Abstract
Objective
Apolipoprotein A5 (APOA5) is associated with plasma triglyceride (TG) levels, a risk factor for coronary heart disease (CHD). This study explored the association between CHD and the APOA5 rs662799 polymorphism.
Methods
We collected 1,521 samples (783 CHD patients and 738 controls) for this case-control study. Meta-analysis was performed using Review Manager Software and Stata Software.
Results
Significant differences were observed between CHD cases and controls at the level of both genotype (χ2 = 8.964, df = 2, P = 0.011) and allele (χ2 = 9.180, df = 1, P = 0.002, OR = 1.275, 95% CI = 1.089–1.492). A breakdown analysis by gender showed a significant association of APOA5 rs662799 with CHD in males (χ2 = 7.770, df = 1, P = 0.005; OR = 1.331, 95% CI = 1.088–1.628). An additional meta-analysis using 21378 cases and 28428 controls established that rs662799 is significantly associated with CHD (P < 0.00001).
Conclusion
Both our case-control study and meta-analysis confirm a significant association between APOA5 rs662799 and CHD. In addition, our results suggest a male-specific association between the APOA5 rs662799 polymorphism and CHD.
Introduction
Coronary heart disease (CHD) is a type of cardiovascular disease that is caused by ischemia and hypoxia in the coronary artery [1] and is the leading cause of human deaths worldwide [2–4]. CHD is the most common cause of death among both men and women over the age of 50 [5]. Environmental factors associated with CHD include obesity, smoking, drinking, diabetes, arterial hypertension and dyslipidemia [6]. In addition, genetic factors are important for CHD [7].
APOA5 is located in the apolipoprotein APOA1/C3/A4 gene cluster [8] on chromosome 11q23 [8,9]. APOA5 is predominantly expressed in hepatocytes and secreted into the blood [10,11]. The APOA5 apolipoprotein plays a key role in the synthesis and removal of triglycerides (TG) [5]. Increased levels of apolipoprotein A5 are correlated with decreased TG levels in the serum [5].
Atherogenic dyslipidemia is a major risk factor for CHD [12–14], as are blood lipid levels [6]. Blood lipids mainly consist of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and TG [12]. In addition to LDL-C and HDL-C levels, APOA5 is associated with TG levels [13]. APOA5 plays an important role in determining TG levels in serum [14]. TG interacts with lipoprotein lipase, an enzyme important for the central regulation of circulating TG levels [15]. In mice, over expression of Apoa5 leads to decreased concentrations of TG in plasma, whereas a shortage of apoA5 causes hypertriglyceridemia, a risk factor for atherosclerosis and CHD [16]. These findings are consistent with observations in humans [17]. Taken together, these studies indicate that APOA5 is associated with CHD [18–20].
APOA5 rs662799 (-1131T>C) is a promoter polymorphism that was shown to be associated with increased levels of TG in young adult Indians [21]. In Italians, APOA5 is associated with TG leaves and acute myocardial infarction (MI) [13]. The significant association of rs662799 with TG and CHD was validated in a Japanese population [22]. According to the HapMap database, there are ethnic differences in APOA5 rs662799 (A>G). The minor allele frequency in European populations (HapMap-CEU) is 1.7%, much lower than the 13.3% observed in individuals of African descent (HapMap-YRI), 26.7% in Chinese (HapMap-CHB) and 28.9% in Japanese (HapMap-JPT). In our previous study, we could not detect a significant association between APOA5 rs662799 and CHD [23], possibly due to a lack of power. Here, we increased the sample size to determine whether APOA5 rs662799 plays a role in the risk of CHD in Han Chinese.
Materials and Methods
Sample collection
Samples from 1,521 unrelated individual inpatients were randomly collected from the Ningbo Lihuili Hospital and the Ningbo Yinzhou People's Hospital, Zhejiang, China. The samples included 783 cases of CHD (537 males and 246 females) and 738 controls (421 males and 317 females). All individuals were free from congenital heart disease, cardiomyopathy and severe liver or kidney disease. Details of the classified criteria have been described in our previous studies [2,24–27]. The study protocol was approved by the Ethical Committees of Ningbo Lihuili Hospital and Ningbo Yinzhou People's Hospital, and informed written consent was obtained from all subjects. The clinical and demographic details of CHD samples are summarized in S1 Table.
SNP Genotyping
Genomic DNA was isolated from peripheral blood lymphocytes using a nucleic acid extraction automatic analyzer (Lab-Aid 820, Xiamen, China). PCR was performed on the ABI GeneAmp PCR System 9700 Dual 384-Well Sample Block Module (Applied Biosystems, Foster City, CA, USA). PCR conditions included an initial denaturation of 95°C for 2 min, followed by 45 cycles of 95°C for 30 sec, 56°C for 30 s, 72°C for 1 min and then a final extension at 72°C for 5 min. After purification by SAP Reaction, we proceeded with primer extension. The primer extension protocol included an initial denaturation at 94°C for 30 s, followed by 40 cycles of amplification (including 94°C for 5 s, 52°C for 5 s, 80°C for 5 s), 5 cycles of amplification (5 s at 52°C, 5 s at 80°C), a final extension at 72°C for 3 min after which samples were held at 4°C. Single nucleotide polymorphism genotyping was performed using the Sequenom Mass-ARRAY iPLEX platform per the manufacturer's instructions [28]. The primer sequences were 5’- ACGTTGGATGAGCATTTGGGCTTGCTCTCC-3’ (first primer), 5’-ACGTTGGATGTCTGAGCCCCAGGAACTGGA-3’ (second primer) and 5’- caGAACTGGAGCGAAAGT-3’ (extended primer).
Publication retrieval and data extraction
The literatures were searched in the online databases including PubMed and Wanfang between Jan 2000 and Jul 2015. The keywords were “coronary heart disease”, “coronary artery disease” or “myocardial infarction” combined with “APOA5” and “rs662799” or “-1131T>C”. All of the case-control studies between APOA5 rs662799 and CHD were retrieved for the consideration of the current meta-analysis. All of the case-control studies between APOA5 (rs662799) and CHD were considered to be eligible for the current meta-analysis. We only included studies that presented data on allele or genotype frequencies for both cases and controls and displayed a genotype distribution meeting Hardy-Weinberg equilibrium (HWE) [29]. Information in the meta-analysis included the first author’s name, publication year, country, ethnic group, number of alleles or genotypes and the total number of cases and controls. The details on the inclusion criteria included as follow:1) only the case-control studies on the association between rs662799 and CHD were included; 2) the eligible studies must contain the odds ratios (ORs) and 95% confidence intervals (CIs), or the genotype or allele information to calculate ORs and 95% Cis; 3) HWE should be met for the genotype distribution in the control group of the eligible studies if they have genotype information. We directly emailed the corresponding authors or called them (only for authors in China) for the missing information in their studies. There were 214 studies retrieved from the Wanfang and CNKI literature databases after searching for the keywords “coronary heart disease”, “coronary artery disease” or “myocardial infarction” combined with “APOA5” and “rs662799” or “-1131T>C”. After a series of selection procedures, we excluded 15 duplicate studies, 5 meta-analysis studies, 120 irrelevant studies, 28 studies on other diseases, and 7 studies without genotyping data (S1 File). In addition, we further downloaded the GWAS dataset from WTCCC research, then we imputed the information of rs662799 genotype by MaCH-Admix in WTCCC database, and added the data to the meta-analysis [30]. The remaining 40 case-control studies were qualified for our meta-analysis (Fig 1) [11,13,17,20,22,31–63].
Fig 1. Flow chart of the meta-analysis.

Statistical analyses
The HWE test was performed using the Arlequin program (version 3.5), and P > 0.05 was considered to be in HWE [64]. Genotype and allele distribution was compared between cases and controls by CLUMP22 software using 10,000 Monte Carlo simulations [65]. The odds ratio (OR) with a 95% confidence interval (CI) were determined using an online program, (http://faculty.vassar.edu/lowry/odds2x2.html) [23]. Meta-analysis was performed using the Review Manager software set to the fixed-effect or random-effect method (version 5.0, Cochrane Collaboration, Oxford, United Kingdom) [66]. Heterogeneity in the meta-analysis was assessed using the Q and I2 tests. An I2 > 50% indicated the existence of heterogeneity among the studies in the meta-analysis. Publication bias was shown by Begg’s funnel plot analysis, which was generated with Stata software (version 11.0, Stata Corporation, College Station, TX, USA). P values < 0.05 were significant.
Results
No departure from HWE was observed for the APOA5 rs662799 polymorphism in cases (P = 0.220) or controls (P = 0.544). Genotypic and allelic comparisons between cases and controls are shown in Table 1. Our data show that rs622799 is associated with the risk of CHD (genotype: χ2 = 8.964, df = 2, P = 0.011; allele: P = 0.002; OR = 1.275, 95% CI = 1.089–1.492). A further gender-stratified association shows that rs662799 is significantly associated with CHD in males (Table 1, genotype: χ2 = 7.486, df = 2, P = 0.024; allele: χ2 = 7.770, df = 1, P = 0.005) but not in females. In addition, frequency of the rs662799-G allele is significantly higher in male cases (31.5%) than in male controls (25.7%, P = 0.005; OR = 1.331, 95% CI = 1.088–1.628; Table 1). A further breakdown analysis by age shows that the frequency of rs662799-G is significantly higher in CHD cases with ages ranging from 55 to 65 years (31.5% versus 25.0%, χ2 = 5.700, df = 1, P = 0.017, OR = 1.383, 95% CI = 1.059–1.805; Table 2).
Table 1. Genotype and allele frequencies in cases and controls.
| Genotype [n, (%)] | χ2 | P (df = 2) | HWE | Allele (counts) | χ2 | P (df = 1) | OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APOA5 | Rs662799 | GG | AG | AA | G | A | ||||||
| All | Cases (N = 783) | 85(10.9%) | 323(41.3%) | 375(47.8%) | 0.220 | 493 | 1,073 | |||||
| Controls (N = 738) | 55(7.5%) | 281(38.1%) | 402(54.4%) | 8.964 | 0.011 | 0.544 | 391 | 1,085 | 9.180 | 0.002 | 1.275 (1.089–1.492) | |
| Male | Cases (N = 537) | 58(10.8%) | 222(41.3%) | 257(47.9%) | 0.335 | 338 | 736 | |||||
| Controls (N = 421) | 32(7.6%) | 152(36.1%) | 237(56.3%) | 7.486 | 0.024 | 0.272 | 216 | 626 | 7.770 | 0.005 | 1.331 (1.088–1.628) | |
| Female | Cases (N = 246) | 27(11.0%) | 101(41.1%) | 118(47.9%) | 0.445 | 155 | 337 | |||||
| Controls (N = 317) | 23(7.3%) | 129(40.7%) | 165(52.0%) | 2.622 | 0.270 | 0.746 | 175 | 459 | 2.040 | 0.153 | 1.206 (0.932–1.561) | |
Table 2. Genotype and allele frequencies in cases and controls with different age ranges.
| Genotype [n, (%)] | χ2 | P (df = 2) | HWE | Allele (counts) | χ2 | P (df = 1) | OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Rs662799 | GG | GA | AA | G | A | ||||||
| 55≤ | Cases (N = 179) | 20(11.2%) | 68(38.0%) | 91(50.8%) | 0.188 | 108 | 250 | |||||
| Controls (N = 239) | 18(7.5%) | 93(38.9%) | 128(53.6%) | 1.660 | 0.436 | 0.846 | 129 | 349 | 1.02 | 0.313 | 1.169 (0.863–1.582) | |
| 55–65 | Cases (N = 271) | 27(10.0%) | 117(43.2%) | 127(46.8%) | 0.994 | 171 | 371 | |||||
| Controls (N = 268) | 17(6.3%) | 100(37.3%) | 151(56.4%) | 5.660 | 0.059 | 0.935 | 134 | 402 | 5.700 | 0.017 | 1.383 (1.059–1.805) | |
| ≥65 | Cases (N = 333) | 38(11.4%) | 138(41.4%) | 157(47.2%) | 0.363 | 214 | 452 | |||||
| Controls (N = 231) | 20(8.7%) | 88(38.1%) | 123(53.2%) | 2.409 | 0.300 | 0.456 | 128 | 334 | 2.530 | 0.112 | 1.235 (0.952–1.603) | |
Meta-analysis
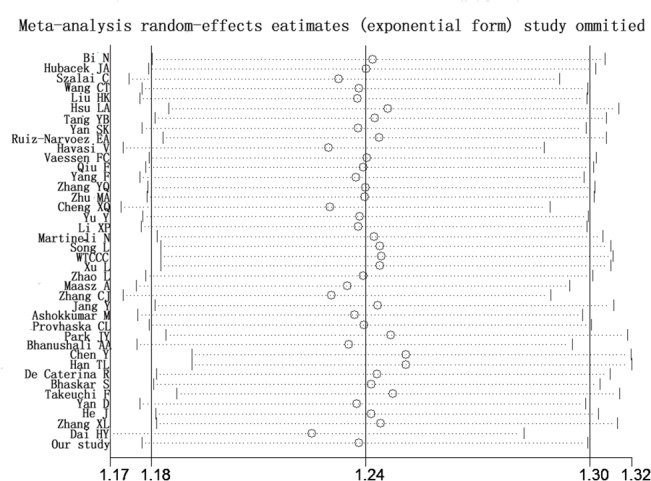
Searching the existing literature databases, we found 40 case-control studies, 30 more cases than were used in the most recently published meta-analysis in 2013 [23]. Therefore, we performed an updated meta-analysis to investigate the link between rs662799 and CHD. Information from these 40 eligible studies and our case-control study are shown in Table 3. Among the 40 eligible studies in the current meta-analysis, 7 studies only had allelic information. Therefore, allele-based model was applied in the meta-analysis. For the meta-analysis with moderate heterogeneity (I2 < 50%), we selected a fixed-effect model for the meta-analysis, otherwise, the random-effect model was used for the meta-analysis with great heterogeneity (I2 > = 50%). The current meta-analysis has great heterogeneity (I2 = 70%), therefore random-effect model was used. As shown in Fig 2, subgroup meta-analysis by major ethnic groups also indicates a significant association between APOA5 rs662799 and CHD in Asians (P = 0.01, I2 = 66%), Chinese (P < 0.000001, I2 = 67%) and Caucasians (P = 0.008, I2 = 60%). The meta-analyses show no publication bias by Begg’s funnel plot analysis (Fig 3). Furthermore, sensitivity analysis suggests that the conclusion is not biased by any individual study (Fig 4).
Table 3. Detailed information of the APOA5 rs662799 cases included in the meta-analysis.
| Year | Author | Ethnic Group | NO. case/controls | NO. A allele | NO. G allele |
|---|---|---|---|---|---|
| 2004 | Bi N [40] | Chinese | 312/317 | 375/423 | 249/211 |
| 2004 | Hubacek JA [41] | Caucasian | 435/2,559 | 778/4,683 | 92/435 |
| 2004 | Szalai C [42] | Hungarian | 308/310 | 549/585 | 67/35 |
| 2004 | Wang CT [33] | Chinese | 286/395 | 405/614 | 167/176 |
| 2005 | Liu HK [43] | Chinese | 483/502 | 588/704 | 378/300 |
| 2005 | Hsu LA [44] | Chinese | 211/317 | 291/446 | 131/188 |
| 2005 | Tang YB [45] | Chinese | 235/262 | 280/344 | 190/180 |
| 2005 | Yan SK [46] | Chinese | 113/155 | 142/224 | 84/86 |
| 2005 | Ruiz-Narvoez EA [47] | Costa Rican | 1,703/1,703 | 3,353/3,352 | 53/54 |
| 2006 | Havasi V [48] | Hungarian | 302/289 | 534/550 | 70/28 |
| 2006 | Vaessen FC [49] | British | 898/1,745 | 1,672/3,295 | 124/195 |
| 2007 | Qiu F [57] | Chinese | 260/316 | 358/477 | 162/155 |
| 2007 | Yang F [31] | Chinese | 168/160 | 196/221 | 140/99 |
| 2007 | Zhang YQ [58] | Chinese | 141/129 | 167/174 | 115/84 |
| 2007 | Zhu MA [39] | Chinese | 119/210 | 141/284 | 97/136 |
| 2007 | Cheng XQ [37] | Chinese | 112/113 | 138/162 | 136/86 |
| 2007 | Yu Y [50] | Chinese | 140/156 | 159/209 | 121/103 |
| 2007 | Li XP[38] | Chinese | 186/268 | 215/364 | 157/172 |
| 2007 | Martineli N [51] | Italian | 669/244 | 1,208/445 | 130/43 |
| 2007 | Song DL [29] | Chinese | 195/181 | 245/241 | 145/121 |
| 2007 | WTCCC [30] | Caucasian | 1926/2938 | 3604/5540 | 248/336 |
| 2008 | Xu L [59] | Chinese | 195/181 | 245/241 | 145/121 |
| 2008 | Zhao L [60] | Chinese | 155/145 | 178/193 | 132/97 |
| 2008 | Maasz A [52] | Hungarian | 378/131 | 676/249 | 80/13 |
| 2009 | Zhang CJ [35] | Chinese | 266/137 | 371/226 | 161/46 |
| 2009 | Jang Y [17] | Korean | 741/741 | 983/1,059 | 499/423 |
| 2010 | Ashokkumar M [53] | Indian | 416/416 | 565/633 | 267/199 |
| 2010 | Provhaska CL [54] | Brazilian | 180/170 | 327/316 | 33/24 |
| 2010 | Park JY [11] | Korean | 807/1,123 | 1,093/1,587 | 521/659 |
| 2010 | Bhanushali AA [55] | Indian | 90/150 | 58/120 | 32/30 |
| 2011 | Chen Y [61] | Chinese | 249/176 | 313/204 | 185/148 |
| 2011 | Han TL [32] | Chinese | 275/289 | 368/364 | 182/214 |
| 2011 | Raffaele De Caterina [13] | Italian | 1,864/1,864 | 3,319/3,423 | 365/313 |
| 2011 | Bhaskar S [56] | Indian | 250/120 | 355/181 | 145/59 |
| 2012 | Takeuchi F [22] | Japanese | 4,399/7,672 | 5,701/10,250 | 3,097/5,094 |
| 2012 | Yan D [20] | Chinese | 229/254 | 269/350 | 189/158 |
| 2012 | He J [30] | Chinese | 54/58 | 83/91 | 25/25 |
| 2012 | Zhang XL [36] | Chinese | 675/636 | 803/831 | 547/441 |
| 2013 | Dai HY [34] | Chinese | 158/130 | 244/138 | 72/122 |
| 2014 | Our study | Chinese | 783/738 | 1,073/1,085 | 493/391 |
Fig 2. Forest plots of APOA5 rs662799 polymorphism with CHD risk in the Chinese, the Caucasian and the Asian populations*.
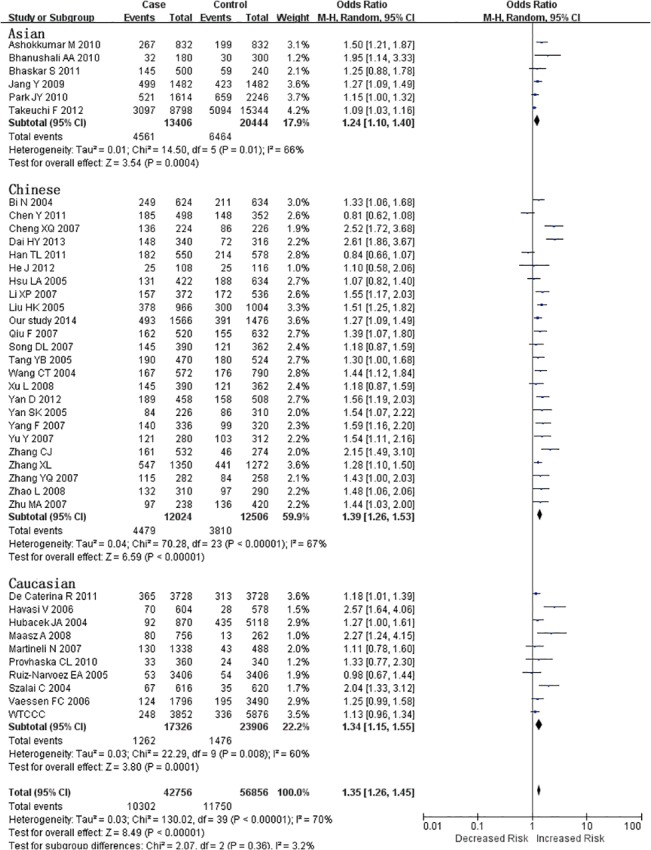
*Events, the number of G alleles; total, total number of A and G alleles; our study: the CHD cases and controls in our study.
Fig 3. Begg’s funnel plot for the association between APOA5 rs662799 and CHD*.
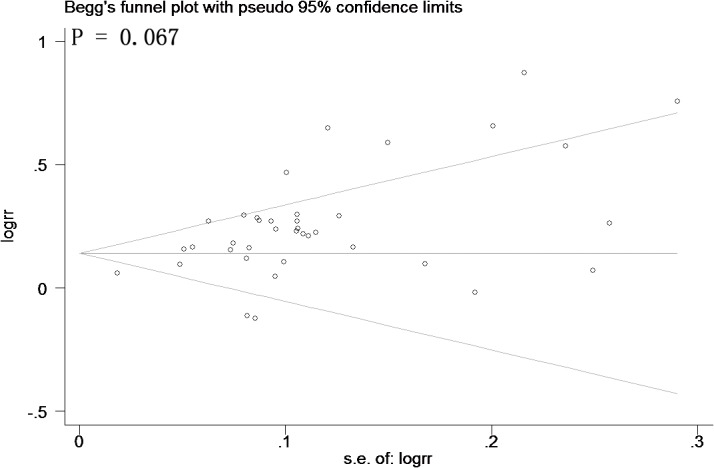
*Horizontal axis represents the standard error of log rr. Vertical axis represents the log rr. The s.e. denotes standard error.
Fig 4. Sensitivity analysis for the APOA5 rs662799 polymorphism with CHD.
Discussion
Our results show that the rs662799 polymorphism in the APOA5 gene is significantly associated with CHD in Han Chinese (P = 0.011). The minor G allele of APOA5 rs662799 may increase the risk of CHD by 27.5% (P = 0.002, OR = 1.275, 95% CI = 1.089–1.492). Consistent with previous reports, the rs662799-G allele is associated with higher leaves of TG in both CHD patients and controls [13,67]. A power calculation for APOA5 rs662799 indicates that our study has 85.9% power to detect significance in the association test.
Environmental factors, such as gender and age, are important factors of CHD. The prevalence of CHD in females was different from males [2,68,69]. Evidence has shown that patients older than 65 years have a higher cardiovascular morbidity and mortality [70,71]. In the current meta-analysis, we were unable to perform the subgroup meta-analysis by the age or gender due to a paucity of related information in the involved studies.
Gender and age are independence risk of CHD [4,72–74]. Epidemiologic evidence suggests that the risk of morbidity and mortality are higher in male CHD patients than in females [75]. Our data show a strong association between APOA5 rs662799 and CHD in the male group, providing a novel molecular explanation for the gender disparity observed in CHD. In addition, we showed a statistically significant difference between rs662799 and CHD in the subgroup aged from 55–65, although the underlying mechanism will require additional studies.
The frequency of the APOA5 rs662799 polymorphism varies greatly among different populations. The rs662799-G allele frequency is 26.7% in Chinese populations, similar to that in Japanese populations (29.1%). However, the Chinese frequency is much higher than that in European populations (1.7%). Nevertheless, accumulating evidence indicates a strong association between APOA5 rs662799 and CHD among different populations. In addition to APOA5 rs662799, there are associations between other APOA5/A4/C3/A1 polymorphisms and CHD, which include APOA5 rs3135506 and APOA/A4/C3/A1 cluster haplotypes [76]. Further functional analysis is needed to discriminate the relationship among these polymorphisms.
There were other seven APOA5 polymorphisms involved in the genetic studies (S2 Table). However, rs3135506 (n = 7) [43,45,49,53,56,76,77] and -12238T/C (n = 1)[78] were tested for the association of CHD. Thus, we only included rs662799 in the current meta-analysis. Among the published GWAS related to the current meta-analysis, we didn’t find any direct information that could be applied in the current meta-analysis [12,67,79]. We further and added the WTCCC data to the meta-analysis. Please see the following figure for the updates (Fig 2). The current meta-analysis includes 40 studies comprised of 21378 cases and 28428 controls from 10 ethnic populations. Our meta-analysis contains at least 26 case studies and 3 ethnic populations more than were included in the last five meta-analyses published [23, 80–83]. All of the meta-analyses indicate that the APOA5 rs662799 polymorphisms associated with CHD in the Chinese population, although many of the studies did not include a subgroup analysis stratified by ethnicity.
Despite the merits of our meta-analysis, there are limitations that must be considered. Our meta-analysis only includes studies from Asian and Caucasian populations. Therefore, it might not be an accurate representation of other ethnicities, such as African populations. Publication and language bias might exist in the case control studies [84]. The current meta-analysis was involved with 10 Caucasian and 30 Asian studies. Among the Asian studies, there were 24 Chinese studies (7 in English and 17 in Chinese). A further check for the minor allele frequency report in the HapMap International Project, we found the MAF in Europeans was 1.7% which was much less than 26.7% in Chinese and 29.1% in Japanese. However, subgroup meta-analyses by ethnicity found significant association of APOA5 rs662799 and CHD in both Europeans and Asians. There may also be a selection bias in our meta-analysis, which only included studies published in English or Chinese. Finally, standards for diagnosis may vary due to differences in the inclusion of CHD cases and non-CHD controls.
In summary, our case-control and meta-analysis demonstrates that the frequency of the APOA5 rs662799-G allele is significantly increased in CHD cases compared with controls. Furthermore, APOA5 rs662799 interacts with both gender and age in the association with CHD.
Supporting Information
(DOC)
(DOCX)
* p values were determined by the Wilcoxon-Mann-Whitney test.
(DOCX)
(DOC)
Acknowledgments
This work was supported by grants from: National Natural Science Foundation of China (31100919 and 81371469), Natural Science Foundation of Zhejiang Province (LR13H020003), the K. C. Wong Magna Fund in Ningbo University, Zhejiang Provincial Bureau of Traditional Chinese Medicine (2013ZZ003) and the Sciences Technology Department of Zhejiang Province (2013F20005).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China (31100919 and 81371469), Natural Science Foundation of Zhejiang Province (LR13H020003), and K. C. Wong Magna Fund in Ningbo University, Zhejiang Provincial Bureau of Traditional Chinese Medicine (2013ZZ003) and the Sciences Technology Department of Zhejiang Province (2013F20005).
References
- 1. Chen Q, Reis SE, Kammerer C, Craig W, McNamara DM, Holubkov R, et al. (2011) Association of anti-oxidized LDL and candidate genes with severity of coronary stenosis in the Women's Ischemia Syndrome Evaluation study. J Lipid Res 52(4): 801–807. 10.1194/jlr.M012963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang Y, Lian J, Huang RS, Wang F, Xu L, Le Y, et al. (2013) Positive association between rs10918859 of the NOS1AP gene and coronary heart disease in male Han Chinese. Genet Test Mol Biomarkers 17(1): 25–29. 10.1089/gtmb.2012.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Lu Z, Liu L. (2008) Coronary heart disease in China. Heart 94(9): 1126–1131. 10.1136/hrt.2007.132423 [DOI] [PubMed] [Google Scholar]
- 4. Zhou J, Huang Y, Huang RS, Wang F, Xu L, Le Y, et al. (2012) A case-control study provides evidence of association for a common SNP rs974819 in PDGFD to coronary heart disease and suggests a sex-dependent effect. Thromb Res 130(4): 602–606. 10.1016/j.thromres.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 5. De Andrade FM, Maluf SW, Schuch JB, Voigt F, Barros AC, Lucatelli JF, et al. (2011) The influence of the S19W SNP of the APOA5 gene on triglyceride levels in southern Brazil: interactions with the APOE gene, sex and menopause status. Nutr Metab Cardiovasc Dis 21(8): 584–590. 10.1016/j.numecd.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 6. Waterworth DM, Ricketts SL, Song K, Chen L, Zhao J, Ripatti S, et al. (2010) Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30(11): 2264–2276. 10.1161/ATVBAHA.109.201020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao Y. (2012) To evaluate the effect of TFPI/FoxO in the incidence of CHD among North pupulation. China people's Liberation Army Medical Institute. [Google Scholar]
- 8. Hubacek JA, Adamkova V, Vrablik M, Kadlecova M, Zicha J, Kunes J, et al. (2009) Apolipoprotein A5 in health and disease. Physiol Res 58(Suppl 2): S101–109. [DOI] [PubMed] [Google Scholar]
- 9. Hadarits F, Kisfali P, Mohas M, Maasz A, Duga B, Janicsek I, et al. (2012) Common functional variants of APOA5 and GCKR accumulate gradually in association with triglyceride increase in metabolic syndrome patients. Mol Biol Rep 39(2): 1949–1955. 10.1007/s11033-011-0942-8 [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Walijee SM, Jin J, Zhao S, Peng D. (2012) Serum apolipoprotein A-V in patients with coronary artery disease and its association with triglyceride. J Clin Lipidol 6(5): 462–468. 10.1016/j.jacl.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 11. Park JY, Paik JK, Kim OY, Chae JS, Jang Y, Lee JH. (2010) Interactions between the APOA5 -1131T>C and the FEN1 10154G>T polymorphisms on omega6 polyunsaturated fatty acids in serum phospholipids and coronary artery disease. J Lipid Res 51(11): 3281–3288. 10.1194/jlr.M010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saleheen D, Soranzo N, Rasheed A, Scharnagl H, Gwilliam R, Alexander M, et al. (2010) Genetic determinants of major blood lipids in Pakistanis compared with Europeans. Circ Cardiovasc Genet 3(4): 348–357. 10.1161/CIRCGENETICS.109.906180 [DOI] [PubMed] [Google Scholar]
- 13. De Caterina R, Talmud PJ, Merlini PA, Foco L, Pastorino R, Altshuler D, et al. (2011) Strong association of the APOA5-1131T>C gene variant and early-onset acute myocardial infarction. Atherosclerosis 214(2): 397–403. 10.1016/j.atherosclerosis.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 14. Vu-Dac N, Gervois P, Jakel H, Nowak M, Bauge E, Dehondt H, et al. (2003) Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator-activated receptor alpha activators. J Biol Chem 278(20): 17982–17985. [DOI] [PubMed] [Google Scholar]
- 15. Kisfali P, Mohas M, Maasz A, Polgar N, Hadarits F, Marko L, et al. (2010) Haplotype analysis of the apolipoprotein A5 gene in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 20(7): 505–511. 10.1016/j.numecd.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 16. Laurila PP, Naukkarinen J, Kristiansson K, Ripatti S, Kauttu T, Silander K, et al. (2010) Genetic association and interaction analysis of USF1 and APOA5 on lipid levels and atherosclerosis. Arterioscler Thromb Vasc Biol 30(2): 346–352. 10.1161/ATVBAHA.109.188912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jang Y, Paik JK, Hyun YJ, Chae JS, Kim JY, Choi JR, et al. (2009) The apolipoprotein A5 -1131T>C promoter polymorphism in Koreans: association with plasma APOA5 and serum triglyceride concentrations, LDL particle size and coronary artery disease. Clin Chim Acta 402(1–2): 83–87. 10.1016/j.cca.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seda O, Sedova L. (2003) New apolipoprotein A-V: comparative genomics meets metabolism. Physiol Res 52(2): 141–146. [PubMed] [Google Scholar]
- 19. Li X, Gong H, Huang X, Huang W, Zhao S. (2013) The influence of statin-fibrate combination therapy on lipids profile and apolipoprotein A5 in patients with acute coronary syndrome. Lipids Health Dis 12: 133 10.1186/1476-511X-12-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding Y, Zhu M, Wang Z, Zhu J, Feng J, Li D. (2012) Associations of polymorphisms in the apolipoprotein APOA1-C3-A5 gene cluster with acute coronary syndrome. J Biomed Biotechnol 2012: 509420 10.1155/2012/509420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramakrishnan L, Sachdev HS, Sharma M, Abraham R, Prakash S, Gupta D, et al. (2011) Relationship of APOA5, PPARgamma and HL gene variants with serial changes in childhood body mass index and coronary artery disease risk factors in young adulthood. Lipids Health Dis 10: 68 10.1186/1476-511X-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi F, Isono M, Katsuya T, Yokota M, Yamamoto K, Nabika T, et al. (2012) Association of genetic variants influencing lipid levels with coronary artery disease in Japanese individuals. PLoS One 7(9): e46385 10.1371/journal.pone.0046385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou J, Xu L, Huang RS, Huang Y, Le Y, Jiang D, et al. (2013) Apolipoprotein A5 gene variants and the risk of coronary heart disease: a case-control study and meta-analysis. Mol Med Rep 8(4): 1175–1182. 10.3892/mmr.2013.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y, Zhou J, Ye H, Xu L, Le Y, Yang X, et al. (2013) Relationship between chemokine (C-X-C motif) ligand 12 gene variant (rs1746048) and coronary heart disease: case-control study and meta-analysis. Gene 521(1): 38–44. 10.1016/j.gene.2013.02.047 [DOI] [PubMed] [Google Scholar]
- 25. Lian J, Huang Y, Huang RS, Xu L, Le Y, Yang X, et al. (2013) Meta-analyses of four eosinophil related gene variants in coronary heart disease[J]. J Thromb Thrombolysis 36(4): 394–401. 10.1007/s11239-012-0862-z [DOI] [PubMed] [Google Scholar]
- 26. Xu L, Zhou J, Huang S, Huang Y, Le Y, Jiang D, et al. (2013) An association study between genetic polymorphisms related to lipoprotein-associated phospholipase A(2) and coronary heart disease[J]. Exp Ther Med 5(3): 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Liu P, Zhou J, Huang RS, Yuan F, Fei L, et al. (2013) Positive correlation between variants of lipid metabolismrelated genes and coronary heart disease. Mol Med Rep 8(1): 260–266. 10.3892/mmr.2013.1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gabriel S, Ziaugra L, Tabbaa D. (2009) SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet Chapter 2: Unit 2 12. [DOI] [PubMed] [Google Scholar]
- 29. Glatt SJ, Faraone SV, Tsuang MT. (2003) Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry 160(3): 469–476. [DOI] [PubMed] [Google Scholar]
- 30.(2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447(7145): 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song D, Guo J, Kang W, Guo X, Zhang Q. (2007) Apolipoprotein gene mutation test and clinical application in Coronary heart disease. Chin J Lab Med 30(1): 65–66. [Google Scholar]
- 32. He J, Gou Z, Dai J, Sun H. (2012) Association Study on Apolipoprotein A5 Gene Polymorphism and Serum Lipid Metabolism and Coronary Heart Disease. J Mod Lab Med 27(2): 15–18. [Google Scholar]
- 33. Yang F, Yang Z, Wang L. (2007) Association of APOA5 gene -1131T/C single nucleotide polymorphism with coronary heart disease among Chinese Han population. Shandong Medicine 47(19): 1–3. [Google Scholar]
- 34. Han T. (2011) A Study on the Haplotype of Apolipoprotein A5/C3 Gene Cluster and Correlation to the Patients with Coronary Artery Disease in Shandong Coastal Area. shandong University Press, JiNan. [Google Scholar]
- 35. Wang C. (2002) Polymorphism in the apoliprotein A5 gene and their association with coronary heart disease in Chinese Fujian Medical University Press, FuZhou. [Google Scholar]
- 36. Dai H, Ye G, Shi Z, Zhao Y. (2013) Relationship of APOA5 Level and APOA5 -1131T/C Locus Genen Polymorphism to Early-oneset CHD and Restenoses after Percutaneous Coronary Intervention in Han population. Chinese General Pratics 16(11B): 3821–3824. [Google Scholar]
- 37. Zhang C. (2009) The associations between Uygu-Han APOA5, APOB, CETPgene and liPids of coronary heart disease with diabetes Xinjiang Medical University Press, Wulumuqi. [Google Scholar]
- 38. Zhang X, Liu T, Cai W, Yan C, Liang Z, Sun Y, et al. (2012) Association of Apolipoprotein T-1131C with Acute Coronary Syndrome in Han Population of North China. Progress in Modern Biomedicine 12(8): 1401–1404. [Google Scholar]
- 39. Cheng X, Yan S, Song Y, Xiao X, Bi N, Chen B. (2007) Relationship between Apolipoprotein AV gene -1131T/C polymorphism and Type 2 Diabates Mellitus with Coronary Heart Disease in Han Nationality. Molecular Cardiology of China 7(4): 189–194. [Google Scholar]
- 40. Li X, Zhao S, Nie S. (2007) Relationship Between Apolipoprotein A5 -1131T>C Polymorphism and Coronary Heart Disease. Chinese Circulation Journal 22(1): 4–8. [Google Scholar]
- 41. Zhu M, Zhou Y, Ding Y, Mao D. (2007) Apolipoprotein A5 gene—1131—t > C polymorphism in Coronary Heart Disease Patient. Chinese journal of gerontology 27(1): 73–75. [Google Scholar]
- 42. Bi N, Yan S, Li G, Yin Z, Chen B. (2004) A single nucleotide polymorphism -1131T>C in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and alters triglyceride metabolism in Chinese. Mol Genet Metab 83(3): 280–286. [DOI] [PubMed] [Google Scholar]
- 43. Hubacek JA, Skodova Z, Adamkova V, Lanska V, Poledne R (2004) The influence of APOAV polymorphisms (T-1131>C and S19>W) on plasma triglyceride levels and risk of myocardial infarction. Clin Genet 65(2): 126–130. [DOI] [PubMed] [Google Scholar]
- 44. Szalai C, Keszei M, Duba J, Prohaszka Z, Kozma GT, Csaszar A, et al. (2004) Polymorphism in the promoter region of the apolipoprotein A5 gene is associated with an increased susceptibility for coronary artery disease. Atherosclerosis 173(1): 109–114. [DOI] [PubMed] [Google Scholar]
- 45. Liu H, Zhang S, Lin J, Li H, Huang A, Xiao C, et al. (2005) Association between DNA variant sites in the apolipoprotein A5 gene and coronary heart disease in Chinese. Metabolism 54(5): 568–572. [DOI] [PubMed] [Google Scholar]
- 46. Hsu LA, Ko YL, Chang C, Hu CF, Wu S, Teng MS, et al. (2006) Genetic variations of apolipoprotein A5 gene is associated with the risk of coronary artery disease among Chinese in Taiwan. Atherosclerosis 185(1): 143–149. [DOI] [PubMed] [Google Scholar]
- 47. Tang Y, Sun P, Guo DP, Li X, Chen Q, Fan L, et al. (2005) Association between apolipoprotein A5—1131T > C polymorphism and susceptibility of coronary artery disease in Chinese. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 22(3): 281–283. [PubMed] [Google Scholar]
- 48. Yan S, Cheng X, Song Y, Xiao X, Bi N, Chen B et al. (2005) Apolipoprotein A5 gene polymorphism -1131T—>C: association with plasma lipids and type 2 diabetes mellitus with coronary heart disease in Chinese. Clin Chem Lab Med 43(6): 607–612. [DOI] [PubMed] [Google Scholar]
- 49. Ruiz-Narvaez EA, Yang Y, Nakanishi Y, Kirchdorfer J, Campos H (2005) APOC3/A5 haplotypes, lipid levels, and risk of myocardial infarction in the Central Valley of Costa Rica. J Lipid Res 46(12): 2605–2613. [DOI] [PubMed] [Google Scholar]
- 50. Havasi V, Szolnoki Z, Talian G, Bene J, Komlosi K, Maasz A, et al. (2006) Apolipoprotein A5 gene promoter region T-1131C polymorphism associates with elevated circulating triglyceride levels and confers susceptibility for development of ischemic stroke. J Mol Neurosci 29(2): 177–183. [DOI] [PubMed] [Google Scholar]
- 51. Vaessen SF, Schaap FG, Kuivenhoven JA, Groen AK, Hutten BA, Boekholdt SM, et al. (2006) Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J Lipid Res 47(9): 2064–2070. [DOI] [PubMed] [Google Scholar]
- 52. Yu Y, Xue L, Zhao C. (2007) Study on polymorphism in the apolipoprotein A5 gene in patients with premature coronary heart disease. Beijing Da Xue Xue Bao 39(6): 576–580. [PubMed] [Google Scholar]
- 53. Martinelli N, Trabetti E, Bassi A, Girelli D, Friso S, Pizzolo F, et al. (2007) The -1131 T>C and S19W APOA5 gene polymorphisms are associated with high levels of triglycerides and apolipoprotein C-III, but not with coronary artery disease: an angiographic study. Atherosclerosis 191(2): 409–417. [DOI] [PubMed] [Google Scholar]
- 54. Maasz A, Kisfali P, Jaromi L, Horvatovich K, Szolnoki Z, Csongei V, et al. (2008) Apolipoprotein A5 gene IVS3+G476A allelic variant confers susceptibility for development of ischemic stroke. Circ J 72(7): 1065–1070. [DOI] [PubMed] [Google Scholar]
- 55. AshokKumar M, Subhashini NG, SaiBabu R, Ramesh A, Cherian KM, Emmanuel C. (2010) Genetic variants on apolipoprotein gene cluster influence triglycerides with a risk of coronary artery disease among Indians. Mol Biol Rep 37(1): 521–527. 10.1007/s11033-009-9728-7 [DOI] [PubMed] [Google Scholar]
- 56. Prochaska CL, Picheth G, Anghebem-Oliveira MI, Costantini CO, de Souza EM, Pedrosa FO, et al. (2010) The polymorphisms -1131T>C and the S19W of the APOA5 gene are not associated with coronary artery disease in a Brazilian population. Clin Chem Lab Med 48(3): 419–422. 10.1515/CCLM.2010.070 [DOI] [PubMed] [Google Scholar]
- 57. Bhanushali AA, Das BR (2010) Influence of genetic variants in the apolipoprotein A5 and C3 gene on lipids, lipoproteins, and its association with coronary artery disease in Indians. J Community Genet 1(3): 139–148. 10.1007/s12687-010-0025-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bhaskar S, Ganesan M, Chandak GR, Mani R, Idris MM, Khaja N, et al. (2011) Association of PON1 and APOA5 gene polymorphisms in a cohort of Indian patients having coronary artery disease with and without type 2 diabetes. Genet Test Mol Biomarkers 15(7–8): 507–512. 10.1089/gtmb.2010.0207 [DOI] [PubMed] [Google Scholar]
- 59. Qiu F. (2007) The association of polymorphisms in apolipopro-teina 5 gene with blood lipids and heart cerebrovascular disease Southeast University Press, Nanjing. [Google Scholar]
- 60. Zhang Y. (2007) Associations of apoA5 polymorphisms, blood lipids and coronary heart disease in the Chinese Zhejiang University Press, Hangzhou. [Google Scholar]
- 61. Xu L, He T. (2008) Correlation of apolipoprotein A5 SNP3 gene single nucleotide polymorphism with coronary artery disease in Chinese. Shandong Med J 48(46): 1–3. [Google Scholar]
- 62. Zhao L. (2008) Association between apolipoprotein A5 -1131T>C polymorphism and coronary heart disease in xinjiang Uygur Xinjiang Medical University Press, Wulumuqi. [Google Scholar]
- 63. Chen Y, Xu L, Zhang J, Li R. (2011) Study on the relationship between apolipoprotein A5 gene polymorphism and coronary artery disease. Chin J Conval Med 20(6): 487–489. [Google Scholar]
- 64. Excoffier L, Lischer HE (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10(3): 564–567. 10.1111/j.1755-0998.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 65. Sham PC, Curtis D (1995) Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann Hum Genet 59(Pt 1): 97–105. [DOI] [PubMed] [Google Scholar]
- 66. Ye H, Li X, Wang L, Liao Q, Xu L, Huang Y, et al. (2013) Genetic associations with coronary heart disease: meta-analyses of 12 candidate genetic variants. Gene 531(1): 71–77. 10.1016/j.gene.2013.07.029 [DOI] [PubMed] [Google Scholar]
- 67. Rafiq S, Venkata KK, Gupta V, Vinay DG, Spurgeon CJ, Parameshwaran S, et al. (2012) Evaluation of seven common lipid associated loci in a large Indian sib pair study. Lipids Health Dis 11: 155 10.1186/1476-511X-11-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mendelsohn ME, Karas RH (2005) Molecular and cellular basis of cardiovascular gender differences. Science 308 (5728): 1583–1587. [DOI] [PubMed] [Google Scholar]
- 69. Ober C, Loisel DA, Gilad Y (2008) Sex-specific genetic architecture of human disease. Nat Rev Genet 9 (12): 911–922. 10.1038/nrg2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rai M, Baker WL, Parker MW, Heller GV (2012) Meta-analysis of optimal risk stratification in patients >65 years of age. Am J Cardiol 110 (8): 1092–1099. 10.1016/j.amjcard.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 71. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123 (4): e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shi K, Wu F, Liu W, Zhao C, Chen C, Xie Y, et al. (2014) Non-alcoholic fatty liver disease and risk of in-stent restenosis after bare metal stenting in native coronary arteries. Mol Biol Rep 41(7): 4713–4720. 10.1007/s11033-014-3342-z [DOI] [PubMed] [Google Scholar]
- 73. Post WS, Budoff M, Kingsley L, Palella FJ Jr., Witt MD, Li X, et al. (2014) Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 160(7): 458–467. 10.7326/M13-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fernandez R, Rolley JX, Rajaratnam R, Sundar S, Patel NC, Davidson PM. (2014) Risk Factors for Coronary Heart Disease Among Asian Indians Living in Australia. J Transcult Nurs. [DOI] [PubMed] [Google Scholar]
- 75. Ginter E, Simko V (2013) Women live longer than men. Bratisl Lek Listy 114(2): 45–49. [DOI] [PubMed] [Google Scholar]
- 76. Dallongeville J, Cottel D, Montaye M, Codron V, Amouyel P, Helbecque N. (2006) Impact of APOA5/A4/C3 genetic polymorphisms on lipid variables and cardiovascular disease risk in French men. Int J Cardiol 106(2): 152–156. [DOI] [PubMed] [Google Scholar]
- 77. Maasz A, Kisfali P, Szolnoki Z, Hadarits F, Melegh B (2008) Apolipoprotein A5 gene C56G variant confers risk for the development of large-vessel associated ischemic stroke. J Neurol 255 (5): 649–654. 10.1007/s00415-008-0768-z [DOI] [PubMed] [Google Scholar]
- 78. Yuan S, Ma Y, Xie X, Yang Y, Fu Z, Ma X, et al. (2011) [Association of apolipoprotein A5 gene polymorphism with coronary heart disease in Uygur population of Xinjiang]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 28 (1): 73–77. 10.3760/cma.j.issn.1003-9406.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 79. Angelakopoulou A, Shah T, Sofat R, Shah S, Berry DJ, Cooper J, et al. (2012) Comparative analysis of genome-wide association studies signals for lipids, diabetes, and coronary heart disease: Cardiovascular Biomarker Genetics Collaboration. Eur Heart J 33 (3): 393–407. 10.1093/eurheartj/ehr225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu H, Shi J. (2011) APOA5 polymorphism and coronary heart disease in Han Chinese: a meta-analysis. Chin J Public Health 27(9): 1195–1196. [Google Scholar]
- 81. Zhai G, Li M, Zhu C. (2011) APOA5 -1131T/C polymorphism is associated with coronary artery disease in a Chinese population: a meta-analysis. Clin Chem Lab Med 49(3): 535–539. 10.1515/CCLM.2011.070 [DOI] [PubMed] [Google Scholar]
- 82. Li Y, Wu X, Xu J, Qian Y, Zhou C, Wang B, et al. (2013) Apo A5 -1131T/C, FgB -455G/A, -148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol Biol Rep 40(2): 1997–2014. 10.1007/s11033-012-2257-9 [DOI] [PubMed] [Google Scholar]
- 83. Zhang Z, Peng B, Gong R, Gao L, Du J, Fang D, et al. (2011) Apolipoprotein A5 polymorphisms and risk of coronary artery disease: a meta-analysis. Biosci Trends 5(4): 165–172. 10.5582/bst.2011.v5.4.165 [DOI] [PubMed] [Google Scholar]
- 84. Pan Z, Trikalinos TA, Kavvoura FK, Lau J, Ioannidis JP (2005) Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med 2 (12): e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
* p values were determined by the Wilcoxon-Mann-Whitney test.
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


