatitis (see below).
Peripheral insulin resistance and cognitive impairment
Growing evidence suggests that peripheral insulin resistance with obesity, T2DM, metabolic syndrome (dyslipidemic states), and NASH mediate brain insulin/IGF resistance, and thereby contribute to the pathogenesis of mild cognitive impairment (MCI), dementia, and AD [12, 58, 158–160]. Obese individuals have increased rates of MCI [161], impaired performance on executive function tests [161, 162], and at least a two-fold increased risk for developing dementia or AD [163]. In experimental models, obesity caused by chronic high fat diet feeding results in mild neurodegeneration and cognitive impairment [12, 127, 164–166]. Although weight loss has not been shown to improve cognitive performance, it does enhance neuropsychiatric function including mood and behavior [167]. Patients with NASH have increased rates of neuropsychiatric diseases including depression and anxiety [168], and they are at increased risk for developing cognitive impairment prior to cirrhosis [169].
The relationship between peripheral insulin resistance and cognitive impairment or sporadic AD was brought to light by the near parallel growths of the obesity and AD epidemics across multiple age groups [3]. Moreover, epidemiologic studies showed that individuals with glucose intolerance, deficits in insulin secretion, T2DM, or obesity/dyslipidemic disorders were at increased risk for developing MCI or AD-type dementia [59, 170, 171]. These human studies are supported by the findings in experimental animal models of chronic high fat diet (HFD) feeding and diet induced obesity with T2DM in which deficits in spatial learning and memory [165, 172] are associated with mild brain atrophy, brain insulin resistance, neuro-inflammation, oxidative stress, and deficits in cholinergic function [127, 164]. Therefore, obesity or T2DM can lead to cognitive impairment, brain atrophy, and some of the biochemical and molecular abnormalities associated with AD-type neurodegeneration. However, it is noteworthy that the extent of neurodegeneration associated with peripheral insulin resistance diseases alone does not fully replicate the pathology of advanced AD. For the most part, T2DM, obesity, and probably other peripheral/systemic insulin resistance states cause modest rather than severe degrees of neurodegeneration in which neurofibrillary tangle pathology is absent, cortical dystrophic neurites are low in abundance, AβPP-Aβ burden is low, and cell loss is subtle. In essence, the neuropathology in obesity and peripheral insulin resistance diseases more closely mimics the findings in MCI or early stage AD. Therefore, although peripheral insulin resistance disease states by themselves mainly cause MCI, their more serious impact is that they can serve as co-factors in the pathogenesis or progression of AD neurodegeneration.
Exogenous ceramides mediate cytotoxicity and insulin resistance in brain
As discussed, cognitive impairment can be mediated by peripheral insulin resistance diseases. More specifically, evidence suggests that cognitive impairment and neuropsychiatric dysfunction correlate more with hepatic steatosis and hepatic insulin resistance rather than obesity or T2DM per se [168, 173–178]. For example, neurocognitive deficits and brain insulin resistance were observed in experimental models of chronic HFD whereby the mice or rats developed visceral obesity with hepatic steatosis or steatohepatitis. Moreover, in the absence of obesity, toxin exposures that cause steatohepatitis with hepatic insulin resistance also promote neurodegeneration and cognitive impairment [17, 127, 151, 164, 179]. A key feature of steatohepatitis with insulin resistance is increased ceramide generation. Since ceramides can promote insulin resistance both locally and at distant sites [28, 30, 43, 104, 180–183], brain insulin resistance and neurodegeneration arising in the setting of hepatic insulin resistance could be mediated by liver-derived cytotoxic ceramides [12]. Correspondingly, in humans and experimental models of chronic steatohepatitis, irrespective of cause, ceramide levels are increased in both liver and peripheral blood. One potential explanation for this phenomenon is that ceramides produced in diseased livers may transit into peripheral blood following hepatocellular injury or death, and subsequently exert their toxic and metabolic effects on distant organs, including brain. We used in vitro and in vivo experiments to test this hypothesis.
Treatment of cultured neurons or cerebellar PCSCs with C2 or C6 synthetic cytotoxic ceramides resulted in significant cell or tissue injury with oxidative stress, inhibition of insulin signaling, and increased expression of genes and proteins associated with AD-type neurodegeneration relative to cultures treated with the C2D inactive ceramide [156]. Moreover, intraperitoneal administration of C2 or C6 cytotoxic ceramides caused cognitive and motor impairments with deficits in insulin/IGF signaling, and insulin/IGF responsive gene expression in the brain [63]. Therefore, exogenous exposure to cytotoxic ceramides can cause neurodegeneration with brain insulin resistance and deficits in cognitive and motor functions.
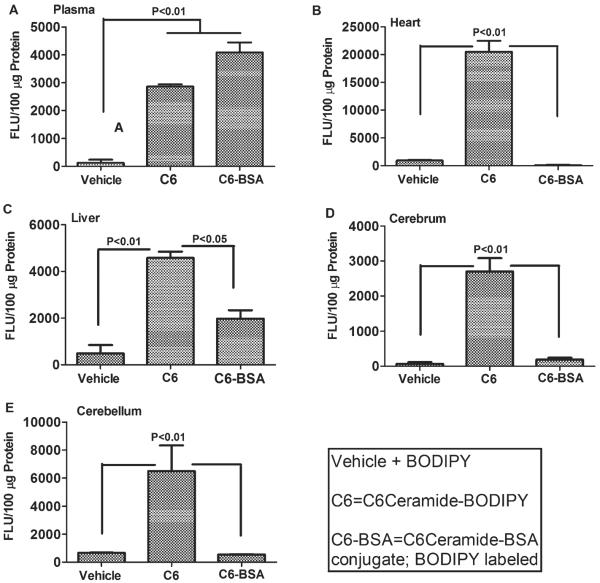
To further assess the likelihood that cytotoxic ceramides present in peripheral blood can cause brain injury/neurodegeneration with insulin resistance, we injected BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-sindacene)-tagged ceramides (50 μg/kg BODIPY-TR-BSA-C5) i.p. or i.v., and 2 hours later, determined the degrees to which BODIPY fluorescence could be detected in brain tissue membranes. BODIPY is an intrinsically lipophilic fluorescent dye that does not interfere with the properties of natural lipids. The study demonstrated that BODIPY-TR-C5 and not the bovine serum albumin (BSA) conjugate, BODIPYTR-C5-BSA, could be recovered from lipid but not aqueous fractions of brain. These findings indicate that the synthetic ceramides had partitioned into lipid containing membranes rather than extracellular fluid or blood (Fig. 1). The absence of BODIPY-TR-C5-BSA fluorescence in brain was expected because the BSA-conjugate would have prevented trafficking of the BODIPY-TR tagged C5 ceramide from crossing the blood brain barrier. In further preliminary studies, we demonstrated that cerebellar PCSCs exposed to lipids extracted from plasma or livers of rats with chronic steatohepatitis exhibited increased neurotoxicity and oxidative stress, and reduced insulin signaling and insulin-responsive gene expression relative to cultures exposed to control sera (data not shown). These findings support the hypothesis that cytotoxic ceramides originating from liver (or other organs/tissues) and entering peripheral blood can cross the blood-brain barrier and exert neurotoxic and neurodegenerative effects on the brain.
Fig. 1.
Ceramide crosses the blood-brain barrier. Long Evans rats were given a single intravenous injection of vehicle, C6-Ceramide-BODIPY, or C6-Ceramide-BSA-BODIPY, and 1 hr later, (A) plasma, (B) liver, (C) cerebrum, (D) cerebellum and (E) heart (myocardium) were harvested to measure fluorescence (Ex 575 nm/Em 590 nm). Plasma fluorescence was measured directly. Tissue fluorescence was measured in membrane (chloroform : methanol) lipid extracts to demonstrate uptake. The BSA-conjugated C6-Cer does not penetrate the blood brain barrier and served as a control. Data were analyzed by one-way ANOVA with the Dunnet post-hoc significane test.
Liver-brain axis of neurodegeneration hypothesis: role of cytotoxic ceramides
In obesity, adipose tissue, skeletal muscle, and liver have abnormal sphingolipid metabolism that results in increased ceramide production, inflammation, and activation of pro-inflammatory cytokines, with impairments in glucose homeostasis and insulin responsiveness [40, 43, 61]. In human [184] and experimental models of NASH [185], ceramide levels in adipose tissue are elevated due to increased activation of serine palmitoyl transferase, and acidic and neutral sphingomyelinases [81]. In addition, liver ceramide synthase and serine palmitoyl transferase mRNA levels are increased in the early stages of hepatic steatosis, but with the development of NASH and neurodegeneration, ceramide synthase mRNA transcripts decline while sphingomyelinase gene expression increases [127]. Since neurodegeneration in models of obesity and diabetes have not been associated with increased CNS expression of pro-ceramide genes, we suspect that the AD-type neurodegeneration with brain insulin/IGF resistance is mediated by secondary effects of peripheral insulin resistance, i.e., dysregulated lipid metabolism, increased production of cytotoxic ceramides, and increased trafficking of cytotoxic ceramides from peripheral blood to brain.
The aggregate results from several studies suggest that chronic steatohepatitis, irrespective of cause, promotes hepatic insulin resistance, oxidative stress, and tissue injury. Insulin resistance drives dysregulation of lipid metabolism that leads to increased ceramide production in liver. Hepatocyte accumulation of cytotoxic ceramides promotes ER stress which exacerbates insulin resistance, inflammation, and oxidative stress. Consequences include, increased DNA damage, mitochondrial dysfunction, energy depletion, ROS production, and eventually the formation lipid, protein, and DNA adducts, which impair cellular functions at multiple levels. Finally, a reverberating cascade of mal-signaling and insulin resistance gets established, and progressively impairs cell survival [12]. Since toxic lipids, including ceramides can cross the blood-brain barrier and cause insulin resistance by interfering with critical phosphorylation events [81, 117, 147] and activating pro-inflammatory cytokines [43, 114, 115], CNS insulin resistance, which is an early and important feature of AD, may be mediated by chronic exposure to cytotoxic ceramides generated from extra-CNS sources [63], mimicking effects of AD. In essence, brain insulin resistance and cognitive impairment in the settings of obesity, Type 2 diabetes, metabolic syndrome, NASH, or other forms of peripheral insulin resistance could be mediated by trafficking of neurotoxic ceramides from liver to brain, i.e., a liver-brain axis of neurodegeneration.
Corresponding with the above concept, mass spectrometry-based lipidomics analysis of plasma detected elevated levels of saturated sphingolipids (N16 : 0 and N21 : 0) in AD relative to control subjects, and linked severity of cognitive impairment with altered levels of specific very long chain ceramides [186]. In addition, elevated plasma levels of very long-chain saturated ceramides (C22 : 0 and C24 : 0) were found to be predictive of memory loss and hippocampal atrophy in patients with MCI [187], whereas increased ratios of dihydrosphingomyelin to dihydroceramide and sphingomyelin to ceramide were shown to be correlated with slower progression of AD [188]. Although these studies did not interrogate the sources of plasma sphingolipids and ceramides or the presence of underlying peripheral insulin resistance diseases, they provide evidence that shifts in plasma spingolipid profiles and levels could be used as peripheral biomarkers for individuals at risk for progression from MCI to dementia. Ideally, it would be beneficial to determine the degree to which peripheral blood very long chain ceramide profiles shift with treatment of AD with insulin, insulin sensitizers, or measures to support neurotransmitter function and metabolic homeostasis in the CNS.
PRIMARY BRAIN INSULIN RESISTANCE, CERAMIDES, ER STRESS AND NEURODEGENERATION
Brain insulin resistance without peripheral insulin resistance in AD
Growing evidence supports the concept that AD is a metabolic disease in which brain glucose utilization and energy production are impaired [6, 15, 16, 189]. Brain insulin and insulin-like growth factor (IGF) resistance disrupt signaling that regulates neuronal survival, metabolism, gene expression, and plasticity [7]. Moreover, inhibition of insulin/IGF signaling contributes to AD-type neurodegeneration by increasing: 1) the activity of kinases that aberrantly phosphorylate tau; 2) expression of AβPP and accumulation of AβPP-Aβ; 3) levels of oxidative and ER stress; 4) the generation of ROS and RNS that damage proteins, RNA, DNA, and lipids; 5) mitochondrial dysfunction; and 6) activation of pro-inflammatory and pro-death cascades. On a functional basis, insulin/IGF resistance down-regulates target genes needed for cholinergic homeostasis, and compromises systems that mediate neuronal plasticity, memory, and cognition [7, 12, 51].
The early stages of AD are marked by deficits cerebral glucose utilization [190–193], and as disease progresses, metabolic and physiological abnormalities worsen [194]. AD is associated with brain insulin resistance and insulin deficiency, with significant abnormalities in the expression of genes and activation of kinases that are regulated by insulin and IGF [15, 16]. As AD progresses, cerebral glucose utilization declines, and deficits in insulin signaling worsen [15]. As is the case for other organs and tissues, brain insulin resistance impairs energy metabolism and increases oxidative stress, ROS production, DNA damage, and mitochondrial dysfunction, all of which drive pro-apoptosis, pro-inflammatory, and pro-AβPP-Aβ cascades. Correspondingly, experimental inhibition of brain insulin receptor expression and/or function results in cognitive impairment and neurodegeneration with features that overlap with AD [17, 195–198].
In brains with AD, deficits in insulin/IGF signaling are due to combined effects of insulin/IGF resistance and deficiency. Insulin/IGF resistance is manifested by reduced levels of insulin/IGF receptor binding and decreased responsiveness to insulin/IGF stimulation, while the trophic factor deficiency is associated with reduced levels of insulin polypeptide and gene expression in brain and cerebrospinal fluid [6, 15, 16, 158, 199, 200]. Therefore, AD may be best regarded as a brain form of diabetes that has elements of both insulin resistance and insulin deficiency. To consolidate this concept, we proposed that AD be referred to as, “Type 3 diabetes” [15, 16].
ER stress and neurodegeneration
As in other organs and tissues, ER stress in the brain is triggered by the accumulation of unfolded or misfolded proteins in the ER lumen. Initially, the ER adapts to stress by triggering the UPR [136, 137], which activates ER stress sensor proteins including IRE-1α, PERK, and ATF-6α. Persistent activation of the UPR causes PERK and IRE-1α to activate ER stress signaling networks [136, 138] that ultimately promote cell death via apoptosis or mitochondrial mechanisms [139]. Increased ER stress is a recognized feature of several major neurodegenerative diseases, including AD and Parkinson's disease, in which mis-folded cytoskeletal proteins accumulate, aggregate and become ubiquitinated, and thereby promote ER and oxidative stress [201–203]. In vitro experiments linked ER stress to increased expression of GRP78/BiP, CHOP, ATF4, ATF6, and phosphorylated PERK and eIF1α in cultured neuronal cells, but only after induction by tunicamycin or thapsigargin [204]. This suggests that ER stress responses in neuronal cells may be driven by calcium release from the ER, together with oxidative stress and possibly mitochondrial dysfunction.
Role of brain-derived ceramides and ER stress in AD progression
Although T2DM, obesity, and other peripheral insulin resistance diseases significantly increase the risk of developing MCI, dementia, or AD [205], the vast majority of patients with AD do not have peripheral insulin resistance diseases. Similarly, in models of neurodegeneration produced by intracerebral injection of Streptozotocin, the cognitive deficits, and the nature and severity of neurodegeneration closely match the findings in AD, and are not associated with T2DM or peripheral insulin resistance [17, 179]. At the same time, postmortem human brain studies demonstrated no significant increase in AD diagnosis among diabetics [206], and in experimental models and humans with obesity, T2DM, or other peripheral insulin resistance diseases, the severity of neurodegeneration and brain insulin resistance tend to be relatively mild, and they do not fully mimic the pathology of moderate or severe AD [127, 164]. Together, these observataions suggest that brain insulin resistance-associated neurodegeneration can be mediated by either peripheral insulin resistance as discussed earlier, or a primary disease process in the brain.
We hypothesize that primary or endogenous brain insulin resistance is mechanistically similar to the same process described earlier with respect to liver in that, insulin resistance, ER stress, and cytotoxic ceramides are pathophysiologically inter-related and together these factors promote chronic injury and neurodegeneration. In support of this concept are the following: 1) sphingomyelins are abundantly expressed in brain and their degradation by sphingomyelinases could readily generate ceramides [56, 112, 207, 208]; 2) oligodendrocyte function, including myelin homeostasis, is regulated by insulin/IGF, and cerebral white matter atrophy and degeneration are early features of AD [209], overlapping with the time course during which brain insulin resistance emerges; 3) earlier studies suggested that sphinolipid metabolism [210] and ceramide levels were increased in AD brains [53]; 4) ER stress responses in AD are thought to be activated by the accumulation, aggregation, and polyubiquitination of mis-folded fibrillar proteins, e.g., hyper-phosphorylated tau and AβPP-Aβ [202, 203]; and 5) dysregulated ceramide generation in brain promotes cell death and AβPP-Aβ deposition [211–214]. Increased ER stress in the brain would exacerbate brain insulin resistance and cytotoxic ceramide production, which in turn would forward neurodegeneration by increasing mitochondrial dysfunction and cell death. In the final segments of this article, we present new data that was generated to more directly examine this hypothesis. In this regard, we measured pro-ceramide and ER stress RNA transcripts, ER stress signaling proteins, and ceramide immunoreactivity in human brains with normal aging, moderate AD, or end-stage AD.
Hypothesis: Insulin resistance can precipitate and propagate progressive AD neurodegeneration via intrinsic and/or extrinsic mechanisms (Fig. 2)
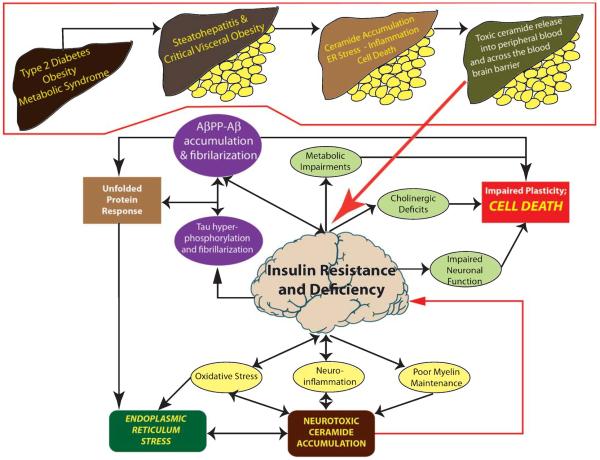
Fig. 2.
Intrinsic versus extrinsic mechanisms of brain insulin/IGF resistance and neurodegeneration. The central portion of this cartoon depicts the intrinsic mechanisms of neurodegeneration mediated by primary brain insulin/IGF resistance and deficiency. The consequences are multi-faceted, contributing tall all aspects of AD-type neurodegeneration. Neurodegeneration progresses because certain effects of brain insulin resistance, e.g., AβPP-Aβ toxicity and neuroinflammation can also promote brain insulin resistance. As injury progresses, the unfolded protein response leads to increased ER stress and neuroinflammation, oxidative stress and ER stress lead to accumulation of neurotoxic ceramides, which further exacerbate brain insulin/IGF resistance and advance the cascade of neurodegeneration. The extrinsic mechanism is particularly relevant to Type 2 diabetes, non-alcoholic steatohepatitis, and visceral obesity (visceral obesity shown as yellow discs below livers), whereby excess lipid accumulation leads to insulin resistnce, which promotes inflammation, ER stress, and oxidative injury. This process establishes a reverberating cascade of mal-signaling and insulin resistance with impaired cell survival that results in leakage of toxic ceramides from liver (or visceral fat) to peripheral blood. Toxic ceramides capable of penetrating the blood brain barrier, cause CNS insulin resistance, oxidative stress, and pro-inflammatory cytokine activation, which ultimately result in dysregulated lipid metabolism, myelin breakdown, increased endogenous ceramide generation, and ER stress.
We propose that the AD neurodegeneration cascade can be initiated and propagated by primary brain insulin/IGF resistance (intrinsic), peripheral insulin/IGF resistance diseases (extrinsic), or combined effects of both.
Intrinsic mechanisms
Brain insulin/IGF deficiency and resistance directly contribute to oxidative stress, neuroinflammation, impaired function and survival of neurons and oligodendrocytes, reduced neurotransmitter functions required for plasticity, learning, and memory, deficient myelin integrity, and disrupted processing and function of tau and AβPP-Aβ. Together, these abnormalities contribute to constitutive activation of ER stress and pro-ceramide pathways. A positive feedback loop leading to progressive neurodegeneration takes hold because each aspect of the cascade reinforces one or more of the key elements. Given the fact that many of the fundamental molecular and cellular abnormalities in AD also occur in other neurodegenerative diseases, such as Parkinson's disease, motor neuron disease, alcoholic brain disease, HIV-AIDS dementia and Niemann-Pick disease, it is likely that perturbations in sphingolipid metabolism with accumulation of neurotoxic ceramides contribute to the pathogenesis and progress of various forms of neurodegeneration [55, 56, 62, 215].
Extrinsic mechanisms
Peripheral, including hepatic insulin resistance with associated chronic injury, inflammation, and metabolic dysfunction leads to dysregulated lipid metabolism with increased ceramide production. Intra-hepatic accumulation of cytotoxic ceramides promotes ER stress, which exacerbates insulin resistance, inflammation, and oxidative stress. Consequences include the establishment of a reverberating cascade of mal-signaling and insulin resistance with impaired cell survival that results in leakage of toxic ceramides from liver to peripheral blood. Toxic ceramides capable of penetrating the blood brain barrier, cause CNS insulin resistance, oxidative stress, and proinflammatory cytokine activation, which ultimately result in dysregulated lipid metabolism, myelin breakdown, increased endogenous ceramide generation, and ER stress. Sporadic AD that occurs independent of peripheral insulin resistance disease is likely mediated by intrinsic brain insulin resistance with increased activation of ER streass and pro-ceramide pathways. On the other hand, the epidemic of peripheral insulin resistance diseases which includes obesity, Type 2 diabetes, and non-alcoholic fatty liver disease, is likely responsible for the staggering increases in morbidity and mortality rates from AD across all age groups, 50-years and older [3]. Mechanistically, we attribute this extrinsic pathway of brain insulin/IGF resistance with attendant ER stress to initiating insults arising from toxic ceramides generated in peripheral tissues, e.g., liver, and trafficking through peripheral blood to the CNS to exert their neurotoxic and degenerative effects. Future strategies for designing treatments for AD should consider multi-pronged approaches that combat this triangulated mechanism of neurodegeneration.
ACKNOWLEDGMENTS
Supported by AA-11431, AA-12908, and AA-16126 from the National Institutes of Health.
Footnotes
The author's disclosure is available online .(http://www.j-alz.com/disclosures/view.php?id=1103).
REFERENCES
- [1].Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G697–G702. doi: 10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- [3].de la Monte SM, Neusner A, Chu J, Lawton M. Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer's disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;17:519–529. doi: 10.3233/JAD-2009-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol. 2011;22:210–215. doi: 10.1097/MOL.0b013e3283453dbe. [DOI] [PubMed] [Google Scholar]
- [5].Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- [6].Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- [7].de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- [8].D'Ercole AJ, Ye P. Expanding the mind: Insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu J, Yeon JE, Chang H, Tison G, Chen GJ, Wands J, de la Monte S. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: Role of PTEN phosphatase. J Biol Chem. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- [11].Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: Roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–1060. [PMC free article] [PubMed] [Google Scholar]
- [13].de la Monte SM, Tong M, Lawton M, Longato L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener. 2009;4:54. doi: 10.1186/1750-1326-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008;23:e477–e486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: Link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- [16].Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- [17].Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- [18].Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [19].Haan MN. Therapy Insight: Type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- [20].Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- [22].Greilberger J, Fuchs D, Leblhuber F, Greilberger M, Wintersteiger R, Tafeit E. Carbonyl proteins as a clinical marker in Alzheimer's disease and its relation to tryptophan degradation and immune activation. Clin Lab. 2010;56:441–448. [PubMed] [Google Scholar]
- [23].Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D, Oettl K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radic Res. 2008;42:633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- [24].Gu F, Zhu M, Shi J, Hu Y, Zhao Z. Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice. Neurosci Lett. 2008;440:44–48. doi: 10.1016/j.neulet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- [25].Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adhesion Migration. 2009;3:88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rahmadi A, Steiner N, Munch G. Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer's disease. Clin Chem Lab Med. 2011;49:385–391. doi: 10.1515/CCLM.2011.079. [DOI] [PubMed] [Google Scholar]
- [27].Krautwald M, Munch G. Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer's disease? Exp Gerontol. 2010;45:744–751. doi: 10.1016/j.exger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- [28].Lipina C, Hundal HS. Sphingolipids: Agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596–1607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- [29].Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: Role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12:163–172. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- [30].Summers SA. Sphingolipids and insulin resistance: The five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- [31].DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- [33].Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- [35].Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer's disease. Biochem Soc Trans. 2004;32:144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- [36].Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- [37].Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: A shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem Res. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- [38].Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- [39].Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- [40].Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- [41].Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–241. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- [43].Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [44].Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- [45].Rosenberg PB. Clinical aspects of inflammation in Alzheimer's disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- [46].Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: Role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- [47].Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: Molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [48].Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–1952. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- [49].Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [50].Yalniz M, Bahcecioglu IH, Ataseven H, Ustundag B, Ilhan F, Poyrazoglu OK, Erensoy A. Serum adipokine and ghrelin levels in nonalcoholic steatohepatitis. Mediators Inflamm. 2006;2006:34295. doi: 10.1155/MI/2006/34295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer's disease. Front Biosci. 2012;E4:1582–1605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer's disease. Biochim Biophys Acta. 2010;1801:774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta. 2010;1801:878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer's disease: New biomarkers and treatment targets? Neuromolecular Med. 2010;12:331–340. doi: 10.1007/s12017-010-8121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ben-David O, Futerman AH. The role of the ceramide acyl chain length in neurodegeneration: Involvement of ceramide synthases. Neuromolecular Med. 2010;12:341–350. doi: 10.1007/s12017-010-8114-x. [DOI] [PubMed] [Google Scholar]
- [56].Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes: Evidence reviewed. J Diabetes Sci Tech. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Craft S. Insulin resistance and Alzheimer's disease pathogenesis: Potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- [59].Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- [60].Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- [61].Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR. The liver-brain axis of alcohol-mediated neurodegeneration: Role of toxic lipids. Int J Environ Res Public Health. 2009;6:2055–2075. doi: 10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J Alzheimers Dis. 2010;21:967–984. doi: 10.3233/JAD-2010-091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hannich JT, Umebayashi K, Riezman H. Distribution and functions of sterols and sphingolipids. Cold Spring Harb Perspect Biol. 2011;3:pii: a004762. doi: 10.1101/cshperspect.a004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cantu L, Del Favero E, Sonnino S, Prinetti A. Gangliosides and the multiscale modulation of membrane structure. Chem Phys Lipids. 2011;164:796–810. doi: 10.1016/j.chemphyslip.2011.09.005. [DOI] [PubMed] [Google Scholar]
- [66].Muller G. Novel applications for glycosylphosphatidylinositol-anchored proteins in pharmaceutical and industrial biotechnology. Mol Membr Biol. 2011;28:187–205. doi: 10.3109/09687688.2011.562557. [DOI] [PubMed] [Google Scholar]
- [67].van Meer G, Hoetzl S. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett. 2010;584:1800–1805. doi: 10.1016/j.febslet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- [68].Quinn PJ. A lipid matrix model of membrane raft structure. Prog Lipid Res. 2010;49:390–406. doi: 10.1016/j.plipres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [69].Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fantini J. Interaction of proteins with lipid rafts through glycolipid-binding domains: Biochemical background and potential therapeutic applications. Curr Med Chem. 2007;14:2911–2917. doi: 10.2174/092986707782360033. [DOI] [PubMed] [Google Scholar]
- [71].Ohno-Iwashita Y, Shimada Y, Hayashi M, Iwamoto M, Iwashita S, Inomata M. Cholesterol-binding toxins and anti-cholesterol antibodies as structural probes for cholesterol localization. Subcell Biochem. 2010;51:597–621. doi: 10.1007/978-90-481-8622-8_22. [DOI] [PubMed] [Google Scholar]
- [72].Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- [73].Soriano JM, Gonzalez L, Catala AI. Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B1. Prog Lipid Res. 2005;44:345–356. doi: 10.1016/j.plipres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [74].Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].van Die I, van Stijn CM, Geyer H, Geyer R. Structural and functional analysis of glycosphingolipids of Schistosoma mansoni. Methods Enzymol. 2010;480:117–140. doi: 10.1016/S0076-6879(10)80006-0. [DOI] [PubMed] [Google Scholar]
- [77].Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91:784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [78].Stiban J, Tidhar R, Futerman AH. Ceramide synthases: Roles in cell physiology and signaling. Adv Exp Med Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- [79].Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: Identifying biochemical connections. Adv Enzyme Regul. 2011;51:51–58. doi: 10.1016/j.advenzreg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fanani ML, Hartel S, Maggio B, De Tullio L, Jara J, Olmos F, Oliveira RG. The action of sphingomyelinase in lipid monolayers as revealed by microscopic image analysis. Biochim Biophys Acta. 2010;1798:1309–1323. doi: 10.1016/j.bbamem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- [81].Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997;8:311–322. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- [82].Nikolova-Karakashian MN, Rozenova KA. Ceramide in stress response. Adv Exp Med Biol. 2010;688:86–108. doi: 10.1007/978-1-4419-6741-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zeidan YH, Hannun YA. The acid sphingomyelinase/ceramide pathway: Biomedical significance and mechanisms of regulation. Curr Mol Med. 2010;10:454–466. doi: 10.2174/156652410791608225. [DOI] [PubMed] [Google Scholar]
- [84].Farooqui AA. Lipid mediators in the neural cell nucleus: Their metabolism, signaling, and association with neurological disorders. Neuroscientist. 2009;15:392–407. doi: 10.1177/1073858409337035. [DOI] [PubMed] [Google Scholar]
- [85].Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal. 2011;15:2501–2517. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mullen TD, Obeid LM. Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152012800228661. in press. [DOI] [PubMed] [Google Scholar]
- [87].Hanada K. Intracellular trafficking of ceramide by ceramide transfer protein. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:426–437. doi: 10.2183/pjab.86.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mencarelli C, Losen M, Hammels C, De Vry J, Hesselink MK, Steinbusch HW, De Baets MH, Martinez-Martinez P. The ceramide transporter and the Goodpasture antigen binding protein: One protein–one function? J Neurochem. 2010;113:1369–1386. doi: 10.1111/j.1471-4159.2010.06673.x. [DOI] [PubMed] [Google Scholar]
- [89].Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hanada K, Kumagai K, Tomishige N, Kawano M. CERT and intracellular trafficking of ceramide. Biochim Biophys Acta. 2007;1771:644–653. doi: 10.1016/j.bbalip.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [92].Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- [93].Perry SW, Norman JP, Gelbard HA. Adjunctive therapies for HIV-1 associated neurologic disease. Neurotox Res. 2005;8:161–166. doi: 10.1007/BF03033827. [DOI] [PubMed] [Google Scholar]
- [94].Yamaji T, Kumagai K, Tomishige N, Hanada K. Two sphingolipid transfer proteins, CERT and FAPP2: Their roles in sphingolipid metabolism. IUBMB Life. 2008;60:511–518. doi: 10.1002/iub.83. [DOI] [PubMed] [Google Scholar]
- [95].Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem. 2006;281:7324–7331. doi: 10.1074/jbc.M508382200. [DOI] [PubMed] [Google Scholar]
- [96].Kornhuber J, Tripal P, Reichel M, Muhle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- [97].Okino N, Ikeda R, Ito M. Expression, purification, and characterization of a recombinant neutral ceramidase from Mycobacterium tuberculosis. Biosci Biotechnol Biochem. 2010;74:316–321. doi: 10.1271/bbb.90645. [DOI] [PubMed] [Google Scholar]
- [98].Zhou Y, Lin XW, Yang Q, Zhang YR, Yuan JQ, Lin XD, Xu R, Cheng J, Mao C, Zhu ZR. Molecular cloning and characterization of neutral ceramidase homologue from the red flour beetle, Tribolium castaneum. Biochimie. 2011;93:1124–1131. doi: 10.1016/j.biochi.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sun W, Jin J, Xu R, Hu W, Szulc ZM, Bielawski J, Obeid LM, Mao C. Substrate specificity, membrane topology, and activity regulation of human alkaline ceramidase 2 (ACER2) J Biol Chem. 2010;285:8995–9007. doi: 10.1074/jbc.M109.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sonnino S, Prinetti A. Lipids and membrane lateral organization. Front Physiol. 2010;1:153. doi: 10.3389/fphys.2010.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang C, Li PL. Membrane raft redox signalosomes in endothelial cells. Free Radic Res. 2010;44:831–842. doi: 10.3109/10715762.2010.485994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li X, Becker KA, Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem. 2010;26:41–48. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- [103].Corre I, Niaudet C, Paris F. Plasma membrane signaling induced by ionizing radiation. Mutat Res. 2010;704:61–67. doi: 10.1016/j.mrrev.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [104].Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- [105].Lingwood CA, Manis A, Mahfoud R, Khan F, Binnington B, Mylvaganam M. New aspects of the regulation of glycosphingolipid receptor function. Chem Phys Lipids. 2010;163:27–35. doi: 10.1016/j.chemphyslip.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [106].Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function - where membrane structure and pathology intersect. FEBS Lett. 2010;584:1879–1886. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- [107].Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50:104–114. doi: 10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Meisen I, Mormann M, Muthing J. Thin-layer chromatography, overlay technique and mass spectrometry: A versatile triad advancing glycosphingolipidomics. Biochim Biophys Acta. 2011;1811:875–896. doi: 10.1016/j.bbalip.2011.04.006. [DOI] [PubMed] [Google Scholar]
- [110].Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, Obeid LM, Bielawska A. Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg Med Chem. 2006;14:7083–7104. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- [111].Ekiz HA, Baran Y. Bioactive sphingolipids in response to chemotherapy: A scope on leukemias. Anticancer Agents Med Chem. 2011;11:385–397. doi: 10.2174/187152011795677571. [DOI] [PubMed] [Google Scholar]
- [112].Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- [113].Gomez-Munoz A, Frago LM, Alvarez L, Varela-Nieto I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem J. 1997;325(Pt 2):435–440. doi: 10.1042/bj3250435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Van Brocklyn JR. Sphingolipid signaling pathways as potential therapeutic targets in gliomas. Mini Rev Med Chem. 2007;7:984–990. doi: 10.2174/138955707782110123. [DOI] [PubMed] [Google Scholar]
- [116].Heffernan-Stroud LA, Obeid LM. p53 and regulation of bioactive sphingolipids. Adv Enzyme Regul. 2011;51:219–228. doi: 10.1016/j.advenzreg.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- [118].Richter C, Ghafourifar P. Ceramide induces cytochrome c release from isolated mitochondria. Biochem Soc Symp. 1999;66:27–31. doi: 10.1042/bss0660027. [DOI] [PubMed] [Google Scholar]
- [119].Rotstein NP, Miranda GE, Abrahan CE, German OL. Regulating survival and development in the retina: Key roles for simple sphingolipids. J Lipid Res. 2010;51:1247–1262. doi: 10.1194/jlr.R003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, Hannun YA, Radin NS, Elias PM, Holleran WM. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- [121].Ekiz HA, Baran Y. Therapeutic applications of bioactive sphingolipids in hematological malignancies. Int J Cancer. 2010;127:1497–1506. doi: 10.1002/ijc.25478. [DOI] [PubMed] [Google Scholar]
- [122].Jessup CF, Bonder CS, Pitson SM, Coates PT. The sphingolipid rheostat: A potential target for improving pancreatic islet survival and function. Endocr Metab Immune Disord Drug Targets. 2011;11:262–272. doi: 10.2174/187153011797881201. [DOI] [PubMed] [Google Scholar]
- [123].Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gomez-Munoz A, Gangoiti P, Granado MH, Arana L, Ouro A. Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv Exp Med Biol. 2010;688:118–130. doi: 10.1007/978-1-4419-6741-1_8. [DOI] [PubMed] [Google Scholar]
- [125].Capeau J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008;34:649–657. doi: 10.1016/S1262-3636(08)74600-7. [DOI] [PubMed] [Google Scholar]
- [126].Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ. Insulin resistance in the Zucker diabetic fatty rat: A metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 2005;42:162–170. doi: 10.1007/s00592-005-0197-8. [DOI] [PubMed] [Google Scholar]
- [127].Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kao Y, Youson JH, Holmes JA, Al-Mahrouki A, Sheridan MA. Effects of insulin on lipid metabolism of larvae and metamorphosing landlocked sea lamprey, Petromyzon marinus. Gen Comp Endocrinol. 1999;114:405–414. doi: 10.1006/gcen.1999.7265. [DOI] [PubMed] [Google Scholar]
- [129].Langeveld M, Aerts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res. 2009;48:196–205. doi: 10.1016/j.plipres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [130].Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- [131].Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125:411–424. [PubMed] [Google Scholar]
- [132].Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: Implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- [133].Nogueira TC, Anhe GF, Carvalho CR, Curi R, Bordin S, Carpinelli AR. Involvement of phosphatidylinositol-3 kinase/AKT/PKCzeta/lambda pathway in the effect of palmitate on glucose-induced insulin secretion. Pancreas. 2008;37:309–315. doi: 10.1097/mpa.0b013e318168dac3. [DOI] [PubMed] [Google Scholar]
- [134].Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- [138].Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- [139].Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E. Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem. 2004;279:50375–50381. doi: 10.1074/jbc.M408493200. [DOI] [PubMed] [Google Scholar]
- [140].Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J, Albano E, Ingelman-Sundberg M, Badger TM. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med (Maywood) 2008;233:344–355. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- [142].Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21(Suppl 3):S7–S9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- [143].Banerjee A, Russell WK, Jayaraman A, Ramaiah SK. Identification of proteins to predict the molecular basis for the observed gender susceptibility in a rat model of alcoholic steatohepatitis by 2-D gel proteomics. Proteomics. 2008;8:4327–4337. doi: 10.1002/pmic.200700368. [DOI] [PubMed] [Google Scholar]
- [144].Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of n-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011;35:2139–2151. doi: 10.1111/j.1530-0277.2011.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Longato L, Ripp K, Setshedi M, Wands JR, de la Monte SM. Oxid Med Cell Longev. 2012. Advanced human alcoholic liver disease is associated with increased pro-ceramide gene expression, ceramide accumulation, endoplasmic reticulum stress, and insulin/IGF resistance. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217:1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- [147].Arboleda G, Huang TJ, Waters C, Verkhratsky A, Fernyhough P, Gibson RM. Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur J Neurosci. 2007;25:3030–3038. doi: 10.1111/j.1460-9568.2007.05557.x. [DOI] [PubMed] [Google Scholar]
- [148].Arboleda G, Cardenas Y, Rodriguez Y, Morales LC, Matheus L, Arboleda H. Differential regulation of AKT, MAPK and GSK3beta during C2-ceramide-induced neuronal death. Neurotoxicology. 2010;31:687–693. doi: 10.1016/j.neuro.2010.08.001. [DOI] [PubMed] [Google Scholar]
- [149].Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- [150].Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J. 2008;410:369–379. doi: 10.1042/BJ20070936. [DOI] [PubMed] [Google Scholar]
- [151].Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- [152].Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61:47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [154].Vistisen B, Hellgren LI, Vadset T, Scheede-Bergdahl C, Helge JW, Dela F, Stallknecht B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;158:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- [155].Zierath JR. The path to insulin resistance: Paved with ceramides? Cell Metab. 2007;5:161–163. doi: 10.1016/j.cmet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [156].Tong M, de la Monte SM. Mechanisms of ceramide-mediated neurodegeneration. J Alzheimers Dis. 2009;16:705–714. doi: 10.3233/JAD-2009-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Setshedi M, Tong M, Feng D, Le T, Wands JR, de la Monte SM. Ceramide inhibitors and PPAR agonists ameliorate alcohol-induced steatohepatitis in an ex-vivo liver slice culture model. Hepatology. 2011;52:121A. [Google Scholar]
- [158].Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: Therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- [159].Craft S. Insulin resistance and cognitive impairment: A view through the prism of epidemiology. Arch Neurol. 2005;62:1043–1044. doi: 10.1001/archneur.62.7.1043-a. [DOI] [PubMed] [Google Scholar]
- [160].Craft S. Insulin resistance syndrome and Alzheimer disease: Pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- [161].Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: A pilot study. Surg Obes Relat Dis. 2009;5:547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- [162].Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [163].Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007;4:123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- [164].Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and Type 2 diabetes mellitus. J Alzheimers Dis. 2008;15:29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [166].Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- [167].Bryan J, Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001;36:147–156. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- [168].Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68:563–569. doi: 10.1097/01.psy.0000221276.17823.df. [DOI] [PubMed] [Google Scholar]
- [169].Felipo V, Urios A, Montesinos E, Molina I, Garcia-Torres ML, Civera M, Del Olmo JA, Ortega J, Martinez-Valls J, Serra MA, Cassinello N, Wassel A, Jorda E, Montoliu C. Metab Brain Dis. 2011. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. doi: 10.1007/s11011-011-9269-3. [DOI] [PubMed] [Google Scholar]
- [170].Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- [171].Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- [172].Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- [173].Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, Schmidt PS. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20:286–291. doi: 10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- [174].Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: Clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- [175].Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008;430:264–268. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: A review. Dig Dis Sci. 2008;53:307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- [177].Karaivazoglou K, Assimakopoulos K, Thomopoulos K, Theocharis G, Messinis L, Sakellaropoulos G, Labropoulou-Karatza C. Neuropsychological function in Greek patients with chronic hepatitis C. Liver Int. 2007;27:798–805. doi: 10.1111/j.1478-3231.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- [178].Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006;3:176–181. doi: 10.1007/s11904-006-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: Relevance to Alzheimer's disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- [180].Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: Assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- [181].Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- [182].Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- [183].Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: Dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- [184].Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lin-dell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- [185].Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983–990. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- [186].Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer's disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PloS one. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6:378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Mielke MM, Haughey NJ, Bandaru VV, Weinberg DD, Darby E, Zaidi N, Pavlik V, Doody RS, Lyketsos CG. Plasma sphingomyelins are associated with cognitive progression in Alzheimer's disease. J Alzheimers Dis. 2011;27:259–269. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: An update. J Neural Transm. 2002;109:341–360. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- [190].Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65:1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- [191].Langbaum JB, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, Alexander GE, Burns CM, Kaszniak AW, Reeder SA, Corneveaux JJ, Allen AN, Pruzin J, Huentelman MJ, Fleisher AS, Reiman EM. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67:462–468. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: A cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3:1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- [195].Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- [196].Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: An update. Exp Gerontol. 2000;35:1363–1372. doi: 10.1016/s0531-5565(00)00156-x. [DOI] [PubMed] [Google Scholar]
- [197].Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasinski A, Grieb P. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: A model of sporadic Alzheimer's disease. Acta Neurochir Suppl. 2010;106:177–181. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- [198].Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- [199].Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J Alzheimers Dis. 2002;4:225–232. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- [200].Blum-Degen D, Frolich L, Hoyer S, Riederer P. Altered regulation of brain glucose metabolism as a cause of neurodegenerative disorders? J Neural Transm Suppl. 1995;46:139–147. [PubMed] [Google Scholar]
- [201].Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am J Pathol. 2009;174:1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Matus S, Lisbona F, Torres M, Leon C, Thielen P, Hetz C. The stress rheostat: An interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med. 2008;8:157–172. doi: 10.2174/156652408784221324. [DOI] [PubMed] [Google Scholar]
- [203].Hoozemans JJ, Stieler J, van Haastert ES, Veerhuis R, Rozemuller AJ, Baas F, Eikelenboom P, Arendt T, Scheper W. The unfolded protein response affects neuronal cell cycle protein expression: Implications for Alzheimer's disease pathogenesis. Exp Gerontol. 2006;41:380–386. doi: 10.1016/j.exger.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [204].Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J. Ethanol promotes endoplasmic reticulum stress-induced neuronal death: Involvement of oxidative stress. J Neurosci Res. 2008;86:937–946. doi: 10.1002/jnr.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- [206].Nelson PT, Smith CD, Abner EA, Schmitt FA, Scheff SW, Davis GJ, Keller JN, Jicha GA, Davis D, Wang-Xia W, Hartman A, Katz DG, Markesbery WR. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ariga T, Jarvis WD, Yu RK. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J Lipid Res. 1998;39:1–16. [PubMed] [Google Scholar]
- [208].Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [209].de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer's disease. Ann Neurol. 1989;25:450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- [210].He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Grosgen S, Grimm MO, Friess P, Hartmann T. Role of amyloid beta in lipid homeostasis. Biochim Biophys Accta. 2010;1801:966–974. doi: 10.1016/j.bbalip.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [213].Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. Journal Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- [214].Tamboli IY, Hampel H, Tien NT, Tolksdorf K, Breiden B, Mathews PM, Saftig P, Sandhoff K, Walter J. Sphingolipid storage affects autophagic metabolism of the amyloid precursor protein and promotes Abeta generation. J Neurosci. 2011;31:1837–1849. doi: 10.1523/JNEUROSCI.2954-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Haughey NJ, Steiner J, Nath A, McArthur JC, Sacktor N, Pardo C, Bandaru VV. Converging roles for sphingolipids and cell stress in the progression of neuro-AIDS. Front Biosci. 2008;13:5120–5130. doi: 10.2741/3068. [DOI] [PMC free article] [PubMed] [Google Scholar]