Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with poor prognosis. In the last decades pirfenidone an anti-inflammatory and anti-fibrotic agent has shown benefit in inhibit collagen production and has also demonstrated benefit in decline progression in IPF in physiological outcomes as Forced vital capacity (FVC), in clinical outcomes such as progression free survival (PFS) and a benefit in mortality but no in clinically relevant outcomes as exacerbations or worsening of IPF. Methods: We conducted a systematic review to evaluate the effectiveness of physiological and clinical outcomes of pirfenidone compared to placebo in IPF. We performed a search with no language restriction. Two researchers performed literature search, quality assessment, data extraction and analysis. And was performed a summary of findings table following the GRADE approach. Results: We included 5 RCTs (Randomized controlled trials) in analysis. The meta-analysis resulted in a decrease in all cause-mortality (RR 0.52 IC 0.32–0.88) and IPF related mortality (RR 0.32 IC 0.14–0.75); other outcomes evaluated were worsening of IPF (RR 0.64 IC 0.50–0.83) and acute exacerbation (RR: 0.72 IC 0.30–1.66 respectively). Also there was a decrease in progression free survival (PFS) (RR 0.83 IC 0.74–0.92) compared to placebo. Conclusions: We observed significant differences in physiologic and clinically relevant outcomes such as reduction in all-cause mortality, IPF related mortality, worsening and exacerbation of IPF and PFS. So pirfenidone treatment should be considered not only for its benefits in pulmonary function tests but also by its clinically relevant outcomes.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare and progressive disease of unknown etiology characterized by dyspnea and deterioration of lung function, with poor quality of life and a median survival time of about 3 years[1]. Several drugs have failed to demonstrate utility in this disease[2], or have been associated with significant adverse effects or even higher mortality[3].
Pirfenidone (5-methyl-1-phenyl-2- [1H]-pyridone) is an agent that combines anti-inflammatory and antifibrotic effects, acting through the regulation of TNFα and TNFβ’s pathways, as well through modulation of cellular oxidation[4,5]. Since the late nineties, some studies have showed that pirfenidone inhibits fibroblast proliferation and collagen synthesis and deposition, both in vitro and in animal models [6–8]. Initial open-label trials showed therapeutic potential for IPF [9,10], and the first prospective clinical studies demonstrated slowing the deterioration of lung function measured as change in lowest SPO2 during 6-minute exercise test (6MET) and vital capacity (VC) (11,12). Based on these findings, Japan approved in 2008 the use of pirfenidone to treat mild to moderate IPF.
The CAPACITY programme (PIPF 004 and 006) included patients with mild to moderate idiopathic pulmonary fibrosis, which was treated for 72 weeks. The PIPF 004 trial, which compared pirfenidone vs. placebo, showed reduction in FVC decline. The PIPF 006 trial, however, did not show significant differences between groups treated with pirfenidone after 48 weeks[11,12]. Based in the pooled analysis of both studies the European Union approved the use of this drug in 2011. Then a Cochrane meta-analysis of these studies besides an effect in FVC, it also found a significant 30% increase in progression free survival, although no effect on mortality was found and other clinically relevant outcomes were not evaluated[13]. A network meta-analysis published in 2014 also showed a possible significant effect on FVC but clinically relevant outcomes were not analyzed.[14]
FDA requested additional evidence to support the use of pirfenidone in USA. Intended to assess and confirm the efficacy and safety of pirfenidone the ASCEND study[15] was performed in IPF patients who received pirfenidone 2403 mg/day vs. placebo for 52 weeks, they observed a relative reduction of 47.9% in the proportion of patients who had an absolute decline of ≥10% in the percentage of the predicted FVC or who died. Pirfenidone also increased the proportion of patients with no decline in FVC and reduced the decline in 6MWT. In addition the ASCEND study pooled all mortality data, incorporating PIPF 004 and 006 studies in the analysis resulting in a significant decrease in this outcome.
After the publication of this last trial pirfenidone was approved by FDA for the treatment of IPF but despite this study showed a mortality benefit at this time the Division of Drug Information in the FDA's Center for Drug Evaluation and Research only consider physiological outcomes (change in FVC) as the efficacy endpoint that was met but no other clinically meaningful outcomes. [16]
The aim of this study is to assess the efficacy and security of pirfenidone on several clinical (including mortality, acute exacerbations and worsening of IPF) and physiological outcomes in IPF, conducting a systematic review and meta-analysis of the current published literature.
Material and Methods
Literature search and clinical eligibility criteria
Two reviewers independently searched the following electronic databases: PubMed, Lilacs, Clinical Trials Registry Platform (ICTRP), clinicaltrials.gov and the Cochrane Controlled Trials Register up to October 30th of 2014. We also searched in two specialized evidence databases: TRIP database and Epistemonikos[17], which conduct searches for systematic reviews in several other databases following PRISMA statement[18].
For maximum sensitivity, we used Mesh term: “pirfenidone” alone and in combination with others term such as: ("pirfenidone" [Supplementary Concept] OR "Anti-Inflammatory Agents, Non-Steroidal"[Mesh]) AND ("Pulmonary Fibrosis"[Mesh]). All meeting abstracts of European respiratory society (ERS) from 2010 to 2014, American thoracic society (ATS) and American college of chest physicians (ACCP) from 2010 to 2014 were searched.
Studies were eligible for inclusion if they met the following criteria: The patients in the selected studies were older than 18 years, and had idiopathic pulmonary fibrosis; the studies were RCTs and compared Pirfenidone against placebo, and had at least one or more clinical outcomes.
Full articles were retrieved, when titles and/or abstracts met this objective. A manual cross-reference search of relevant articles was conducted.
There was no language restriction on publications. Discordance about study inclusion between the two reviewers (CA and GL) was resolved through discussion until 100% agreement was reached on the final interpretation of the data.
Quality assessment of retrieved articles
The selected studies were appraised by two reviewers (AA and GL), who independently assessed their quality using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions.[19]
Outcome measure
The included outcomes in the analysis were: 1) Change in all cause- mortality 2) Change in IPF related mortality 3) Progression-free Survival (PFS) 4) Decrease in predicted Forced Vital Capacity (FVC) 5) Worsening of Idiopathic pulmonary fibrosis 6) Acute exacerbation 7) Change in Six-Minute Walk Test (6MWT) Distance 8) Adverse Effect (all included adverse events, skin related adverse events and change in aminotransferases. In addition, a subgroup analysis evaluating worsening of IPF (Japanese and no-Japanese studies) was performed.
Data extraction and analysis
Data extraction and analysis was performed by two independent reviewers (GL and AA). The studies were tabulated and methodologically evaluated to assess homogeneity. Any heterogeneity between the studies would not be justified to pool the assessed outcomes. A customized data-extraction form, as described in the Cochrane Handbook for Systematic Reviews of Interventions was used to record the duration of the trial, sample size, dropouts, and effect of interventions.
Quantitative data were analyzed using the Cochrane Review Manager (RevMan) version 5.2 software. Summary estimates, including 95% CIs, were calculated. For continuous outcome data, means and standard deviations were used to calculate a weighted mean difference (WMD). For dichotomous outcomes, the RR was calculated.
Statistical heterogeneity was tested using the Q statistic of the c2 value test and I2 test. Fixed-effects models were used, unless significant evidence of statistical heterogeneity or clinical diversity was found. For results showing significant heterogeneity (I2>50%)[19], A random-effects meta-analysis was performed by the Der Simoniane Laird method. Outcome measures were assessed on an intent-to-treat basis. A p value of <0.05 was considered statistically significant. A sensitivity analysis was performed and Publication bias was assessed by visually inspecting a funnel plot.
Finally, we created a summary of findings table following the GRADE approach, using GRADEpro software.
Results
Summary of main results
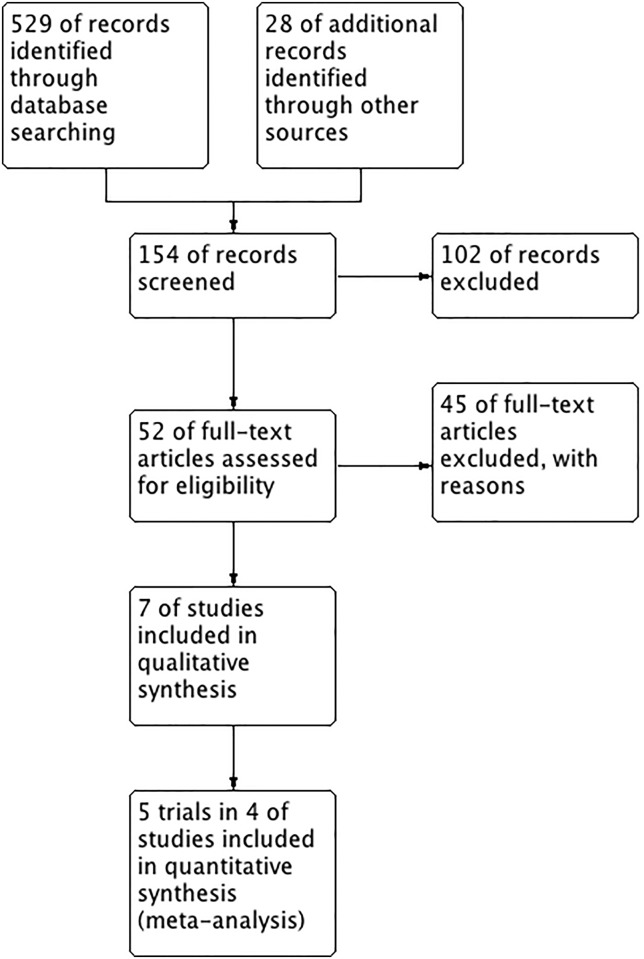
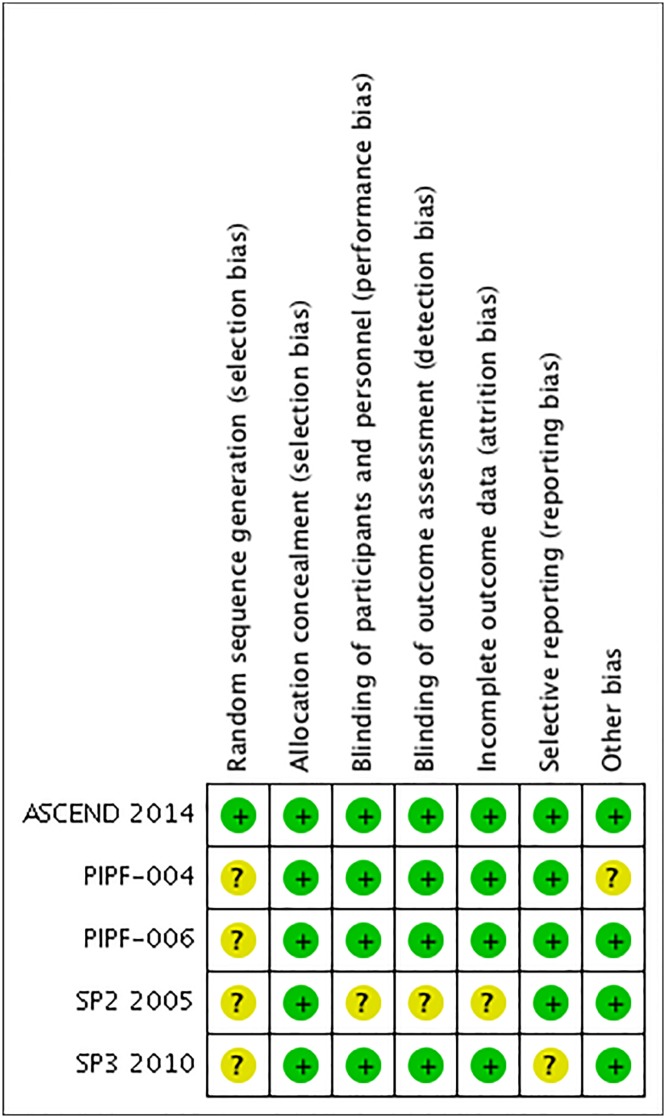
A total of 529 studies were identifiable for library search and 28 were identifiable for others sources. A total of 5 RCTs included in four publications were analyzed. See Table 1 and Fig 1 for a summary of the characteristics of these studies. Excluded studies are shown in supplemental data (S1 Table)[9,20,21]. Quality assessment and risk of BIAS are shown in Fig 2.
Table 1. Characteristics of included studies in this systematic review.
| Trial | Year | N° subjects (intervention/placebo) | Type of studies | Intervention | Comparison | Primary outcome | GRADE |
|---|---|---|---|---|---|---|---|
| CAPACITY (PIPF 004) | 2011 | 174/174 | Parallel | Pirfenidone 1197 mg/day or pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 72 in predicted FVC | MODERATE |
| CAPACITY (PIPF 006) | 2011 | 171/173 | Parallel | Pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 72 in predicted FVC | MODERATE |
| SP2 | 2005 | 72/35 | Parallel | Pirfenidone 200 mg TID for 2 days, 400 mg TID for 2 days and 600 mg TID for 3 days | Placebo pills | Change in the lowest spo2 during 6 mwt | LOW |
| SP3 | 2010 | 163/104 | Parallel | Pirfenidone in stepwise doses; 1800 mg/day in high dose and 1200 mg/day in low dose | Placebo pills | Change from baseline to week 52 in predicted FVC | LOW |
| ASCEND | 2014 | 278/277 | Parallel | Pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 52 in predicted FVC | MODERATE |
Fig 1. PRISMA flow diagram.
Fig 2. Risk of bias summary: review authors' judgment’s about each risk of bias item for each included study.
Effect of interventions
The summary of findings table (GRADE) is shown in Table 2. Funnel plots from all comparisons included in our meta-analysis are shown as supplemental data S1–S10 Figs and PRISMA checklist is shown on S1 PRISMA Checklist.
Table 2. Summary of finding form Pirfenidone for idiopathic pulmonary fibrosis.
| Outcomes | Anticipate absolute effects (Study population) (95% CI) | Relative Effect | NO of participants | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with placebo | Risk with Pirfenidone | ||||
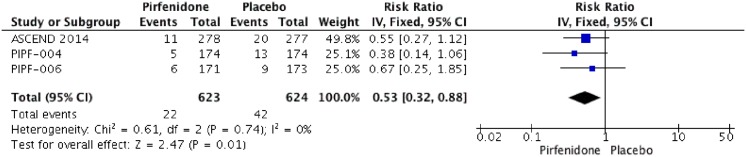
| All cause-mortality | 67 per 1000 | 36 per 1000 (22 to 59) | RR 0.53 (0.32 to 0.88) | 1247 (3 RCTs) | ⨁⨁⨁◯ MODERATE1 |
| Progression free-survival | 442 per 1000 | 372 per 1000 (332 to 416) | RR 0.83 (0.75 to 0.94) | 728 (3 RCTs) | ⨁⨁⨁◯ MODERATE1 |
| Acute exacerbation | 26 per 1000 | 15 per 1000 (5 to 47) | RR 0.59 (0.19 to 1.84) | 235 (2 RCTs) | ⨁⨁◯◯ LOW1,2 |
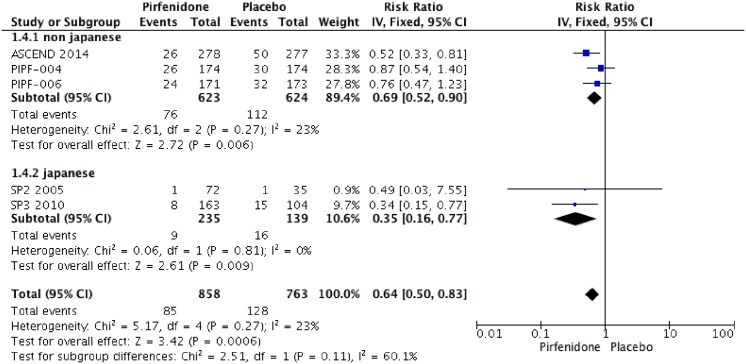
| Worsening of IPF | 168 per 1000 | 107 per 1000 (84 to 139) | RR 0.64 (0.50 to 0.83) | 1615 (5 RCTs) | ⨁⨁⨁◯ MODERATE1 |
| Change on 6MWT | 417 per 1000 | 308 per 1000 (267 to 358) | RR 0.74 (0.64 to 0.86) | 1236 (3 RCTs) | ⨁⨁⨁⨁ HIGH |
| Change on aminotransferases | 30 per 1000 | 68 per 1000 (40 to 115) | RR 2.26 (1.33 to 3.83) | 764 (5 RCTs) | ⨁⨁⨁◯ MODERATE1 |
1: Non primary outcome from RCTs, 2: High heterogeneity; 6MWT: Six minutes walk test; RCT: Randomized controlled trial; RR: Risk ratio; CI: confidence interval.
Mortality
Three RCTs (1247 patients) were identified that reported the effect of pirfenidone and mortality (ASCEND 2014; PIPF004 2011 and PIPF006 2011)[11,15,22,23]. The meta-analysis includes 623 patients in intervention group and 624 in placebo group (Figs 3 and 4). Pirfenidone compared to placebo decreased all cause-mortality (RR: 0.53 IC 0.32–0.88, I2:0%) and IPF related mortality (RR: 0.32, IC 0.14–0.75; I2: 0%) at week 52. We rated the quality of evidence as moderate, because this outcome was not of primary interest in the different studies (indirectness).
Fig 3. Comparison 1.
All cause-mortality at week 52.
Fig 4. Comparison 2.
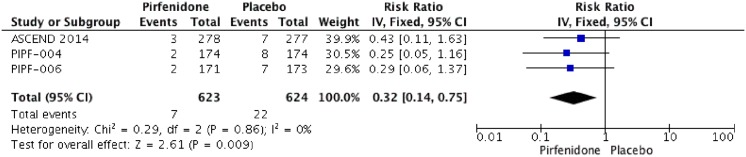
Idiopathic pulmonary fibrosis (IPF) related mortality at week 52.
Progression-free Survival (PFS)
Five RCTs (PIPF004, PIPF006, ASCEND, SP3 and SP2) were identified that reported the effect of pirfenidone and Progression-free Survival. Pooled data from all studies were evaluated at week 52. When PFS was not reported at week 52, data were extracted from Kaplan-Mayer curves and number of events at this week. The meta-analysis includes 850 patients in intervention group and 863 in placebo group (Fig 5). Pirfenidone decreased PFS at week 52 (RR: 0.83 IC 0.74–0.92, I2:0%) compared to placebo. We rated the quality of evidence as moderate, because of indirectness.
Fig 5. Comparison 3.
Change on Progression-free Survival (PFS).
Acute exacerbation of IPF
Four RCTs (SP2, SP3, PIPF004, PIPF006) reported acute exacerbation of IPF. However, in CAPACITY trial (PIPF005 and PIPF 006) exacerbation was reported as “time to acute exacerbation” was part of a composite secondary endpoint and the events were not analyzed separately. The meta-analysis includes 235 patients in intervention group and 139 in placebo group (Fig 6). Pirfenidone do not improves acute exacerbation of IPF with a RR of 0.59 (IC 0.19–1.84, I2: 73%) compared to placebo. We rated the quality of evidence as LOW, because of indirectness and imprecision between results.
Fig 6. Comparison 4.
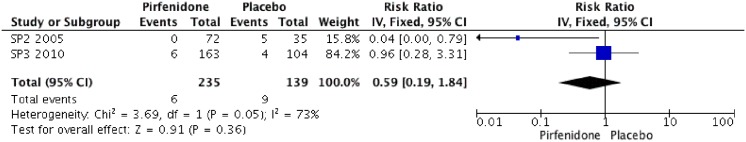
Acute exacerbation.
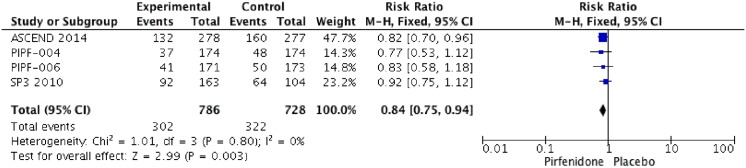
Worsening of IPF
Three RCTs (PIPF004, PIPF006, ASCEND) reported worsening of IPF. This secondary endpoint evaluated in 3 RCTs is related to a composite outcome that included acute IPF exacerbation, IPF related death, lung transplantation or respiratory hospitalization. The meta-analysis includes 786 patients in intervention group and 728 in placebo group (Fig 7). Pirfenidone improves worsening of IPF with a RR of 0.84 (IC 0.74–0.85, I2:0%). compared to placebo. We rated the quality of evidence as moderate, because of indirectness.
Fig 7. Comparison 5.
Worsening of IPF, subgroup analysis from Japanese and non- Japanese studies.
Predicted Forced Vital Capacity (FVC)
Five RCTs (PIPF004, PIPF006, ASCEND, SP3 and SP2) were identified that reported the effect of pirfenidone and FVC or vital capacity (VC). In three RCTs (ASCEND, SP3 and SP2) change of percentage of predicted forced vital capacity >10% were reported. The meta-analysis includes 623 patients in intervention group and 624 in placebo group (Fig 8). Pirfenidone decrease the risk of change >10% of FVC with a Risk ratio of 0.63 (IC 0.47–0.85%, I2: 53%) compared to placebo. We rated the quality of evidence as Moderate due imprecision.
Fig 8. Comparison 6.
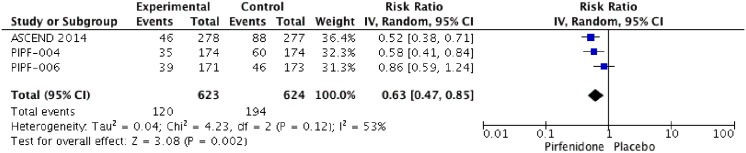
Change >10% of Forced Vital capacity (FVC).
Change in Six-Minute Walk Test (6MWT) Distance
Three RCTs (PIPF004, PIPF006, ASCEND) reported change in 6MWT distance. The meta-analysis includes 617 patients in intervention group and 619 in placebo group (Fig 9). Pirfenidone improves 6MWT distance with a RR of 0.74 (IC 0.64–0.86, I2:0%) compared to placebo. We rated the quality of evidence as high.
Fig 9. Comparison 7.
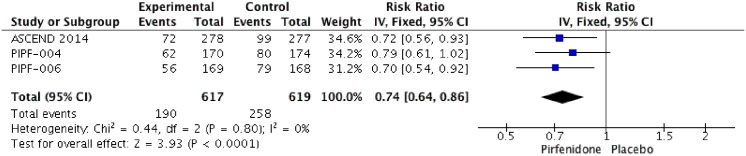
Change on 6-minutes walked test (6MWT) distance.
Adverse events
Five RCTs (PIPF004, PIPF006, ASCEND, SP3 and SP2) were identified that reported the effect of pirfenidone and adverse events. Pooled data from all studies were evaluated at the end of each trial. The meta-analysis includes 857 patients in intervention group and 766 in placebo group (Fig 10). Pirfenidone is not associated with severe adverse events RR: 1.02 (IC 0.93–1.11, I2: 2%) compared to placebo. But other adverse events such as photosensitivity (RR: 4.92; IC 2.10–11.53, I2: 57%) or change on aminotransferases (RR: 2.26; IC 1.33–3.83, I2: 23%) were more frequent than placebo. We rated the quality of evidence as Moderate, because of imprecision between results. Characteristics of treatment-emergent adverse events are shown in Figs 11, 12 and Table 3.
Fig 10. Comparison 8.
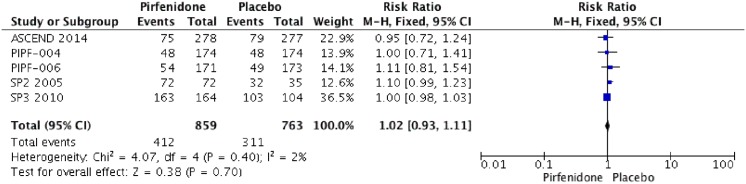
Adverse events.
Fig 11. Comparison 9.
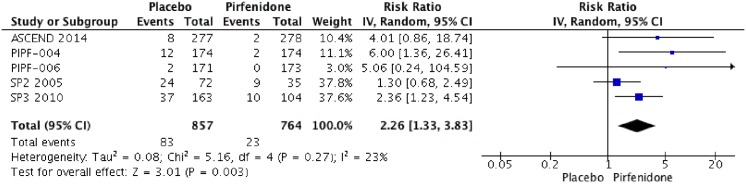
Change on aminotransferase secondary to treatment.
Fig 12. Comparison 10.
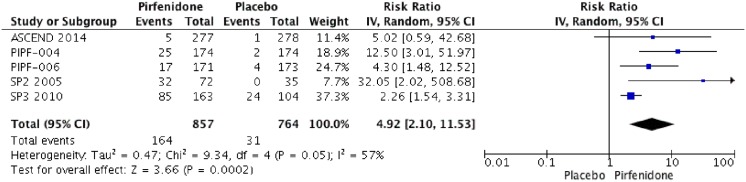
Skin related adverse events.
Table 3. Summary of adverse events.
| SP2 | SP3 | (PIPF-004 and PIPF-006) | ASCEND | |
|---|---|---|---|---|
| Nausea | 72/2 | ND | 125–60 | 100/37 |
| Rash | ND | ND | 111/40 | 78/24 |
| Dyspepsia | ND | ND | 66/26 | 41/49 |
| Vomiting | ND | ND | 47/15 | 36/24 |
| Photosensitivity | 32/0 | 85/24 | 37/6 | 5/1 |
| Anorexia | 23/2 | 24/3 | 37/13 | 44/18 |
| Decreased appetite | 22/3 | 7–0 | 30/10 | 49/17 |
| Weight loss | ND | ND | 28/12 | 35/22 |
| Asthenia | ND | ND | 24–13 | 58/48 |
| Upper airway infection | 12/3 | 04/9 | ND | 61/56 |
| Elevation in aminotransferase | 24/9 | 37/10 | 14/2 | 8/2 |
Right: Pirfenidone group; left: placebo group N.D: No data.
Discussion
Idiopathic pulmonary fibrosis is a chronic, progressive disorder that affects predominantly older patients and has poor prognosis with death rates even worse than many cancers[24].
Before the publication of ASCEND study there was no clear consensus about treatment for this disease. In the 2011 ATS/ERS Guidelines[25], despite have some of data from CAPACITY programme and the potential benefits in physiological outcomes the authors did not recommend the use of any specific pharmacologic therapy for patients with IPF, and only suggest support and adjunctive management, like long-term oxygen, pulmonary rehabilitation, gastroesophageal reflux control and palliative care. While this may be caused because the guidelines are out of date, it may also be due to lack of clinically significant outcomes to strongly support the use of a drug. Related to this reflection the use of systematic reviews with meta-analysis looking for clinical meaningful outcomes may be helpful to develop new clinical practice guidelines.
Unfortunately, previously used drugs (prednisone, azathioprine and N-acetyl cysteine among others) are nowadays discredited after the publication of trials that showed higher mortality rates and/or no improvement in slowing decline of lung function[3,26,27].
Pirfenidone has been a promising drug after initial research that showed its anti-inflammatory, anti-fibrotic and anti-oxidant activity[28–30]. After those in vitro and animal trials, Phase 2 studies and Phase 3 trials were conducted to further elucidate the impact of pirfenidone in patients with IPF[11,23]. The first pooled analysis of these data showed modest results, mostly in lung function, with no benefits in clinically relevant outcomes as mortality, although improvements in progression-free survival and mortality appear promising. This inconsistent effect may be due the limited number of patients, disease phase (recent or former diagnosis) or insufficient follow up. After the recent publication of ASCEND study that confirmed the significance in different physiological and clinical outcomes, this provided more power to determine the potential benefit in mortality that affects these patients.[29]
In this meta-analysis, we observed benefits in several outcomes. The slower decline in lung function test can be seen in the change in FVC and in the change in the 6-minute walk distance the first two of them have a high quality grade, so the estimate of effect is reliable. We also observed differences in clinically relevant outcomes such as reduction in all-cause mortality, IPF related mortality, worsening of IPF and progression free survival; but no benefit on acute exacerbation of IPF: these outcomes have a moderate quality grade and we should consider that future studies may change the estimate of effect. The main limitations of this Systematic review were: the scarce studies that evaluate this intervention; the trials analyzed in this study were performed in different populations; and there were different inclusion criteria in all of them. For example some differences between ASCEND and CAPACITY studies were: ASCEND patients had a poorer baseline lung function, were less likely to have concurrent airflow limitation, had a more likely refined population because underwent to a central review with radiological and pathological analysis prior to enrolment and patients in ASCEND were treated for 52 as opposed to 72 weeks[31]. In the other hand in statistical analysis we did not find significant heterogeneity in several outcomes included all clinical outcomes.
In relation to adverse effects treatment with pirfenidone has been well tolerated in all phase 3 trials. The main adverse effects are photosensitivity (12% in CAPACITY Trials and 51% in the Japanese study), gastrointestinal symptoms and dizziness; the last two were reported only in the CAPACITY program. In this meta-analysis, there is no difference in relative risk of severe adverse effects, but we must consider that is a low quality estimator, so Phase IV clinical trials should be conducted to answer this specific question.
The included systematic review was prepared according to the guidelines of The Cochrane Collaboration and was of high quality in most respects. The quality of the evidence reported by the primary studies in the included reviews was rated using the Cochrane methods and the body of evidence was rated using GRADE and ranged from very low to moderate. The main reasons for the quality of the evidence being downgraded were bias in the primary studies (inadequate reporting of allocation concealment and randomization methods, lack of blinding) and imprecision. The evidence was frequently restricted to a single small trial. No biases were identified during the overview process and no other systematic reviews were identified. We consider this list as the actual body of evidence for this question.
Conclusion
Finally, given the results of this Systematic Review on a poor prognosis disease without previously proven treatment, the associated lower risk and benefits in physiological and clinically relevant outcomes, and considering a future RCT with a mortality primary endpoint is not feasible because the necessary population size, duration and cost. [32] The use of this drug should be highly considered. However, according to our data, this drug does not decrease the risk of acute exacerbation, but more evidence from future RCT is need to improve this outcome.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816. [DOI] [PubMed] [Google Scholar]
- 2. Douglas WW, Ryu JH, Swensen SJ, Offord KP, Schroeder DR, et al. (1998) Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. A randomized prospective study. Members of the Lung Study Group. Am J Respir Crit Care Med 158: 220–225. [DOI] [PubMed] [Google Scholar]
- 3. Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE Jr., Lasky JA, et al. (2012) Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 366: 1968–1977. 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R (2002) Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 446: 167–176. [DOI] [PubMed] [Google Scholar]
- 5. Misra HP, Rabideau C (2000) Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem 204: 119–126. [DOI] [PubMed] [Google Scholar]
- 6. Kaneko M, Inoue H, Nakazawa R, Azuma N, Suzuki M, et al. (1998) Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin Exp Immunol 113: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee BS, Margolin SB, Nowak RA (1998) Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab 83: 219–223. [DOI] [PubMed] [Google Scholar]
- 8. Iyer SN, Margolin SB, Hyde DM, Giri SN (1998) Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model. Exp Lung Res 24: 119–132. [DOI] [PubMed] [Google Scholar]
- 9. Raghu G, Johnson WC, Lockhart D, Mageto Y (1999) Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med 159: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 10. Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, et al. (2002) Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 41: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 11. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, et al. (2011) Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377: 1760–1769. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 12.nice.org.uk (2011) Available: http://www.nice.org.uk/guidance/ta282/documents/idiopathic-pulmonary-fibrosis-pirfenidone-pre-meeting-briefing2.
- 13. Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, et al. (2010) Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev: CD003134 10.1002/14651858.CD003134.pub2 [DOI] [PubMed] [Google Scholar]
- 14. Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, et al. (2014) The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol 15: 63 10.1186/2050-6511-15-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, et al. (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 16.fda.gov (2015) Available: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf.
- 17. Rada G, Perez D, Capurro D (2013) Epistemonikos: a free, relational, collaborative, multilingual database of health evidence. Stud Health Technol Inform 192: 486–490. [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shuster JJ (2011) Review: cochrane handbook for systematic reviews for interventions, Version 5.1. 0, published 3/2011. Julian PT Higgins and Sally Green, Editors. Wiley Online Library. [Google Scholar]
- 20. Azuma A, Taguchi Y, Ogura T, Ebina M, Taniguchi H, et al. (2011) Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res 12: 143 10.1186/1465-9921-12-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taniguchi H, Kondoh Y, Ebina M, Azuma A, Ogura T, et al. (2011) The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir Res 12: 93 10.1186/1465-9921-12-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, et al. (2005) Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 171: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 23. Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, et al. (2010) Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 35: 821–829. 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 24. Vancheri C, Failla M, Crimi N, Raghu G (2010) Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 35: 496–504. 10.1183/09031936.00077309 [DOI] [PubMed] [Google Scholar]
- 25. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, et al. (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, et al. (2005) High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353: 2229–2242. [DOI] [PubMed] [Google Scholar]
- 27. Idiopathic Pulmonary Fibrosis Clinical Research N, Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr., et al. (2014) Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 370: 2093–2101. 10.1056/NEJMoa1401739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antoniou KM, Margaritopoulos GA, Siafakas NM (2013) Pharmacological treatment of idiopathic pulmonary fibrosis: from the past to the future. Eur Respir Rev 22: 281–291. 10.1183/09059180.00002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, et al. (2008) Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 590: 400–408. 10.1016/j.ejphar.2008.06.046 [DOI] [PubMed] [Google Scholar]
- 30. Carter NJ (2011) Pirfenidone: in idiopathic pulmonary fibrosis. Drugs 71: 1721–1732. 10.2165/11207710-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 31. Ryerson CJ, Collard HR (2014) Hot off the breath: A big step forward for idiopathic pulmonary fibrosis. Thorax 69: 791–792. 10.1136/thoraxjnl-2014-205892 [DOI] [PubMed] [Google Scholar]
- 32. King TE Jr, Albera C, Bradford WZ, Costabel U, du Bois RM, et al. (2014) All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med 189: 825–831. 10.1164/rccm.201311-1951OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.