Abstract
Ectoplasmic specialization (ES) is an actin-rich adherens junction in the seminiferous epithelium of adult mammalian testes. ES is restricted to the Sertoli-spermatid (apical ES) interface, as well as the Sertoli cell-cell (basal ES) interface at the blood-testis barrier (BTB). ES is typified by the presence of an array of bundles of actin microfilaments near the Sertoli cell plasma membrane. These actin microfilament bundles require rapid debundling to convert them from a bundled to branched/unbundled configuration and vice versa to confer plasticity to support the transport of 1) spermatids in the adluminal compartment and 2) preleptotene spermatocytes at the BTB while maintaining cell adhesion. Plastin 3 is one of the plastin family members abundantly found in yeast, plant and animal cells that confers actin microfilaments their bundled configuration. Herein, plastin 3 was shown to be a component of the apical and basal ES in the rat testis, displaying spatiotemporal expression during the epithelial cycle. A knockdown (KD) of plastin 3 in Sertoli cells by RNA interference using an in vitro model to study BTB function showed that a transient loss of plastin 3 perturbed the Sertoli cell tight junction-permeability barrier, mediated by changes in the localization of basal ES proteins N-cadherin and β-catenin. More importantly, these changes were the result of an alteration of the actin microfilaments, converting from their bundled to branched configuration when examined microscopically, and validated by biochemical assays that quantified actin-bundling and polymerization activity. Moreover, these changes were confirmed by studies in vivo by plastin 3 KD in the testis in which mis-localization of N-cadherin and β-catenin was also detected at the BTB, concomitant with defects in the transport of spermatids and phagosomes and a disruption of cell adhesion most notably in elongated spermatids due to a loss of actin-bundling capability at the apical ES, which in turn affected localization of adhesion protein complexes at the site. In summary, plastin 3 is a regulator of actin microfilament bundles at the ES in which it dictates the configuration of the filamentous actin network by assuming either a bundled or unbundled/branched configuration via changes in its spatiotemporal expression during the epithelial cycle.—Li, N., Mruk, D. D., Wong, C. K. C., Lee, W. M., Han, D., Cheng, C. Y. Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis.
Keywords: actin microfilaments, blood-testis barrier, spermatid adhesion
During spermatogenesis, extensive remodeling takes place at the Sertoli-germ cell interface to facilitate germ cell transport, in particular spermatids during spermiogenesis, across the seminiferous epithelium (1). Thus, spermatids can be transported across the adluminal compartment while differentiating into spermatozoa that are then lined up at the edge of the tubule lumen at late-stage VIII of the epithelial cycle in rodents to prepare for spermiation (2–4). Similarly, restructuring of the Sertoli cell-cell interface also takes place at the blood-testis barrier (BTB) near the basement membrane to accommodate the transport of preleptotene spermatocytes across the barrier at stage VIII of the cycle (4–6). Thus, the timely occurrence of these events is essential for spermatogenesis. In the testis, there is a unique actin-based cell-cell anchoring junction known as ectoplasmic specialization (ES) at the Sertoli-spermatid and Sertoli cell-cell interface known as apical ES and basal ES, respectively (7–9). Unlike other anchoring junctions, ES is typified by the presence of an array of actin microfilament bundles that lie perpendicular to the Sertoli cell plasma membrane, sandwiched between the cisternae of endoplasmic reticulum and the apposing Sertoli-spermatid (apical ES) or Sertoli-Sertoli (basal ES) plasma membranes (8, 10). Basal ES coexists with tight junction (TJ) and gap junction, and together with desmosome, they constitute the BTB (5, 8). The presence of these actin microfilament bundles at the ES also confers the ES its unusual adhesive strength (11). Interestingly, the occurrence of germ cell transport as well as endocytic vesicle-mediated trafficking events (e.g., endocytosis, transcytosis, and recycling) pertinent to spermatogenesis requires rapid reorganization of these microfilament bundles so that they can be efficiently converted from their “bundled” to “unbundled/branched” configuration and vice versa. Thus, it is envisioned that the dynamic changes of the actin microfilament bundles are conferred by the spatiotemporal expression of actin-bundling proteins, branched actin polymerization proteins, and depolymerization-inducing proteins during the epithelial cycle of spermatogenesis (3, 12). In order to better understand the molecular mechanism that regulates the dynamic nature of microfilament bundles at the ES, we sought to examine the role of an actin-bundling protein, plastin 3, in the testis.
Plastin, also known as fimbrin, is an actin-bundling protein family, involved in the formation of filamentous actin (F-actin) bundles in mammalian cells. This family consists of 3 known members that are evolutionarily conserved from yeast to plant and mammalian cells called plastin 1 (I-plastin), plastin 2 (L-plastin), and plastin 3 (T-plastin), with a Ca2+-binding site near the N terminus (13–15). There is ∼75% sequence similarity among all 3 plastins in mouse and human (13, 15). Plastins are ∼65–68 kDa proteins in which plastin 1 is predominantly found in small intestine, colon, and kidney. Plastin 2 is mostly expressed by hemopoietic cell lineages, such as leukocytes, and tumor cells. Plastin 3 is highly expressed in normal cells of solid tissues such as neurons, fibroblasts, endothelial cells, epithelial cells, and melanocytes. Mutation defects of plastin 3 in humans are associated with osteoporosis and bone fracture (16), possibly due to defects in actin filament bundles in bone cells, thereby impeding the conversion of mechanical signals to intracellular biochemical signals in osteoblasts and osteoclasts (17), causing osteoporosis (18). The best-studied plastin is plastin 2 in immune cells (13, 14, 19, 20). For instance, plastin 2 is known to be involved in T-cell motility and activation (19), and plastin 2-deficient neutrophils failed to kill bacterial pathogens (21) because these cells lacked the ability to quickly rearrange their actin cytoskeleton in response to the pathogens. Studies have shown that phosphorylation of plastin 2 at Ser5 is involved in inducing adhesion in neutrophils (22), and the actin-bundling activity of plastin 2 is an intracellular Ca2+-dependent event (23). However, the function of plastins in the testis remains virtually unknown because there is no report in the literature on this subject except an earlier article with SDS-PAGE analysis supporting the presence of plastin 1 (fimbrin) in the rat testis (24) almost 26 years ago. Herein, we demonstrated that plastin 3 is restrictively expressed by Sertoli cells, but not germ cells, in the seminiferous epithelium. We also examined the likely involvement of plastin 3 in ES dynamics in light of its intrinsic actin-bundling activity in mammalian cells.
MATERIALS AND METHODS
Animals and antibodies
Male Sprague-Dawley rats used for different experiments reported herein were purchased from Charles River Laboratories (Kingston, NY, USA). The use of animals was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol Number 12-506.
Primary Sertoli cell cultures
Sertoli cells were isolated from 20-d-old rat testes as earlier described (25). Sertoli cells were cultured in serum-free Ham’s F12 Nutrient Mixture/DMEM (1:1, vol/vol) (F12/DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with growth factors and gentamicin at 35°C with 95% air-5% CO2 (vol/vol) in a humidified atmosphere. Cells were seeded at a density of 0.04–0.08 × 106 cells/cm2 on 18 mm (diameter) round cover glasses (placed in 12-well dishes with 2 ml F12/DMEM per well; for immunofluorescence analysis), 0.5 × 106 cells/cm2 in 6-well dishes (with 5 ml F12/DMEM per well; for lysate preparation and RNA isolation), or 1.2 × 106 cells/cm2 on Millicell cell culture inserts (HA type, mixed cellulose esters, 12 mm diameter, effective surface area ∼0.6 cm2, 0.45 µm pore size; EMD Millipore, Billerica, MA, USA) for transepithelial electrical resistance (TER) measurements (with 0.5 ml F12/DMEM in the apical and basal chamber) to monitor the TJ-permeability barrier function. About 36 h thereafter, cultures were subjected to a brief hypotonic treatment using 20 mM Tris (pH 7.4) at 22°C for 2.5 min as described (26) to lyse residual germ cells, and Sertoli cells were rinsed twice with F12/DMEM to remove cellular debris. These cultures were ∼98% pure, with negligible contaminations of Leydig cells, peritubular myoid cells, or germ cells using specific markers for these cells by either immunoblotting (IB) or RT-PCR as described (27). All dishes or cover glasses were coated with Matrigel (BD Biosciences, San Jose, CA, USA) diluted with F12/DMEM at 1:7 (for bicameral inserts, a dilution of 1:5 was used) (25). After Sertoli cells were cultured for ∼2 d, a functional TJ-permeability barrier was established, and ultrastructures of TJ, basal ES, gap junction, and desmosome were detected by electron microscopy that mimic the Sertoli cell BTB in vivo (28–30). In fact, this in vitro system was widely used by investigators in the field to study Sertoli cell BTB function (31–35), and these results were subsequently reproduced in studies in vivo (34, 36, 37), illustrating the physiologic relevancy of this in vitro system.
Knockdown of plastin 3 in primary Sertoli cells in vitro by RNA interference
Sertoli cells were cultured in vitro for 3 d to allow the establishment of a functional TJ-permeability barrier that mimic the Sertoli cell BTB in vivo, containing ultrastructures of TJ, basal ES, gap junction, and desmosome as earlier described (25, 29, 30). Thereafter, cells were transfected with plastin 3-specific small interfering RNA (siRNA) duplexes vs. nontargeting negative control siRNA duplexes at 100 nM [for immunofluorescence microscopy (IF) and IB] or 150 nM (for TJ-barrier function assessment) using Lipofectamine RNAiMAX Reagent (Life Technologies, Norwalk, CT, USA) as transfection medium. The desired concentrations of siRNA duplexes for different experiments were selected based on results of pilot experiments that yielded detectable phenotypes without detectable cytotoxicity based on an XTT (sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) assay (Cell Proliferation Kit II, Roche Life Sciences, Branford, CT, USA) as described (29). siRNA duplexes that specifically targeted plastin 3 were obtained from Ambion (Austin, TX, USA): sense, 5′-GCCUAUUUCCAUCUACUCAtt-3′, antisense, 5′-UGAGUAGAUGGAAAUAGGCtt-3′ (s135651); sense, 5′-CACCCUUCAUCAUUCAGGAtt-3′, antisense, 5′-UCCUGAAUGAUGAAGGGUGta-3′ (s135652); and sense, 5′-CCUCUUUAAUAAAUAUCCAtt-3′, antisense, 5′-UGGAUAUUUAUUAAAGAGGtt-3′ (s135653). However, only s135651 siRNA duplexes were used in all subsequent experiments because pilot experiments had demonstrated that the efficacy of s135652 and s135653 in silencing plastin 3 in Sertoli cells was ˂50% vs. 70% obtained with s135651 siRNA duplexes. Nontargeting siRNA duplex (Silencer Select Negative Control #1 siRNA; Ambion) that served as a negative control was included in all experiments, which was comprised of a 19 bp nontargeting sequence with 3 dT overhangs, bearing no significant homology to any known human, mouse, or rat gene sequences as indicated by the manufacturer, which also failed to induce gross changes in gene expression in transfected Sertoli cells as noted in our studies herein. After transfection for 24 h, cells were washed twice and cultured with fresh F12/DMEM including supplements for an additional 24 h before termination to be used for IB, IF, and biochemical assays. For fluorescence microscopy, cells were cotransfected with 1 nM siGLO Red Transfection Indicator (Dharmacon, GE Healthcare Life Sciences, Lafayette, CO, USA) to track successful transfection.
Assessment of TJ-permeability barrier in vitro
Sertoli cell TJ-permeability barrier that assessed the barrier integrity was monitored by quantifying TER across the cell epithelium on the bicameral unit using a Millicell electrical resistance system (EMD Millipore) as described (25). Each time point had quadruplicate culture inserts, and each experiment was repeated 3 times using different batches of Sertoli cells.
Actin-bundling assay
Actin-bundling assay was prepared as earlier described (38) with some modifications. F-actin was first obtained using an Actin Binding Protein Biochem Kit (catalog #BK013; Cytoskeleton, Denver, CO, USA) as follows. In brief, purified nonmuscle actin was reconstituted to 10 mg/ml at 4°C with sterile water. A total of 25 µl of this actin solution was diluted to 1 mg/ml with 225 µl general actin buffer [5 mM Tris-HCl, 0.2 mM CaCl2, and 0.2 mM ATP (pH 8.0)]. The actin solution was then placed on ice for 30 min to obtain G-actin, then supplemented with 25 µl actin polymerization buffer [500 mM KCl, 20 mM MgCl2, 100 mM Tris, and 10 mM ATP (pH 7.5)] and incubated at room temperature (22°C) for 1 h to allow the assembly of F-actin from G-actin, which was then used as the substrate to assess the ability of Sertoli cell lysate to induce actin microfilament bundling. Sertoli cell lysates were first obtained from cells previously transfected with plastin 3-specific siRNA duplexes vs. nontargeting negative control duplexes using a Tris lysis buffer [20 mM Tris, 20 mM NaCl, and 0.5% Triton X-100 (vol/vol) (pH 7.5) at 22°C, freshly supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich) at a 1:100 dilution (vol/vol)], and cellular debris was removed by centrifugation at 20,800 g at 4°C for 1 h. A total of 10 μl Sertoli cell lysates (∼40–50 µg protein) from both groups (clear supernatant from the above step) containing equal amounts of proteins (based on protein estimation using a Bio-Rad DC Protein Assay kit; Hercules, CA, USA) vs. 10 μl Tris lysis buffer (served as a negative control) was then added into 40 μl freshly prepared F-actin obtained above. This mixture was incubated for 1 h at room temperature to allow actin bundling, centrifuged at 14,000 g for 5 min at 24°C to sediment bundled F-actin in the pellet, whereas the linear and unbundled actin microfilaments remain in the supernatant. The whole pellet and 5 μl supernatant of each sample including both groups vs. negative control were analyzed by immunoblot using a specific β-actin antibody. A total of 40 μg of the same cell lysate from each sample was also analyzed by immunoblot to monitor the efficiency of plastin 3 knockdown (KD) and to serve as a protein loading control. This experiment was repeated 5 times using different cell preparations.
Actin polymerization assay
Actin polymerization assay that also assessed the kinetics of actin polymerization was performed as earlier described (39, 40). Effects of plastin 3 KD vs. nontargeting control on actin polymerization were assessed through the initial rate of fluorescence increase that occurs during pyrene-conjugated G-actin incorporation into existing actin microfilaments using Actin Polymerization Biochem Kits from Cytoskeleton. In brief, on d 5 (i.e., 2 d after transfection), Sertoli cells were lysed in Tris lysis buffer in which cellular debris was removed by centrifugation at 20,800 g at 4°C for 1 h. The clear supernatant that contained lysates from Sertoli cells following plastin 3 KD vs. control was immediately subjected to actin polymerization assay as described (39, 40). Cell lysates (∼30 μl) from control and plastin 3-silenced groups with equal amounts of protein (∼120–150 µg) were added to the final reaction mix (100 μl) containing 60 μl pyrene-conjugated G-actin stock and 10 μl of 10× actin polymerization buffer. The kinetics of fluorescence increase were monitored in a Corning 96 well black flat bottom polystyrene microplate (via top reading; Corning, Lowell, MA, USA) by enhanced fluorescence emission at 395–440 nm as described (39) using a FilterMax F5 Multi-Mode Microplate Reader and the Multi-Mode Analysis Software (Molecular Devices, Sunnyvale, CA, USA). A kinetics reading was taken at room temperature every 30 s for 100 cycles with an excitation filter/emission filter at 360/430 nm, and 50 μs integration time was used. The initial rate of actin polymerization (0–12 min) assessed by the rate of increase in fluorescence intensity was obtained by linear regression analysis using Microsoft Excel (Redmond, WA, USA). Each sample was run in duplicate, and this experiment was repeated 3 times, excluding pilot experiments that yielded similar results.
Plastin 3 silencing in adult rat testis in vivo
To knock down plastin 3 in vivo, rats [∼350–375 g body weight (b.w.)] were transfected with siRNA duplexes via intratesticular injection using a 28 gauge needle of an insulin syringe on d 0, to be followed by a second transfection on d 1 as earlier described (41, 42). One testis of a rat received nontargeting negative control siRNA duplexes, whereas the other testis received plastin 3-specific siRNA duplexes shown to perturb the Sertoli cell TJ barrier in vitro. siRNA duplexes at desired concentration (100 nM) were constituted in a transfection mix containing 3 μl Lipofectamine RNAiMAX siRNA transfection reagent and 193 μl Opti-MEM Reduced Serum Medium (Invitrogen, Life Technologies) to a final volume of 200 µl. Thus, each testis (∼1.6 g, with a volume of ∼1.6 ml) of an adult rat received 200 μl of this transfection solution. In short, the 28 gauge (13 mm long) needle attached to a 1 ml insulin syringe containing the transfection mix was inserted from the apical to near the basal end of the testis vertically. As the needle was withdrawn apically, transfection mixture was released gently and gradually from the syringe so that the entire testis was slowly filled with the siRNA mixture that spread throughout the testis to avoid an acute rise in intratesticular hydrostatic pressure. Rats were euthanized on d 3 (i.e., 2 d after the second transfection) by slow (20–30%/min) displacement of chamber air with compressed CO2 via a CO2 tank with a desired regulator. Pilot experiments showed that the phenotypes were similar when rats were terminated on d 3 vs. d 4; thus, animals euthanized on d 3 and d 4 were pooled for analysis with 6 rats. For RNA extraction (for RT-PCR), lysate preparation (for IB), and fluorescence analysis, testes were snap frozen in liquid nitrogen and stored at −80°C until used. Some testes (n = 3) were fixed in Bouin’s fixative to obtain paraffin sections for histologic analysis using hematoxylin and eosin staining.
IB, coimmunoprecipitation, RT-PCR, and quantitative PCR
Lysates of Sertoli cells, germ cells, and testes were obtained by using immunoprecipitation (IP) lysis buffer [10 mM Tris, 0.15 M NaCl, 1% NP-40 (vol/vol), and 10% glycerol (vol/vol) (pH 7.4) at 22°C] freshly supplemented with protease and phosphatase inhibitor cocktails at a 1:100 dilution (vol/vol) as described (39, 42). Immunoblot analysis was performed as earlier described (39) using corresponding specific antibodies (Table 1). Chemiluminescence was performed using a homemade kit in our laboratory as described (44). Coimmunoprecipitation (co-IP) was performed using lysates (∼600 μg protein) from testes as described (39, 42) to assess the binding partners of plastin 3. RNA extraction, PCR, and quantitative PCR (qPCR) using specific primer pairs (Table 2) were performed as described (45). The authenticity of PCR products was verified by direct DNA sequencing performed at Genewiz (South Plainfield, NJ, USA). qPCR was performed at The Rockefeller University Genomics Resource Center (New York, NY, USA).
TABLE 1.
Antibodies used for different experiments in this report
| Antibody | Host species | Vendor | Catalog number | Working dilution |
|
|---|---|---|---|---|---|
| IB | IF | ||||
| Plastin 3 | Rabbit | Abcam | ab137585 | 1:500 | |
| Plastin 3 | Mouse | Santa Cruz Biotechnology | sc-166208 | 1:100 | |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | 1:3000 | 1:50 |
| Eps8 | Mouse | BD Biosciences | 610143 | 1:5000 | 1:50 |
| N-cadherin | Rabbit | Santa Cruz Biotechnology | sc-7939 | 1:200 | |
| N-cadherin | Mouse | Invitrogen, Life Technologies | 33-3900 | 1:100 | |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | 1:300 | |
| α-Catenin | Rabbit | Santa Cruz Biotechnology | sc-7894 | 1:200 | |
| α-Catenin | Mouse | Invitrogen, Life Technologies | 13-9700 | 1:100 | |
| β-Catenin | Rabbit | Invitrogen, Life Technologies | 71-2700 | 1:250 | 1:100 |
| Claudin-11 | Rabbit | Invitrogen, Life Technologies | 36-4500 | 1:50 | |
| JAM-A | Rabbit | Invitrogen, Life Technologies | 36-1700 | 1:250 | |
| Laminin-γ3 | Rabbit | C.Y.C. lab | Reference (43) | 1:50 | |
| Occludin | Rabbit | Invitrogen, Life Technologies | 71-1500 | 1:250 | |
| ZO-1 | Rabbit | Invitrogen, Life Technologies | 61-7300 | 1:250 | 1:100 |
| Vimentin | Mouse | Santa Cruz Biotechnology | sc-6260 | 1:300 | 1:100 |
| Vimentin | Rabbit | Cell Signaling Technology | 3932 | 1:50 | |
| Nectin-3 | Rabbit | Santa Cruz Biotechnology | sc-28637 | 1:50 | |
| Nectin-3 | Goat | Santa Cruz Biotechnology | sc-14806 | 1:200 | |
| β1-Integrin | Rabbit | Santa Cruz Biotechnology | sc-8978 | 1:200 | 1:50 |
| Formin 1 | Mouse | Abcam | ab68058 | 1:500 | |
| Palladin | Rabbit | Proteintech | 10853-1-AP | 1:1000 | 1:50 |
| Filamin A | Mouse | Abcam | ab80837 | 1:500 | |
| CAR | Rabbit | Santa Cruz Biotechnology | sc-15405 | 1:200 | 1:100 |
| p-FAK-Tyr397 | Rabbit | Invitrogen, Life Technologies | 44-625G | 1:50 | |
| GAPDH | Mouse | Abcam | ab824 | 1:1000 | |
Abcam, Cambridge, MA, USA; Santa Cruz Biotechnology, Santa Cruz, CA, USA; Cell Signaling Technology, Danvers, MA, USA; Proteintech, Chicago, IL, USA. CAR, coxsackievirus and adenovirus receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
TABLE 2.
Primers used for different RT-PCR experiments in this report
| Gene | GenBank accession number | Primer sequence (5′–3′) | Nucleotide position | Expected size (bp) |
|---|---|---|---|---|
| Plastin 1 | NM_001108178.2 | Sense: TGAACCAGACACGATTGACG | 61–80 | 188 |
| Antisense: TGATGATCTGCCAGAGAAGC | 229–248 | |||
| Plastin 2 | NM_001012044.1 | Sense: GTCACAGCCACTGATGTTGT | 1093–1112 | 219 |
| Antisense: GTCGGATAAGTCGCTGTACA | 1292–1311 | |||
| Plastin 3 | NM_031084.1 | Sense: CTGAATTCTGCTTCTGCC | 685–702 | 153 |
| Antisense: CAGTGCTTCATTCCTGCT | 820–837 | |||
| S-16 | NM_001169146.1 | Sense: TCCGCTGCAGTCCGTTCAAGTCTT | 87–110 | 385 |
| Antisense: GCCAAACTTCTTGGATTCGCAGCG | 448–471 |
Dual-labeled immunofluorescence analysis
Dual-labeled immunofluorescence analysis was performed using frozen cross sections of testes and specific primary antibodies and corresponding secondary antibodies (Table 1). Negative controls were performed using normal IgG of either mouse or rabbit (depending on the source of primary antibody) or the omission of secondary antibody, which yielded no detectable staining, illustrating the specificity of the antibody. F-actin was stained by rhodamine-conjugated phalloidin as described (46). Cell nuclei were visualized by DAPI (Invitrogen, Life Technologies). Fluorescence images were obtained in an Olympus BX61 fluorescence microscope with images captured using the built-in Olympus DP-71 digital camera (Tokyo, Japan). Image files were analyzed using Photoshop in Adobe Creative Suite (version 3.0; San Jose, CA, USA) such as for image overlay to assess protein colocalization.
Confocal microscopy
Confocal microscopy was performed at the Rockefeller University Bio-Imaging Resource Center (New York, NY, USA) as earlier described (47, 48). It was used to examine changes in the distribution of basal ES protein N-cadherin vs. TJ protein zonula occludens 1 (ZO-1) at the Sertoli cell-cell interface following plastin 3 KD by RNA interference (RNAi). Sertoli cells were cultured at 0.8 × 106 cells/cm2 on Matrigel-coated Transwell Permeable Supports (Costar polyester membrane inserts, 24 mm diameter, 0.4 µm pore size; Corning) for 3 d and transfected with plasmin 3-specific siRNA duplexes vs. nontargeting control duplexes for 24 h, thereafter, cells were washed twice and allowed to culture for an additional day before being fixed in paraformaldehyde and processed for confocal microscopy including staining with primary and secondary antibodies as described (47). Images were obtained using an inverted Zeiss LSM 780 laser-scanning confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped with multiple laser lines (diode, 405 nm; argon/2, 458, 477, 488, and 514 nm; HeNe1, 543 nm; and HeNe2, 633 nm) and the Zeiss LSM 780 software package. Optical sections <0.8 µm were collected at 0.41 µm intervals along the z axis to obtain a series of images (Z-stack). Image deconvolution of Z-stacks was performed using 3D Huygens Deconvolution Software (Scientific Volume Imaging, Hilvesum, The Netherlands) as described (48, 49). Data reported herein were representative findings of an experiment that we repeated 3 times that yielded similar results.
Image analysis
To assess changes in protein localization in Sertoli cells following in vitro plastin 3 KD, at least 200 cells were randomly selected and examined in control vs. experimental groups with 3 experiments. Fluorescence intensity of a target protein in Sertoli cells or in the seminiferous epithelium of testes following in vivo silencing was quantified using ImageJ 1.45 (NIH, Bethesda, MD, USA; http://rsbweb.nih.gov/ij) as earlier described (40). At least 200 randomly selected stage VII vs. stage VIII tubules from cross sections of a rat testis were examined with 3 rats (a total of ∼600 tubules). Analysis was focused on these 2 stages because plastin 3 restrictively expressed at the apical ES in stage VII tubules, and defects in spermatid adhesion and transport that rendered stage VII to become VIII tubules that compromised spermiation were readily detected in stage VIII tubules.
Statistical analysis
For studies using Sertoli cell cultures, triplicate coverslips, dishes, or bicameral units were used. Each data point or bar graph is a mean ± sd of 3–5 experiments (or n = 5 rats). Statistical analysis was performed using the GB-STAT software package (version 7.0; Dynamic Microsystems, Silver Spring, MD, USA) by 1-way ANOVA, followed by Dunnett’s test. In selected experiments, Student’s t test was used for paired comparisons.
RESULTS
Plastin 3 is expressed stage specifically by Sertoli cells in the seminiferous epithelium of adult rat testes during the epithelial cycle
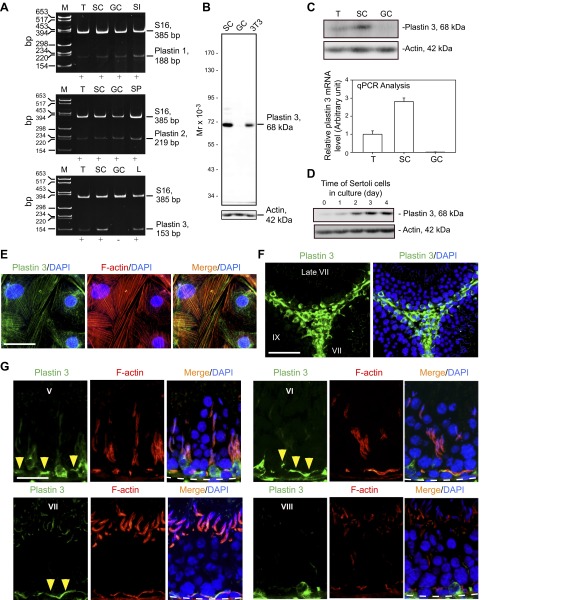
Using primer pairs specific to plastin 1, 2, and 3 (Table 2) for RT-PCR, it was shown that plastin 1 and 2 were expressed by both Sertoli and germ cells, but plastin 3 was expressed only by Sertoli cells, but not germ cells, in the seminiferous epithelium (Fig. 1A). A specific anti-plastin 3 antibody (Table 1) illustrated by immunoblot analysis using lysate of Sertoli cells (Fig. 1A) was then used for our studies. Using lysates of testes and germ cells vs. Sertoli cells and a specific plastin 3 antibody (Fig. 1B and Table 1) for immunoblot analysis, plastin 3 was found to be expressed by Sertoli cells, but not germ cells (Fig. 1C, upper panel), consistent with RT-PCR data shown in Fig. 1A and qPCR analysis shown in Fig. 1C (lower panel). There was a time-dependent expression of plastin 3 by Sertoli cells when these cells were cultured in vitro during the assembly of a functional TJ-permeability barrier (Fig. 1D). When Sertoli cells were cultured alone for 4 d, plastin 3 was found to partially colocalize with actin microfilaments (Fig. 1E). The pattern of plastin 3 distribution in Sertoli cells was not uniform across all cells as shown in Fig. 1E, plausibly the result of the cyclic nature of these cells because they were isolated from tubules of different stages. Besides Sertoli cells, Leydig cells also expressed plastin 3 in the interstitial space in the seminiferous epithelium (Fig. 1F). The expression of plastin 3 by Sertoli cells is most predominant at the F-actin-rich ultrastructure—the basal ES/BTB near the basement membrane and the apical ES at the Sertoli-spermatid interface (Fig. 1G). Although plastin 3 was detected at the basal ES/BTB (annotated by yellow arrowheads in Fig. 1G) in virtually all stages of the epithelial cycle with similar intensity such as at stages V–VII, its expression considerably diminished at stage VIII. Interestingly, its expression at the apical ES was highly stage specific and limited to VII tubules since it was weakly expressed at the apical ES in V–VI tubules and not detectable at any other stages (Fig. 1G). Plastin 3 also partially colocalized with F-actin at both apical and basal ES (Fig. 1G).
Figure 1.
Expression of plastins in the rat testis. A) Relative expression of plastin 1, plastin 2, and plastin 3 in adult rat testes (T), Sertoli cells (SC), and germ cells (GC) vs. the corresponding positive controls of small intestine (SI), spleen (SP), and liver (L) that are known to express these plastins was analyzed by RT-PCR using specific primer pairs (Table 2). S-16 served as a loading and PCR control. M, DNA markers in base pair (bp). B) Specificity of the anti-plastin 3 antibody (Table 1) was assessed by IB using lysates of Sertoli cells (SC) vs. germ cells (GC) and NIH 3T3 cells (mouse embryonic fibroblast cell line, positive control). C) Steady-state protein level of plastin 3 in adult rat testes (T), Sertoli cells (SC), and germ cells (GC) was examined by IB with β-actin serving as a protein loading control (upper panel) vs. steady-state mRNA level by qPCR (lower panel). Each bar in the histogram shown in the lower panel is a mean ± sd of 3 qPCR experiments. D) The relative steady-state protein level of plastin 3 expressed by Sertoli cells that were cultured in vitro for 0–4 d during the establishment of a functional TJ-permeability barrier with actin serving as a protein loading control. E) Staining of Sertoli cells with anti-plastin 3 antibody (green) and rhodamine phalloidin (red) to visualize plastin 3 and F-actin, respectively, illustrating partial colocalization of plastin 3 and F-actin in Sertoli cell cytosol. Sertoli cell nuclei were visualized by DAPI (blue). Scale bar, 40 µm. F) Localization of plastin 3 (green) in the seminiferous epithelium and interstitium of adult rat testis; cell nuclei were visualized by DAPI (blue). Plastin 3 was expressed by Sertoli cells and interstitial cells, most notably Leydig cells, endothelial cells of microvessels, and resident macrophages. Scale bar, 100 µm. G) Stage-specific expression of plastin 3 (green) and its colocalization with F-actin (red) in the seminiferous epithelium of adult rat testes; cell nuclei were visualized by DAPI (blue). Plastin 3 (yellow arrowheads) colocalized with F-actin at the BTB near the basement membrane (annotated by dashed white line) in virtually all stages of the epithelial cycle such as V–VII but considerably diminished in stage VIII; however, its expression at the apical ES was limited mostly to stage VII. Data shown herein are representative findings of an experiment, but each experiment was repeated 3–4 times using different samples or cell preparations excluding pilot experiments. Scale bar, 70 µm.
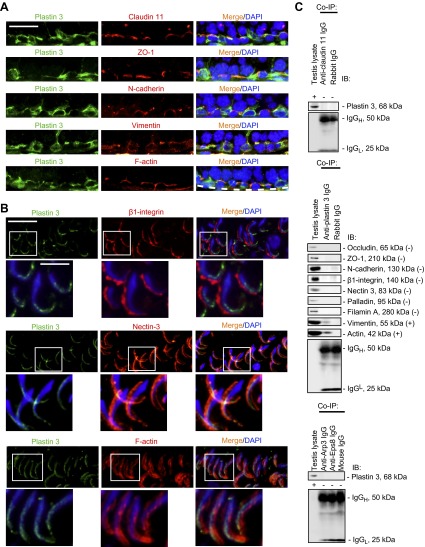
Plastin 3 is associated with ES in adult rat testes
At the basal ES/BTB near the basement membrane, plastin 3 was found to colocalize, at least in part, with integral membrane proteins of TJ (e.g., claudin-11) or basal ES (e.g., N-cadherin), as well as TJ adaptor (e.g., ZO-1) and also vimentin and F-actin in stage VII tubules (Fig. 2A). Plastin 3 also partially colocalized with integral membrane protein and nectin-3 (but not β1-integrin), as well as F-actin at the apical ES (Fig. 2B). Although plastin 3 colocalized in part with basal ES (Fig. 2A) and apical ES (Fig. 2B) proteins, a study by co-IP illustrated that plastin 3 only structurally interacts with actin and vimentin, but not constituent proteins nor actin regulatory proteins at the apical and/or basal ES (Fig. 2C), such as those shown in Fig. 2A, B. This is not entirely unexpected because basal ES protein N-cadherin (a basal ES protein), whereas it colocalizes with occludin (a TJ protein) at the BTB, does not display any structural interaction with occludin by co-IP (50).
Figure 2.
Plastin 3 is a component of the basal ES at the BTB and the apical ES at the Sertoli-Sertoli and Sertoli-spermatid interface in adult rat testes. A) Dual-labeled immunofluorescence analysis confirmed partial colocalization of plastin 3 (green) with TJ (claudin-11 and ZO-1) (red) and basal ES (N-cadherin) (red) proteins, as well as cytoskeletal proteins of the intermediate filament (vimentin) (red) and microfilament (F-actin) (red), at the BTB in stage VII tubules. Cell nuclei were visualized by DAPI (blue). Scale bar, 70 µm. B) Plastin 3 (green) was not colocalized with apical ES protein β1-integrin (red) because β1-integrin was restricted to the convex (dorsal) side of spermatid heads, but plastin 3 was localized mostly to the tip of the spermatid head in stage VII tubules. However, plastin 3 (green) colocalized, at least in part, with nectin-3 (red), which predominantly localized to the convex side of spermatid heads but spread to the tip of spermatid heads where plastin 3 was found. Plastin 3 also colocalized with F-actin, at least in part, at the apical ES. Boxed areas in micrographs were enlarged and shown as insets below. Scale bars, 70 and 35 µm (insets). C) A study by co-IP to assess protein-protein interactions between plastin 3 and selected component proteins of the BTB and the apical ES including cytoskeletal elements using corresponding antibodies as immunoprecipitating antibodies. Rabbit or mouse IgG served as a negative control, and testis lysate without subjection to co-IP served as a positive control. Data shown herein are representative findings of an experiment that was repeated 3–4 times and yielded similar results.
Plastin 3 KD in Sertoli cells with an established TJ barrier by RNAi perturbs the TJ-permeability barrier via mislocalization of basal ES proteins
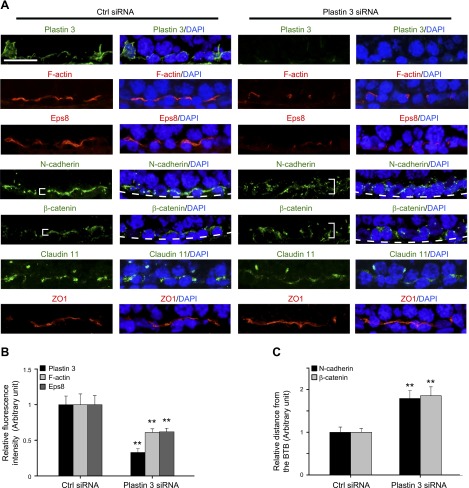
Using siRNA duplexes specific to plastin 3 vs. nontargeting negative control duplexes to knock down plastin 3 in Sertoli cells, plastin 3 was selectively silenced without affecting the expression of either plastin 1 or plastin 2 when RNAs extracted from these cells were used for RT-PCR to examine the steady-state mRNA levels of all 3 plastins (Fig. 3A). These findings were confirmed by immunoblot analysis when the steady-state protein level of plastin 3 was quantified in plastin 3 KD cells vs. nontargeting negative control cells, illustrating an ∼70% KD (Fig. 3B). Furthermore, the expression of several integral membrane proteins, adaptors, actin regulatory proteins, and cytoskeletal proteins at the BTB was also not affected, illustrating that there was no apparent off-target effects (Fig. 3B). When plastin 3 was knocked down by ∼70%, the Sertoli TJ-permeability barrier was found to be perturbed (Fig. 3C), likely the result of a loss of actin filament-bundling ability conferred by plastin 3 at the Sertoli cell barrier. It was noted that a KD of plastin 3 by ∼70% based on IF led to changes in the distribution of basal ES proteins (e.g., N-cadherin and β-catenin) at the Sertoli cell-cell interface, but not TJ proteins (claudin-11 and ZO-1). Both N-cadherin and β-catenin were no longer tightly restricted to the Sertoli cell-cell interface; instead, they were diffusely localized at the site in plastin 3 KD cells vs. control cells (Fig. 3D, see white brackets), likely due to an increase in protein endocytosis. These changes in localization were summarized semiquantitatively in histograms shown in Fig. 3D (see right panel), suggesting that the plastin 3 KD-induced TJ-barrier disruption is mediated by changes in localization of basal ES proteins at the BTB. These observations were further supported by a study using confocal microscopy that assessed changes in the localization of basal ES protein N-cadherin vs. TJ protein ZO-1 in both x-y (horizontal view) and x-z (vertical view) planes (Fig. 4A). Changes in N-cadherin localization following plastin 3 KD were semiquantitatively assessed by averaging the width of green fluorescence (N-cadherin) on 4 sides of a Sertoli cell in plastin 3-silenced cells at the x-z plane vs. control cells from 3 independent experiments (Fig. 4B), supporting findings shown in Fig. 3D.
Figure 3.
A KD of plastin 3 by RNAi perturbs the Sertoli cell TJ-permeability barrier function via changes in the localization of basal ES proteins at the cell-cell interface. Sertoli cells were cultured for 3 d alone to form a functional TJ-permeability barrier. Thereafter, cells were transfected with rat plastin 3-specific siRNA duplexes vs. nontargeting negative control duplexes (Ctrl siRNA) for 24 h. Subsequently, cells were washed twice and cultured for an additional 24 h and terminated for RNA extraction, lysate preparation, or IF analysis. A) A study by RT-PCR confirmed the specific KD of plastin 3 by RNAi because the expression of either plastin 1 or plastin 2 was unaffected. B) A study by IB also illustrated the specific KD of plastin 3 by up to ∼70%, and several BTB-associated proteins including actin-regulating proteins were unaffected without any apparent off-target effect. Each bar in the histogram is a mean ± sd of 4 experiments. **P < 0.01. C) A KD of plastin 3 was found to perturb the Sertoli cell TJ permeability. Each data point is a mean ± sd of triplicates of a representative experiment, which was repeated 3 times and yielded similar results. *P < 0.05. D) A study by IF to confirm the silencing of plastin 3 by ∼70%. The KD of plastin 3 also affected the localization of basal ES proteins N-cadherin and β-catenin, but not TJ proteins claudin-11 and ZO-1, at the Sertoli cell-cell interface in which these proteins moved away from the cell junction site (see white brackets). Scale bar, 40 µm.
Figure 4.
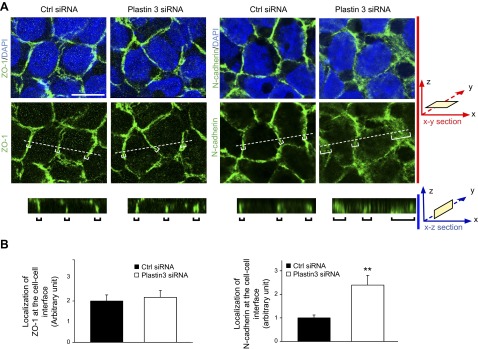
Confocal microscopy analysis of TJ vs. basal ES proteins at the Sertoli cell-cell interface following plastin 3 KD by RNAi. A) Sertoli cells transfected with nontargeting negative control siRNA duplexes vs. plastin 3-specific duplexes for plastin 3 KD and stained for either TJ protein ZO-1 (green fluorescence) or basal ES protein N-cadherin (green fluorescence) are shown in the top 2 panels, which are the horizontal views of the Sertoli cell epithelium, showing the optical slice from the x-y plane (i.e., parallel to the plane of Sertoli cell attachment). See x-y section orientation annotated in the graph (in red) on the right. The bottom panel in (A) illustrates the vertical view of the Sertoli cell epithelium, showing the reconstructed optical slide from the x-z plane (i.e., perpendicular to the plane of Sertoli cell attachment). See x-z section orientation annotated in the graph (in blue) on the right. Corresponding optical slice positions on the x-y plane are annotated by white brackets marked by dotted lines (middle panel) and shown in the bottom x-z plane and annotated by black brackets. These findings illustrate that basal ES protein N-cadherin considerably endocytosed following plastin 3 KD, thereby destabilizing the Sertoli cell-permeability barrier as shown in Fig. 3C. Scale bar, 8 µm. B) About 80 Sertoli cells in each experimental group of 3 independent experiments (i.e., 240 cells total) were randomly selected and analyzed as shown in (A) and summarized in the histogram in which the localization of N-cadherin (green fluorescence) or ZO-1 (green fluorescence) at the cell-cell interface was assessed in plastin 3 KD cells vs. control cells. Localization refers to the mean of the width of green fluorescence on 4 sides of a Sertoli cell of 240 randomly selected cells from 3 experiments, and the width of N-cadherin signal in control cells was arbitrarily set at 1. Each bar is a mean ± sd of 3 experiments. **P < 0.01.
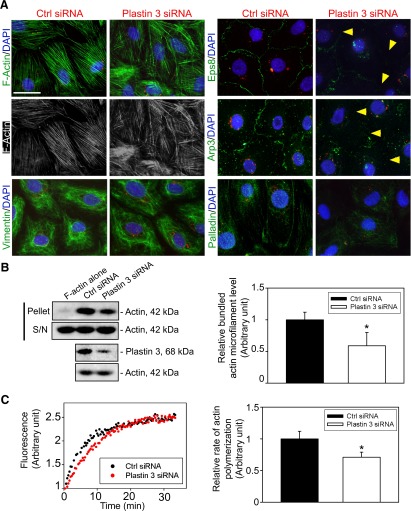
Plastin 3 KD perturbs F-actin organization at the Sertoli cell BTB mediated by a disruption of intrinsic actin-bundling and actin polymerization activity
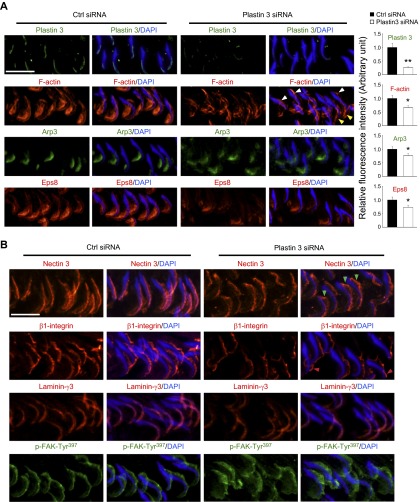
Because basal ES is an actin microfilament-rich adherens junction (8, 10) and plastin 3 is an actin-bundling protein, we next investigated changes in F-actin organization in Sertoli cells following plastin 3 KD in Sertoli cells. As noted in control Sertoli cells, actin microfilaments appeared as a properly organized F-actin network, analogous to bundles of F-actin (Fig. 5A). Following plastin 3 KD, however, extensively branched actin microfilaments were detected, but vimentin-based intermediate filaments were not affected (Fig. 5A). It was also noted that whereas the level of epidermal growth factor receptor pathway substrate 8 (Eps8; an actin barbed end capping and bundling protein) and actin-related protein (Arp; a branched actin nucleation protein, effectively converting bundled actin microfilaments to an unbundled/branched configuration) 3 was unaffected following plastin 3 KD (Fig. 3B), the localization of both Eps8 and Arp3 was perturbed in which these actin regulatory proteins were relocated to the cell cytosol, no longer found conspicuously at or near the Sertoli cell-cell interface (Fig. 5A) to support the proper organization of F-actin at or near the TJ barrier. Based on a biochemical actin-bundling assay, the KD of plastin 3 indeed perturbed actin-bundling activity in Sertoli cells vs. control cells (Fig. 5B). Furthermore, the actin polymerization kinetics was also perturbed (Fig. 5C). Collectively, these data illustrate that plastin 3 is a crucial regulator of actin microfilament bundles at the basal ES/BTB by conferring actin bundling and polymerization of microfilaments.
Figure 5.
KD of plastin 3 leads to disorganization of actin microfilaments in Sertoli cells. A) Following the KD of plastin 3, the F-actin (green or gray) network in Sertoli cells became branched, truncated, and intersected with each other, illustrating a reduced bundling capability, and the vimentin-based intermediate filaments were mostly unaffected. These changes in actin microfilament organization following plastin 3 KD also associated with a mislocalization of actin barbed end capping and bundling protein Eps8, branched actin polymerization protein Arp3, and actin-bundling protein palladin. For instance, both Eps8 and Arp3 were not found at the Sertoli cell-cell interface (see yellow arrowheads). B) A study by biochemical assay to assess actin-bundling activity illustrated that the KD of plastin 3 led to a reduced actin-bundling capability (left panel). Pellet contained bundled F-actin, whereas supernatant (S/N) contained single unbundled actin microfilaments (see Materials and Methods for details). Each bar in the histogram (right panel) is a mean ± sd of 4 experiments. *P < 0.05. C) A KD of plastin 3 by RNAi also perturbed the kinetics of actin polymerization (left panel) in Sertoli cells. In brief, Sertoli cells (0.4 × 106 cells/cm2) cultured for 3 d were transfected with corresponding siRNA duplexes for 24 h. Thereafter, siRNA duplexes were removed, and Sertoli cells were cultured alone in fresh F12/DMEM for another 24 h and harvested on d 5 to obtain lysates and subjected to a fluorometric-based actin polymerization assay, in which the polymerization of pyrene-labeled actin was monitored by enhanced fluorescence emission at 395–440 nm over a 35-min period. The relative rate of actin polymerization (right panel) (i.e., increase in fluorescence intensity over time) during the initial linear phase in the first 12 min based on the kinetics data shown on the left panel was estimated by linear regression. The rate of actin polymerization of control cells (transfected with nontargeting negative control duplexes) was arbitrarily set as 1. This experiment was repeated 4 times and yielded similar results. Each bar in the histogram is a mean ± sd of 4 experiments. *P < 0.05.
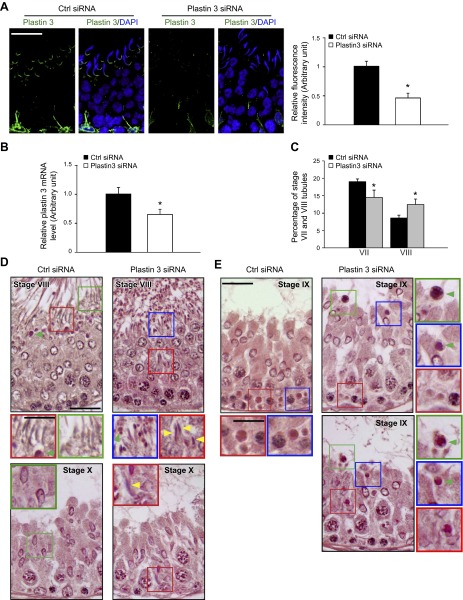
Plastin 3 KD in the testis in vivo perturbs spermatogenesis
To better understand the role of plastin 3 in the testis during spermatogenesis, plastin 3 was silenced by transfecting adult rat testes with plastin 3-specific siRNA duplexes to knock down plastin 3 by ∼55% based on fluorescence analysis (Fig. 6A), which is consistent with results of qPCR (Fig. 6B). Testes from these rats were examined for any changes in the status of spermatogenesis. Because plastin 3 was highly expressed at the apical ES in stage VII tubules, and in vitro studies illustrate its involvement in bundled actin microfilaments at the ES (Fig. 5), we assessed if its KD would impede the transport of elongated spermatids in the adluminal compartment, accelerating the progression of stage VII to VIII tubules, because one of the primary functions of apical ES is to confer spermatid transport besides adhesion. It was noted that in Sprague-Dawley rats transfected with nontargeting negative control siRNA duplexes, the frequencies of stage VII and VIII tubules were ∼20 and ∼8%, respectively, among the 600 tubules randomly scored from 3 rats, consistent with findings of an earlier report (51). Indeed, the KD of plastin 3 led to a considerable reduction in the number of VII and a concomitant increase in VIII tubules (Fig. 6C), supporting the concept that a disruption of actin microfilament bundles at the apical ES perturbed spermatid transport and adhesion, such that stage VII tubules underwent spermiation, making them analogous to stage VIII tubules morphologically (Fig. 6D). Histologic analysis of the testis following plastin 3 KD vs. the nontargeting control testis indeed supports this concept because multiple elongated spermatids were found to be embedded deep inside the seminiferous epithelium in an apparent stage VIII tubule (top panel in Fig. 6D), which in fact was a stage VII tubule. Also, residual elongated spermatids were found to be embedded deep inside the seminiferous epithelium in stage X tubules (lower panel in Fig. 6D). Furthermore, defects in the transport of phagocytes were also noted. For instance, phagosomes that were found near the basement membrane—a typical feature of stage IX tubules in control testes (left panel in Fig. 6E)—remained near the tubule lumen in plastin 3 KD testes in stage IX tubules including a late-stage IX tubule as shown herein (Fig. 6E), illustrating a failure of phagosome transport that is known to be an actin-based cytoskeleton-dependent event (52). In short, these data illustrate that the ES function was compromised due to plastin 3 KD in the testis.
Figure 6.
A KD of plastin 3 in adult rat testes in vivo impairs spermatid adhesion and spermatid transport. Testes of adult rats (∼70 d postpartum; ∼325 –350 g b.w.) were transfected with plastin 3-specific siRNA duplexes vs. nontargeting negative controls. About 2–3 d thereafter, testes were collected either to obtain frozen sections or fixed in Bouin’s fixative to obtain paraffin sections for histologic analysis. A) Frozen sections of testes were stained for plastin 3 (green); cell nuclei were visualized with DAPI (blue). Due to the stage-specific expression of plastin 3 at the apical ES in stage VII tubules, we focused our analysis on this stage. At least 30 stage VII tubules of each testis from the plastin 3 RNAi group (n = 4 rats) and control group (n = 4 rats) were analyzed. Plastin 3 fluorescence staining intensity was considerably diminished following the KD of plastin 3 in vivo. Each bar in the histogram shown on the right is a mean ± SD of n = 4 rats, and the fluorescence intensity of control rats was arbitrarily set as 1. *P < 0.05. B) The steady-state mRNA level of plastin 3 in the testis of the plastin 3 KD group vs. nontargeting control group was examined by qPCR using glyceraldehyde 3-phosphate dehydrogenase as a loading control and reference gene. The mRNA level of plastin 3 was reduced by 40% with 4 rats in each group when testes were transfected by siRNA duplexes in vivo. *P < 0.05. C) The number of stage VII tubules expressed as percentage of all tubules examined was found to be reduced concomitant with an increase in stage VIII tubules in the testis after plastin 3 KD due to defects in spermatid adhesion following a loss of actin-bundling protein plastin 3 at the apical ES, in which spermiation took place in stage VII tubules, rendering them to appear as stage VIII tubules. About 200 tubules from each testis with 3 rats from each group were randomly counted to score stage VII and VIII tubules. *P < 0.05. D) In the control testis that was transfected with nontargeting negative control siRNA duplexes, elongated spermatids line up near the tubule lumen edge as shown in this stage VIII tubule to prepare for spermiation (left top panel), and phagosome (green arrowhead) was also detected near the tubule lumen that was derived from residual bodies phagocytosed by the Sertoli cell to be transported to the base of the seminiferous epithelium at stage IX (see Ctrl siRNA panel in E). However, after plastin 3 KD, in this apparent stage VIII tubule (right top panel), elongated spermatids remained entrapped in the seminiferous epithelium (yellow arrowheads), which appeared to be spermatids from a putative stage VII tubule that failed to be transported to the tubule lumen following plastin 3 KD. But spermatids near the tubule lumen were induced to undergo spermiation prematurely due to defects of actin microfilament organization at the apical ES as a result of plastin 3 KD. In the bottom panel in (D), an elongated spermatid also remained entrapped deep inside the epithelium (yellow arrowhead) in this stage X tubule (right panel) vs. the control (left panel). Scale bars, 70 and 40 μm (insets). E) In the control testis, phagosomes were transported to the base of the epithelium in stage IX (left panel); however, a KD of plastin 3 in the testis caused defects in actin microfilaments, impeding phagosome transport so that many phagosomes remained near the tubule lumen (annotated by green arrowheads), and this failure in phagosome transport persisted in late-stage IX (bottom-right panel). In short, these defects were widespread in many of the apparent stage VIII/IX tubules following plastin 3 KD resulting from defects in spermatid adhesion/transport due to disorganization of actin microfilaments at the ES. Insets are magnified images of the corresponding boxed areas in micrographs. Scale bars, 70 and 40 µm (insets).
Changes in the basal ES protein distribution at the BTB following plastin 3 in vivo KD in the testis
KD of plastin 3 in the testis in vivo led to a reduced expression of plastin 3 at the BTB; however, plastin 3 KD also led to reduced F-actin and Eps8 at the BTB (Fig. 7A, B). Consistent with findings in vitro shown in Fig. 3D, the localization of basal ES proteins N-cadherin and β-catenin, but not TJ proteins claudin-11 and ZO-1, was no longer tightly restricted to the BTB and diffused away from the site (Fig. 7A, C).
Figure 7.
Changes in the distribution of F-actin, actin regulatory protein Eps8, and basal ES proteins following a KD of plastin 3 in adult rat testes in vivo. Frozen sections of rat testes transfected with siRNA duplexes to knock down plastin 3 vs. nontargeting negative control duplexes were stained for plastin 3, F-actin, actin barbed end capping and bundling protein Eps8, as well as basal ES proteins N-cadherin and β-catenin vs. TJ proteins claudin-11 and ZO-1. Cell nuclei were visualized with DAPI (blue). Stage VII/VIII/IX tubules were randomly selected for analysis. A) KD of plastin 3 was verified in these tubules in which plastin 3 fluorescence staining was considerably diminished. The fluorescence intensity of both F-actin and Eps8 at the BTB was also weakened after plastin 3 KD vs. control, supporting the notion that there were less F-actin and microfilament bundles in plastin 3 KD testes. Consistent with the findings in vitro, the basal ES proteins N-cadherin and β-catenin were no longer restrictively localized to the BTB near the basement membrane (annotated by dashed white line); instead, they displayed a considerably diffused pattern (see white brackets in plastin 3 KD testes vs. control testes). Interestingly, the TJ proteins claudin-11 and ZO-1 were not affected by the plastin 3 KD. Scale bar, 40 µm. B and C) Semiquantitative analysis of fluorescence image data shown in (A) including fluorescence intensity (B) or the mislocalization of basal ES proteins by diffusing away from the BTB (C). Data in the control group were arbitrarily set at 1. Each bar is a mean ± sd of 3 rats. **P < 0.01.
Changes in the apical ES protein distribution in vivo following plastin 3 KD in the testis
Because the expression of plastin 3 is limited to stage VII tubules, the following analysis was performed using stage VII tubules. KD of plastin 3 in the testis in vivo that led to a reduced expression of plastin 3 at the apical ES also perturbed the organization and/or localization of F-actin at the apical ES (Fig. 8A). For instance, F-actin is no longer confined predominantly to the concave (ventral) side of spermatid heads as in control testes but mostly to the tip (yellow arrowheads in Fig. 8A) or on the convex (dorsal) side (white arrowheads) of spermatid heads. These findings are consistent with data obtained in vitro (Fig. 5). Furthermore, the expression of Arp3 and Eps8 at the apical ES near the concave side of spermatid heads was also considerably down-regulated (Fig. 8A). These changes in actin regulatory protein localization and/or expression that impeded F-actin organization following plastin 3 KD thus perturbed the localization of apical ES proteins nectin-3, β1-integrin, and laminin-γ3, as well as an apical ES regulatory protein kinase p-focal adhesion kinase (FAK)-Tyr397, at the Sertoli-spermatid interface (Fig. 8B). For instance, nectin-3 no longer tightly associated with the convex (dorsal) side of spermatid heads as found in control testes. Instead, it became disengaged and moved away from the site in many spermatids (green arrowheads in Fig. 8B), whereas β1-integrin that was found covering the convex side of spermatid heads in control testes became limited to the tip of spermatid heads in some spermatids (red arrowheads), and laminin-γ3 chain was generally down-regulated in plastin 3-silenced testes (Fig. 8B). p-FAK-Tyr397 was no longer tightly associated with the convex side of spermatid heads but diffusely localized at the apical ES (Fig. 8B). These changes thus support findings shown in Fig. 6C–E regarding defects in spermatid adhesion and transport of spermatids and phagosomes following plastin 3 KD in the testis.
Figure 8.
Changes in the distribution of F-actin, actin regulatory proteins, and apical ES proteins following a KD of plastin 3 in adult rat testes in vivo. Frozen sections of testes transfected with siRNA duplexes to knock down plastin 3 vs. nontargeting negative control duplexes were stained for plastin 3, F-actin, branched actin polymerization protein Arp3, and actin barbed end capping and bundling protein Eps8, as well as apical ES constituent proteins nectin-3, β1-integrin, and laminin-γ3, and apical ES regulatory protein p-FAK-Tyr397. Nuclei were stained with DAPI (blue). A) In stage VII tubules, plastin 3 staining was considerably diminished at the apical ES following plastin 3 KD in the testis in vivo vs. control. Actin microfilaments prominently localized to the concave (ventral) side of spermatid heads as shown in the seminiferous epithelium of control testes were found to become diffusively localized at the site and considerably diminished; and in some cases, F-actin was mostly expressed on the convex (dorsal) side of spermatid heads but near the base (white arrowheads) and also near the tip (yellow arrowheads) of spermatid heads, considerably different from the control testes. Furthermore, both Arp8 and Eps8 were considerably diminished at the apical ES following plastin 3 KD in the testis. Right panel illustrates semiquantitative analysis of the IF data shown on the left panel. *P < 0.05, **P < 0.01. B) Apical ES proteins nectin-3 (an integral membrane protein in spermatids) and β1-integrin (an integral membrane in Sertoli cells) were also found to be mislocalized in which nectin-3 appeared to be detached from the convex side of spermatid heads (green arrowheads), whereas β1-integrin shifted its expression mostly to the tip of spermatids in some cases (red arrowheads). For laminin-γ3 chain (a spermatid-specific apical ES protein), its expression was considerably down-regulated, whereas apical ES regulatory protein p-FAK-Tyr397 no longer tightly localized to the convex side of spermatid heads; instead, it was diffusely localized. Scale bars, 40 µm.
DISCUSSION
Mammalian cells usually express just one form of plastins, such as plastin 1 in small intestine, colon, and kidney, plastin 2 in leukocytes and tumor cells, and plastin 3 in normal cells of solid tissues (e.g., neurons, fibroblasts, endothelial cells, epithelial cells, and melanocytes) (13–15, 20). Sertoli cells, however, express all 3 plastins with plastin 3 being the most predominant form. Germ cells also express plastin 1 and 2, but not plastin 3, in the seminiferous epithelium as reported herein. Recent studies in humans have illustrated the physiologic and pathophysiologic significance of plastin 3. For instance, plastin 3 is a reliable marker for Sézary syndrome (a type of cutaneous lymphoma) diagnosis, in which the expression of plastin 3 by CD4+ T cells is several orders of magnitude vs. CD4+ T cells in normal subjects (53). Plastin 3 is also identified as a protective modifier of spinal muscular atrophy (SMA) because higher expression of plastin 3 was detected in peripheral blood of patients who had lesser clinical severity of SMA, such as the capability of walking unaided in some female patients (54). Plastin 3 is also becoming a novel marker for circulating tumor cells (CTCs) in blood, possessing important prognostic value for metastasis because it is highly expressed in metastatic CTCs (55). These findings also support the notion that plastin 3 is crucial to confer actin-based cytoskeletal dynamics to mammalian cells, such as metastatic cells for locomotion. Although the Sertoli cell in vivo is not a motile cell type per se, unlike fibroblasts, macrophages, or neutrophils, Sertoli cells in the seminiferous epithelium throughout the epithelial cycle make direct contacts with germ cells to support their development or with each other such as at the BTB via cell junctions to coordinate cellular events across the epithelium, many of which (e.g., ES, gap junction, and TJ) are using actin microfilaments for attachment (1, 8, 56). Thus, there is extensive remodeling of the actin microfilaments at the Sertoli-germ and Sertoli-Sertoli cell-cell interface during the epithelial cycle, in particular at the ES, which requires the presence of actin microfilament bundles for its optimal function. In fact, besides an integrated component of the ES, such as basal ES/BTB at the Sertoli cell-cell interface, a transient KD of plastin 3 in Sertoli cells leads to a disruption of actin microfilament bundles in which microfilaments became extensively branched and unbundled when examined by fluorescence microscopy, which is also supported by a biochemical-bundling assay, as shown herein. These findings also illustrate that efficient reorganization of actin microfilaments at the ES (e.g., rapid conversion between a bundled and an unbundled configuration) in response to changes in the epithelial cycle to support BTB remodeling, spermatid adhesion, and spermatid/preleptotene spermatocyte or phagosome transport may not necessarily require the involvement of branched actin-inducing proteins such as the Arp2/3 complex/neuronal Wiskott-Aldrich syndrome protein (57, 58); a timely or spatiotemporal down-regulation of plastin 3 could effectively convert bundled actin microfilaments to the unbundled/branched F-actin network. It is likely that plastin 3 can also be working in concert with the Arp2/3 protein complex to confer or fine-tune proper configuration of actin microfilaments at the ES because an earlier report using HeLa cells or CCL-81 (a monkey kidney cell line) has shown that plastin 3 stimulates the Arp2/3-mediated cell movement and also inhibits cofilin-mediated depolymerization of actin filaments in vitro (59).
Besides conferring spermatid transport in the adluminal compartment, apical ES is also an anchoring device (6, 9, 60, 61). In fact, it is known that once apical ES appears at the Sertoli-spermatid (step 8–19) interface, it is the only anchoring device, replacing desmosome and gap junction (10, 56, 62). Thus, in vivo KD of plastin 3 in the testis led to the premature release of spermatids into the tubule lumen such as at stage VII, causing the appearance of more stage VIII tubules in plastin 3 KD testes. This increase in stage VIII tubules is the result of stage VII tubules being pushed to undergo spermiation, making them analogous to stage VIII tubules. The premature release of spermatids, as shown here, is not entirely unexpected because actin microfilament bundles at the apical ES are essential to confer adhesive function. It is known that the release of sperm that occurs at spermiation is a complicated cellular process, involving multiple signal proteins (2, 63), and studies have shown that this event is heavily dependent on actin-based cytoskeleton, requiring the involvement of multiple actin-binding proteins and regulatory signaling protein kinases (3, 64). Here, KD of plastin 3 in vivo indeed induces structural defects of the F-actin network at the apical ES as visualized by rhodamine-phalloidin staining, involving mis-localization of other actin regulatory proteins, Arp3 and Eps8. These changes thus contribute to the disruption of adhesion junction proteins nectin-3 (65), β1-integrin (66–68), and laminin-γ3 (43, 69) via changes in their spatiotemporal localization/expression, leading to premature release of spermatids, causing stage VII tubules to become stage VIII alike, and impeding the relative percentage of stage VII tubules in plastin 3 KD testes vs. control testes.
Plastin 3, also known as T-plastin, similar to plastin 1 (I-plastin) and plastin 2 (L-plastin), is a Ca2+-dependent actin-bundling protein even though its reliance on Ca2+ is less strict vs. the other 2 plastins (23, 70, 71). Furthermore, activation of plastins, at least plastin 2, to elicit their intrinsic actin-bundling activity likely involves phosphorylation such as at Ser5 in plastin 2 (72). Thus, p-FAK-Tyr397, the activated form of FAK known to be a component of the apical ES that persists from stage VII through late-stage VIII until shortly before spermiation (39, 73, 74), is likely to be involved in the regulation of plastin 3 because both plastin 3 and p-FAK-Tyr397 were shown to be partially colocalized at the apical ES. This postulate is further supported by the observation that a KD of plastin 3 in the testis perturbed the localization of p-FAK-Tyr397 at the apical ES. Recent reports have shown that a disruption on the expression of p-FAK-Tyr397 perturbs spermatid adhesion (75), and FAK is involved in mediating actin polymerization kinetics at the ES (39), illustrating that plastin 3 and p-FAK-Tyr397 may be working synergistically to regulate actin microfilament bundles at the ES. This possibility must be carefully evaluated in future studies.
In summary, plastin 3 is spatiotemporally expressed by Sertoli cells at the ES in the seminiferous epithelium during the epithelial cycle of spermatogenesis. Plastin 3 is also a regulator of actin microfilaments at the ES, maintaining actin filament bundles in Sertoli cells that confers spermatid and Sertoli cell-cell adhesion, and facilitates spermatid transport during spermatogenesis.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD056034 and U54 HD029990 Project 5) both to C.Y.C.; Hong Kong Baptist University (LSK/14-15/P06) to C.K.C.W.; National Natural Science Foundation of China Research Grants Council of Hong Kong Joint Research Scheme (Grant N_HKU 717/12, RGC 771513); and University of Hong Kong Committee on Research and Conference Grants Seed Funding, both to W.M.L.
Glossary
- Arp3
actin-related protein 3
- BTB
blood-testis barrier
- b.w.
body weight
- co-IP
coimmunoprecipitation
- CTC
circulating tumor cell
- Eps8
epidermal growth factor receptor pathway substrate 8
- ES
ectoplasmic specialization
- F12/DMEM
Ham’s F12 Nutrient Mixture/DMEM (1:1, vol/vol)
- F-actin
filamentous actin
- FAK
focal adhesion kinase
- IB
immunoblotting
- IF
immunofluorescence microscopy
- IP
immunoprecipitation
- KD
knockdown
- qPCR
quantitative PCR
- RNAi
RNA interference
- siRNA
small interfering RNA
- SMA
spinal muscular atrophy
- TER
transepithelial electrical resistance
- TJ
tight junction
- ZO-1
zonula occludens 1
REFERENCES
- 1.Cheng C. Y., Mruk D. D. (2002) Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell L., Nicholls P. K., O’Bryan M. K., McLachlan R. I., Stanton P. G. (2011) Spermiation: The process of sperm release. Spermatogenesis 1, 14–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian X., Mruk D. D., Cheng Y. H., Tang E. I., Han D., Lee W. M., Wong E. W., Cheng C. Y. (2014) Actin binding proteins, spermatid transport and spermiation. Semin. Cell Dev. Biol. 30, 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess R. A., Renato de Franca L. (2008) Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 636, 1–15 [DOI] [PubMed] [Google Scholar]
- 5.Cheng C. Y., Mruk D. D. (2012) The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 64, 16–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao X., Mruk D. D., Wong C. K. C., Cheng C. Y. (2014) Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda) 29, 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell L. D., Peterson R. N. (1985) Sertoli cell junctions: morphological and functional correlates. Int. Rev. Cytol. 94, 177–211 [DOI] [PubMed] [Google Scholar]
- 8.Vogl A. W., Vaid K. S., Guttman J. A. (2008) The Sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 636, 186–211 [DOI] [PubMed] [Google Scholar]
- 9.Yan H. H. N., Mruk D. D., Lee W. M., Cheng C. Y. (2007) Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays 29, 36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng C. Y., Mruk D. D. (2010) A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 6, 380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolski K. M., Perrault C., Tran-Son-Tay R., Cameron D. F. (2005) Strength measurement of the Sertoli-spermatid junctional complex. J. Androl. 26, 354–359 [DOI] [PubMed] [Google Scholar]
- 12.Cheng C. Y., Mruk D. D. (2011) Actin binding proteins and spermiogenesis: Some unexpected findings. Spermatogenesis 1, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinomiya H. (2012) Plastin family of actin-bundling proteins: its functions in leukocytes, neurons, intestines, and cancer. Int. J. Cell Biol. 2012, 213492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babich A., Burkhardt J. K. (2013) Coordinate control of cytoskeletal remodeling and calcium mobilization during T-cell activation. Immunol. Rev. 256, 80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delanote V., Vandekerckhove J., Gettemans J. (2005) Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol. Sin. 26, 769–779 [DOI] [PubMed] [Google Scholar]
- 16.Van Dijk F. S., Zillikens M. C., Micha D., Riessland M., Marcelis C. L., de Die-Smulders C. E., Milbradt J., Franken A. A., Harsevoort A. J., Lichtenbelt K. D., Pruijs H. E., Rubio-Gozalbo M. E., Zwertbroek R., Moutaouakil Y., Egthuijsen J., Hammerschmidt M., Bijman R., Semeins C. M., Bakker A. D., Everts V., Klein-Nulend J., Campos-Obando N., Hofman A., te Meerman G. J., Verkerk A. J., Uitterlinden A. G., Maugeri A., Sistermans E. A., Waisfisz Q., Meijers-Heijboer H., Wirth B., Simon M. E., Pals G. (2013) PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 369, 1529–1536 [DOI] [PubMed] [Google Scholar]
- 17.Weinbaum S., Duan Y., Thi M. M., You L. (2011) An integrative review of mechanotransduction in endothelial, epithelial (renal) and dendritic cells (osteocytes). Cell. Mol. Bioeng. 4, 510–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvihill B. M., Prendergast P. J. (2010) Mechanobiological regulation of the remodelling cycle in trabecular bone and possible biomechanical pathways for osteoporosis. Clin. Biomech. (Bristol, Avon) 25, 491–498 [DOI] [PubMed] [Google Scholar]
- 19.Morley S. C. (2013) The actin-bundling protein L-plastin supports T-cell motility and activation. Immunol. Rev. 256, 48–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samstag Y., Eibert S. M., Klemke M., Wabnitz G. H. (2003) Actin cytoskeletal dynamics in T lymphocyte activation and migration. J. Leukoc. Biol. 73, 30–48 [DOI] [PubMed] [Google Scholar]
- 21.Chen H., Mocsai A., Zhang H., Ding R. X., Morisaki J. H., White M., Rothfork J. M., Heiser P., Colucci-Guyon E., Lowell C. A., Gresham H. D., Allen P. M., Brown E. J. (2003) Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity 19, 95–104 [DOI] [PubMed] [Google Scholar]
- 22.Jones S. L., Wang J., Turck C. W., Brown E. J. (1998) A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function. Proc. Natl. Acad. Sci. USA 95, 9331–9336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namba Y., Ito M., Zu Y., Shigesada K., Maruyama K. (1992) Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J. Biochem. 112, 503–507 [DOI] [PubMed] [Google Scholar]
- 24.Grove B. D., Vogl A. W. (1989) Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J. Cell Sci. 93, 309–323 [DOI] [PubMed] [Google Scholar]
- 25.Mruk D. D., Cheng C. Y. (2011) An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol. Biol. 763, 237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galdieri M., Ziparo E., Palombi F., Russo M. A., Stefanini M. (1981) Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J. Androl. 2, 249–254 [Google Scholar]
- 27.Lee N. P. Y., Mruk D. D., Conway A. M., Cheng C. Y. (2004) Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J. Androl. 25, 200–215 [DOI] [PubMed] [Google Scholar]
- 28.Lee N. P. Y., Cheng C. Y. (2003) Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology 144, 3114–3129 [DOI] [PubMed] [Google Scholar]
- 29.Li M. W. M., Mruk D. D., Lee W. M., Cheng C. Y. (2009) Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 41, 2302–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lie P. P. Y., Cheng C. Y., Mruk D. D. (2010) Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int. J. Biochem. Cell Biol. 42, 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janecki A., Jakubowiak A., Steinberger A. (1992) Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol. Appl. Pharmacol. 112, 51–57 [DOI] [PubMed] [Google Scholar]
- 32.Nicholls P. K., Harrison C. A., Gilchrist R. B., Farnworth P. G., Stanton P. G. (2009) Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology 150, 2481–2490 [DOI] [PubMed] [Google Scholar]
- 33.Kaitu’u-Lino T. J., Sluka P., Foo C. F., Stanton P. G. (2007) Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 133, 1169–1179 [DOI] [PubMed] [Google Scholar]
- 34.Qiu L., Zhang X., Zhang X., Zhang Y., Gu J., Chen M., Zhang Z., Wang X., Wang S. L. (2013) Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol. Sci. 135, 229–240 [DOI] [PubMed] [Google Scholar]
- 35.Lui W.-Y., Lee W. M., Cheng C. Y. (2003) Transforming growth factor β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol. Reprod. 68, 1597–1612 [DOI] [PubMed] [Google Scholar]
- 36.Lui W. Y., Wong C. H., Mruk D. D., Cheng C. Y. (2003) TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 144, 1139–1142 [DOI] [PubMed] [Google Scholar]
- 37.Su L., Mruk D. D., Lie P. P. Y., Silvestrini B., Cheng C. Y. (2012) A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat. Commun. 3, 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ip C. K., Cheung A. N., Ngan H. Y., Wong A. S. (2011) p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 30, 2420–2432 [DOI] [PubMed] [Google Scholar]
- 39.Lie P. P. Y., Mruk D. D., Mok K. W., Su L., Lee W. M., Cheng C. Y. (2012) Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc. Natl. Acad. Sci. USA 109, 12562–12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan H. T., Mruk D. D., Wong C. K. C., Cheng C. Y. (2014) Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr(407): an in vitro study. Endocrinology 155, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mok K. W., Mruk D. D., Lee W. M., Cheng C. Y. (2013) Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 27, 1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian X., Mruk D. D., Wong E. W. P., Lie P. P. Y., Cheng C. Y. (2013) Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology 154, 1907–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan H. H. N., Cheng C. Y. (2006) Laminin α 3 forms a complex with β3 and γ3 chains that serves as the ligand for α 6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 281, 17286–17303 [DOI] [PubMed] [Google Scholar]
- 44.Mruk D. D., Cheng C. Y. (2011) Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 1, 121–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee N. P. Y., Mruk D., Lee W. M., Cheng C. Y. (2003) Is the cadherin/catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis? Biol. Reprod. 68, 489–508 [DOI] [PubMed] [Google Scholar]
- 46.Xiao X., Mruk D. D., Lee W. M., Cheng C. Y. (2011) c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int. J. Biochem. Cell Biol. 43, 651–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X., Mruk D. D., Cheng C. Y. (2013) c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am. J. Physiol. Endocrinol. Metab. 304, E145–E159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lie P. P. Y., Cheng C. Y., Mruk D. D. (2011) Interleukin-1α is a regulator of the blood-testis barrier. FASEB J. 25, 1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su L., Mruk D. D., Lui W. Y., Lee W. M., Cheng C. Y. (2011) P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc. Natl. Acad. Sci. USA 108, 19623–19628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan H. H. N., Cheng C. Y. (2005) Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl. Acad. Sci. USA 102, 11722–11727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hess R. A., Schaeffer D. J., Eroschenko V. P., Keen J. E. (1990) Frequency of the stages in the cycle of the seminiferous epithelium in the rat. Biol. Reprod. 43, 517–524 [DOI] [PubMed] [Google Scholar]
- 52.Clermont Y., Morales C., Hermo L. (1987) Endocytic activities of Sertoli cells in the rat. Ann. N. Y. Acad. Sci. 513, 1–15 [DOI] [PubMed] [Google Scholar]
- 53.Michel L., Jean-Louis F., Begue E., Bensussan A., Bagot M. (2013) Use of PLS3, Twist, CD158k/KIR3DL2, and NKp46 gene expression combination for reliable Sézary syndrome diagnosis. Blood 121, 1477–1478 [DOI] [PubMed] [Google Scholar]
- 54.Yanyan C., Yujin Q., Jinli B., Yuwei J., Hong W., Fang S. (2014) Correlation of PLS3 expression with disease severity in children with spinal muscular atrophy. J. Hum. Genet. 59, 24–27 [DOI] [PubMed] [Google Scholar]
- 55.Yokobori T., Iinuma H., Shimamura T., Imoto S., Sugimachi K., Ishii H., Iwatsuki M., Ota D., Ohkuma M., Iwaya T., Nishida N., Kogo R., Sudo T., Tanaka F., Shibata K., Toh H., Sato T., Barnard G. F., Fukagawa T., Yamamoto S., Nakanishi H., Sasaki S., Miyano S., Watanabe T., Kuwano H., Mimori K., Pantel K., Mori M. (2013) Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 73, 2059–2069 [DOI] [PubMed] [Google Scholar]
- 56.Mruk D. D., Cheng C. Y. (2004) Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 [DOI] [PubMed] [Google Scholar]
- 57.Suetsugu S. (2013) Activation of nucleation promoting factors for directional actin filament elongation: allosteric regulation and multimerization on the membrane. Semin. Cell Dev. Biol. 24, 267–271 [DOI] [PubMed] [Google Scholar]
- 58.Cheng C. Y., Mruk D. D. (2011) Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem. J. 435, 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giganti A., Plastino J., Janji B., Van Troys M., Lentz D., Ampe C., Sykes C., Friederich E. (2005) Actin-filament cross-linking protein T-plastin increases Arp2/3-mediated actin-based movement. J. Cell Sci. 118, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 60.Russell L. (1977) Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell 9, 475–498 [DOI] [PubMed] [Google Scholar]
- 61.Russell L. D. (1993) Morphological and functional evidence for Sertoli-germ cell relationships. In The Sertoli Cell (Russell L. D., Griswold M. D., eds.), pp. 365–390, Cache River Press, Clearwater, FL [Google Scholar]
- 62.Vogl A. W., Pfeiffer D. C., Mulholland D., Kimel G., Guttman J. (2000) Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch. Histol. Cytol. 63, 1–15 [DOI] [PubMed] [Google Scholar]
- 63.Cheng C. Y., Mruk D. D. (2015) Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In Sertoli Cell Biology (Griswold M. D., ed.), pp. 333–383, Elsevier, New York [Google Scholar]
- 64.Wan H. T., Mruk D. D., Tang E. I., Xiao X., Cheng Y. H., Wong E. W., Wong C. K., Cheng C. Y. (2014) Role of non-receptor protein tyrosine kinases in spermatid transport during spermatogenesis. Semin. Cell Dev. Biol. 30, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozaki-Kuroda K., Nakanishi H., Ohta H., Tanaka H., Kurihara H., Mueller S., Irie K., Ikeda W., Sakai T., Wimmer E., Nishimune Y., Takai Y. (2002) Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 12, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 66.Salanova M., Ricci G., Boitani C., Stefanini M., De Grossi S., Palombi F. (1998) Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol. Reprod. 58, 371–378 [DOI] [PubMed] [Google Scholar]
- 67.Palombi F., Salanova M., Tarone G., Farini D., Stefanini M. (1992) Distribution of β 1 integrin subunit in rat seminiferous epithelium. Biol. Reprod. 47, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 68.Siu M. K. Y., Cheng C. Y. (2004) Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 70, 945–964 [DOI] [PubMed] [Google Scholar]
- 69.Koch M., Olson P. F., Albus A., Jin W., Hunter D. D., Brunken W. J., Burgeson R. E., Champliaud M. F. (1999) Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J. Cell Biol. 145, 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyakawa T., Shinomiya H., Yumoto F., Miyauchi Y., Tanaka H., Ojima T., Kato Y. S., Tanokura M. (2012) Different Ca²⁺-sensitivities between the EF-hands of T- and L-plastins. Biochem. Biophys. Res. Commun. 429, 137–141 [DOI] [PubMed] [Google Scholar]
- 71.Lyon A. N., Pineda R. H., Hao T., Kudryashova E., Kudryashov D. S., Beattie C. E. (2014) Calcium binding is essential for plastin 3 function in Smn-deficient motoneurons. Hum. Mol. Genet. 23, 1990–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janji B., Giganti A., De Corte V., Catillon M., Bruyneel E., Lentz D., Plastino J., Gettemans J., Friederich E. (2006) Phosphorylation on Ser5 increases the F-actin-binding activity of L-plastin and promotes its targeting to sites of actin assembly in cells. J. Cell Sci. 119, 1947–1960 [DOI] [PubMed] [Google Scholar]
- 73.Siu M. K. Y., Mruk D. D., Lee W. M., Cheng C. Y. (2003) Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology 144, 2141–2163 [DOI] [PubMed] [Google Scholar]
- 74.Beardsley A., Robertson D. M., O’Donnell L. (2006) A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 190, 759–770 [DOI] [PubMed] [Google Scholar]
- 75.Wan H. T., Mruk D. D., Li S. Y. T., Mok K. W., Lee W. M., Wong C. K. C., Cheng C. Y. (2013) p-FAK-Tyr(397) regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am. J. Physiol. Endocrinol. Metab. 305, E687–E699 [DOI] [PMC free article] [PubMed] [Google Scholar]