Abstract
Objective
Patients’ unawareness of their cancer diagnosis (PUAW) and their tendency for non-disclosure (TTND) to relatives leads to a lack of cancer visibility among familial networks. Lack of familial cancer visibility could affect the accuracy of family cancer history (FCH) reports. In this study, we investigated familial cancer visibility and its potential determinants.
Patients and Methods
A sample of patients with a confirmed cancer diagnosis was interviewed. Participants were asked about their number of relatives, number of their relatives who are aware about the cancer diagnosis, and the number of relatives from whom they intended to conceal their diagnosis. PUAW was also assessed. Point estimates and 95% confidence intervals were calculated using the bootstrap technique. Multivariate analyses were conducted using mixed Poisson and logistic regression analyses.
Results
A total of 415 participants with a mean age of 53±15 years and a male to female ratio of 0.53 were enrolled in this study. The rates of PUAW, TTND, and familial cancer visibility in the total sample were 0.20 (95% confidence interval (CI): 0.16, 0.24), 0.16 (95% CI: 0.12, 0.19), and 0.86 (95% CI: 0.83, 0.89), respectively. PUAW (adjusted rate ratio (RR) = 1.32, 95% CI: 1.27, 1.38), TTND (RR = 0.92, 95% CI: 0.91, 0.93), and the patients’ gender (RR = 0.92, 95% CI: 0.82, 0.95) were the most important determinants of familial cancer visibility.
Conclusion
Familial cancer visibility may be a point of concern among the Iranian population. Self-reported cancer histories and FCHs may have low sensitivities (not exceeding 80% and 86%, respectively) in this population. However, these estimates may vary across different societies, because of societal and cultural contexts.
Introduction
Family cancer history (FCH) reports are a frequently used data source in epidemiological studies [1–3]. They are also crucial in genetic risk assessments and predictive models when making referrals for genetic counseling and inherited cancer risk categorization [4–7]. Although FCHs are widely used and dozens of studies have examined their precision, the accuracy of FCH is still a subject of controversy [8–12]. Recent evidence has shown that, because of misreporting on FCHs, risk prediction models (such as BRCAPRO) could underestimate the risk of a mutation in the BRCA1/BRCA2 genes for hereditary breast and ovarian cancer syndrome [13, 14]. Clinicians, genetic consultants, and preventive specialists have the responsibility to accurately detect inherited cancer risks and encourage familial information transmission [15]. Therefore, in order to improve FCH accuracy, we need to identify its determinants.
Although the evidence has shown a high specificity for FCHs [16], reported values for the sensitivity of FCHs vary widely (from 36 to 92 percent) [8, 16, 17]. Therefore, FCH reports mainly suffer from false negatives (FN) rather than false positives.
A FN FCH may be due to a lack of accuracy in patient self-reports, a lack of awareness about his/her relative’s cancer diagnosis, and a lack of closeness to the affected relative, as well as the patients’ recall bias, age, and gender [9, 16, 18].
The accuracy of self-reporting has been addressed in several studies and self-reports are often compared to medical records or cancer registry data (the gold standards) [19–21]. In addition, a recent study by Inoue et al. [22] reported an overall FN rate of 47% for self-reported cancer history. An FN self-report may be the result of the patients’ unawareness about her/his cancer diagnosis (PUAW) or recall bias [19–21, 23, 24]. PUAW, in turn, is the result of a physician’s reticence to give patients their diagnosis. Therefore, non-disclosure to patients might be a distal determinant of FCH accuracy.
In order to examine the factors associated with lack of FCH accuracy, we mapped a web of known determinants. The hypothesized factor, tendency for non-disclosure (TTND), was incorporated into the web (Fig 1). The tendency for non-disclosure (TTND) of a given patient represents the tendency of a patient to not disclose her/his cancer to relatives, which is the result of cultural issues such as cancer stigmatization or desire to protect loved ones [25–27]. Therefore, we believe that the tendency for non-disclosure (TTND) may be a determinant of lack of FCH accuracy.
Fig 1. Schematic web of the determinants for the accuracy of family cancer history reports.
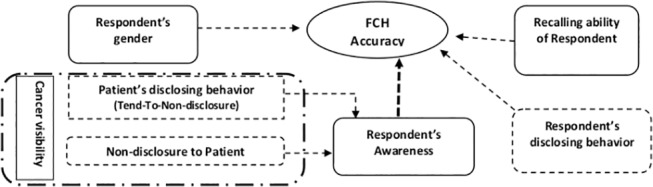
Solid line box: Previously reported determinants. Dashed arrow: Potential association. Long dash-dotted line box: What we investigated in this study. Dashed line box: Hypothetical determinants
Social scientists believe that the TTND results in a visibility bias for population size estimation efforts [28, 29]. This bias causes us to underestimate the size of hard-to-count populations [30]. Inspired by sociologists’ jargon, a FN FCH may be the result of lack of familial cancer visibility (FCV). Sociologists use a bias correction factor to compensate for the visibility bias which is dependent on the societal and cultural context [28, 29].
Despite the abundance of evidence regarding the lack of FCH sensitivity, widespread usage is common and it is unclear how we can improve the accuracy of FCH. Therefore, we conducted this study to investigate familial cancer visibility (FCV) as a potential determinant of FCH sensitivity in an Iranian population and to assess the hypothetical determinants of familial cancer visibility (FCV) including patients’ unawareness about her/his cancer diagnosis (PUAW) and TTND.
Methods
Study population
This cross- sectional study was part of an ongoing study in Iran (KCRNSU: Assessing the Completeness of Case Ascertainment of the Kerman Cancer Registry Using the Network Scale-Up Method). The main study was designed to use the network scale-up method to assess the completeness of case ascertainment of a cancer registry in a middle-sized province in Kerman, Iran.
Patients who had a confirmed malignancy (e.g., breast, colorectal, lung, leukemia, lymphoma, kidney, gastric, esophagus, ovarian, uterine, eye, sarcoma, laryngeal, liver, skin, bone marrow, thyroid, brain, bladder, testicular, bile duct, prostate or pancreatic) with the ability to speak Persian were eligible to participate in the study. A representative sample of oncology centers in Kerman was selected, including the six referral centers with the highest number of patient visits. In each center, all eligible patients were referred to our interviewers by the receptionist. Sampling was conducted during working hours in August 2014.
Data collection & measurements
Data were collected after obtaining informed verbal consent via a face-to-face, structured interview conducted in a private room near the sampling site. Informed consent was obtained verbally because Iranians are frequently reluctant to provide written consent. If the patient consented, the verbal consent checkbox was checked by the interviewer after informing eligible patients about the study, its aims, and the study questions. All ethical considerations, including obtaining verbal consent, were approved by the Ethical Committee of Kerman University of Medical Sciences as part of approval for the KCRNSU study (KMU 9350).
All interviewers were trained in a workshop that included a role-playing interview component. Interviewees were either patients or, in cases where the patient was unaware of her/his cancer diagnosis, the most informed caregiver. If the caregiver was the interviewee, then he/she was questioned on behalf of the patient. Two questions, one for the patient’s doctor and one for the caregiver, were used to assess patients’ unawareness about her/his cancer diagnosis (PUAW). If one or both of them answered “No, the patient is not aware of his/her cancer,” that patient was coded as unaware. Interviews were gender-matched for genital cancers and for female respondents who were younger than 40. Interviews lasted around ten to fifteen minutes.
To assess FCV and TTND, an interview form was designed and validated for this study. The first section introduced the study and its aims while the second assessed PUAW, type of cancer, date of current cancer diagnosis, patients’ age, gender, marital status, and medical record code. The third section examined kinship relations using a table with relationships listed in the rows (i.e., descent or affinity (related by marriage) groups: parents, siblings and their spouses, children, grandparents, aunts and their spouses, uncles and their spouses, nephews, nieces, first cousins, spouse and his/her first-degree relatives). Table columns included the “number of living relatives,” “number of surely informed relatives," and “number of relatives who are intended to be left uninformed.” This section was modified from the standard method for visibility bias estimation [29]. Section four assessed the patients’ type of residence, monthly income, and tumor-node-metastasis (TNM) staging information. Data regarding the date of diagnosis and TNM staging of the current tumor were extracted from medical records by the patients’ oncologist.
The interview form was used in three sequential pilot studies, and several minor corrections were made regarding its validity. Its test-retest reliability was assessed around the one-month interval by 26 participants (R: mean = 0.73, 95% confidence interval (CI): 0.69, 0.76). Despite an incentive ($6.50) for the retest interview, the participation rate was 0.60.
Data management & statistical methods
We calculated “time from diagnosis” using the date of the diagnosis and the interview date. FCV was estimated using the following formula:
| (1) |
TTND was defined according to the following formula:
| (2) |
The proportion of PUAW, FCV, and TTND as well as the 95% CIs were computed using the bootstrap method with 1000 resamplings. These proportions were also estimated across different subgroups based on their potential determinants.
Two or K-proportion comparison tests were used for the univariate analysis. To model potential determinants of PUAW, a mixed effects logistic regression analysis was used. Mixed effects Poisson regression was used for modeling of the FCV and TTND determinants. In both Poisson and logistic regression models, cancer type was included as a random intercept factor. To assess the significance effect of cancer type, we estimated median odds ratio (MOR) or median incidence rate ratio (MIRR) for the intercept-only models as well as for the final models.
All variables with a P-value less than 0.3 in the univariate analyses entered the multivariate model. However, those with a P-value greater than 0.05 were eliminated using a backward approach and only those with a P-value less than 0.05 were kept in the model. The stability of the models was checked using the bootstrap technique. The data analysis was conducted using Stata software (Version 11.2; StataCorp, Texas, USA).
Results
Participant characteristics
A total of 415 cancer patients were enrolled in the study. Participants had a mean age of 53 years (standard deviation (SD) = 15 years) and 65.5% (n = 272) were female while 34.5% (n = 143) were male. Eighty-one percent (n = 331) were from an urban area and 19% (n = 84) were from rural areas. The majority of participants (93.3%) were married (Table 1). Males were approximately 5 years older than females (P<0.001). Time from diagnosis varied from 0.1 to 280 months (mean = 28.2, SD = 40, median = 12). Time from diagnosis was significantly longer for females than males (mean = 32, SD = 44 and mean = 21, SD = 28, respectively, P = 0.009).
Table 1. Sampling distribution and patients’ unawareness of their cancer diagnosis.
| Factor | Groups | n | Unawareness (%) | 95% CI (%) | Univariate P-value |
|---|---|---|---|---|---|
| Stage at Diagnosis | I | 42 | 12 | 2, 22 | 0.022 |
| II | 90 | 17 | 10, 24 | ||
| III | 85 | 19 | 11, 27 | ||
| IV | 99 | 27 | 19, 36 | ||
| Unknown † | 60 | 23 | 16, 30 | ||
| Gender | Female | 272 | 16 | 12, 21 | 0.64 |
| Male | 143 | 27 | 20, 35 | ||
| Age Group | 0–39 | 87 | 6 | 1, 10 | <0.001 |
| 40–60 | 187 | 15 | 9, 20 | ||
| 60+ | 141 | 36 | 28, 43 | ||
| Marital Status | Single | 28 | 28 | 11, 43 | 0.299 |
| Married | 387 | 20 | 16, 24 | ||
| Cancer Type ‡ | Breast | 160 | 5.6 | 2, 9 | <0.001 |
| Colorectal | 33 | 44 | 27, 61 | ||
| Lung | 33 | 30 | 15, 46 | ||
| Leukemia | 25 | 28 | 10, 46 | ||
| Lymphoma | 28 | 18 | 4, 33 | ||
| Time from Diagnosis | Up to six M | 138 | 27 | 20, 34 | <0.001 |
| Seven to 18 M | 98 | 20 | 12, 28 | ||
| 19 to 30 M | 43 | 21 | 9, 33 | ||
| 31 to 42 M | 34 | 9 | 0, 18 | ||
| 42+ M | 102 | 14 | 7, 20 | ||
| Overall | 415 | 20 | 16, 24 | - | |
Abbreviations: CI, confidence interval; M, month.
†Hematologic cancers and others, the TNM data of which was not available.
‡First five common cancers.
Patients’ unawareness about their cancer (PUAW)
Twenty percent (95% CI: 16%, 24%) of all patients were unaware of their cancer diagnosis. The rate of unawareness varied widely (from 5.6% (95% CI: 2%, 9%) for breast cancer to 62.5% (95% CI: 51%, 74%) for gastric cancer). Males were more likely to be unaware than females (PUAW: 27.3%, 95% CI: 20, 35; and 16.2%, 95% CI: 12, 21, respectively; P = 0.64). In the univariate analysis, the stage of tumor at diagnosis (P = 0.022), time from diagnosis (P<0.001), metastasis of tumor (P = 0.030), type of cancer (P<0.001), and age (P<0.001) were associated with PUAW. PUAW had no association with the patient’s monthly income or marital status (P = 0.32 and 0.299, respectively; Table 1). There was no significant difference in unawareness between patients from rural and urban areas (P = 0.90; Table 1).
In the adjusted multivariate model, age (crude odds ratio (OR) = 0.71, 95% CI: 0.62, 0.82; adjusted OR = 0.71, 95% CI: 0.62, 0.82) and cancer type (crude MOR = 2.16; adjusted MOR = 2.04; P<0.0001) were significantly associated with PUAW.
The patients’ tendency for non-disclosure to relatives (TTND)
The data showed that the patients tended to withhold disclosure of their cancer from up to 16% (95% CI: 11.7, 19.4) of their relatives (Table 2). Females tended to withhold disclosure more than males (TTND = 17%, 95% CI: 12.3, 22.3 and TTND = 12%, 95% CI: 6.9, 17.5, respectively), and the lowest TTND was reported for spouses (TTND = 4%, 95% CI: 1.7, 5.4), children (TTND = 8%, 95% CI: 5.1, 11.3), parents (TTND = 13%, 95% CI: 7.9, 13.1), and siblings (TTND = 13%, 95% CI: 9.5, 17.0). Patients with lymphoma (TTND = 27%, 95% CI: 10.6, 43.7) or colorectal cancer (TTND = 21%, 95% CI: 5.0, 37.1) reported a higher TTND. Younger participants (up to 39 years) reported a higher TTND (22%, 95% CI: 11.9, 30.9) than middle-aged (14%, 95% CI: 9.4, 13.3) or elderly (12%, 95% CI: 6.5, 16.6; Table 2) participants.
Table 2. Familial cancer visibility and tendency for non-disclosure.
| Factor | n | FCV% (95% CI) | TTND% (95% CI) |
|---|---|---|---|
| Closeness of Relationship | |||
| Very close | 415 | 88 (86, 90) | 11 (7.9, 13.2) |
| Close | 415 | 86 (83, 89) | 15 (10.8, 18.5) |
| Distant | 415 | 84 (81, 88) | 19 (13.6, 24.0) |
| Patient Self-Awareness | |||
| Yes | 332 | 89 (86, 92) | 15 (11.0, 19.5) |
| No | 83 | 69 (60,78) | 17 (8.5, 26.3) |
| Gender | |||
| Female | 272 | 87 (84, 91) | 17 (12.3, 22.3) |
| Male | 143 | 83 (77, 88) | 12 (6.9, 17.5) |
| Age Group | |||
| 0–39 | 87 | 83 (76, 89) | 22 (11.9, 30.9) |
| 40–60 | 187 | 88 (84, 92) | 14 (9.4, 13.3) |
| 60+ | 141 | 85 (81, 89) | 12 (6.5, 16.6) |
| Time from Diagnosis | |||
| Up to six M | 138 | 80 (74, 86) | 17 (9.9, 23.1) |
| Seven to 18 M | 98 | 88 (82, 93) | 12 (5.6, 17.8) |
| 19 to 30 M | 43 | 90 (82, 98) | 10 (0.3, 19.1) |
| 31 to 42 M | 34 | 94 (89, 99) | 19 (0.3, 38.0) |
| 42+ M | 102 | 87 (81, 92) | 19 (11.3, 26.6) |
| Cancer Type † | |||
| Breast | 160 | 88 (83, 92) | 16 (9.3, 23.4) |
| Colorectal | 33 | 79 (66, 93) | 21 (5.0, 37.1) |
| Lung | 33 | 83 (75, 95) | 15 (2.0, 27.5) |
| Leukemia | 25 | 81 (67, 95) | 15 (1.2, 28.5) |
| Lymphoma | 28 | 82 (67, 97) | 27 (10.6, 43.7) |
| Overall | 415 | 86 (83, 89) | 16 (11.7, 19.4) |
Abbreviations: FCV, familial cancer visibility; TTND, tendency for non-disclosure to relatives; CI, confidence interval; M, month.
†First five common cancers.
Relatives’ gender, gender matching with the relatives, and stage of tumor were not included in the multivariate model (univariate P<0.3; results not shown), and the age group was ignored after adjustment (P = 0.066). Multivariate, mixed Poisson modeling showed that the patient’s gender, type of residency, marital status, monthly income, closeness to relatives, time from diagnosis, and cancer type were significantly associated with TTND (P<0.0001; Table 3). Cancer type was the most important determinant of TTND (adjusted MIRR = 14.45; P<0.0001).
Table 3. Determinants of cancer visibility and disclosing behavior of cancer patients.
| Determinant | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | P-value | ||
| Tendency for Non-Disclosure to Relatives | ||||||
| Gender (Ref: Female) | 0.84 | 0.77, 0.91 | 0.87 | 0.76, 0.99 | <0.0001 | |
| Residency (Ref: Urban) | 1.74 | 1.58, 1.92 | 1.80 | 1.60, 2.00 | <0.0001 | |
| Marital Status (Ref: Single) | 0.61 | 0.55, 0.68 | 0.55 | 0.47, 0.64 | <0.0001 | |
| Monthly Income (Ref: <100$) | 0.82 | 0.80, 0.84 | 0.70 | 0.67, 0.73 | <0.0001 | |
| Time from Diagnosis (Year) | 1.02 | 1.01, 1.03 | 1.02 | 1.01, 1.03 | <0.0001 | |
| Closeness of Relationship (Ref: Very close) | Close | 1.39 | 1.26, 1.53 | 1.33 | 1.16, 1.52 | <0.0001 |
| Distant | 1.75 | 1.60, 1.92 | 1.41 | 1.23, 1.61 | <0.0001 | |
| Cancer Type (Random Intercept) | 15.7 † | - | 14.45 † | - | <0.0001 | |
| Familial Cancer Visibility | ||||||
| Patients’ Awareness (Ref: No) | 1.31 | 1.26, 1.36 | 1.32 | 1.27, 1.38 | <0.0001 | |
| Gender (Ref: Female) | 0.94 | 0.93, 0.95 | 0.92 | 0.89, 0.95 | <0.0001 | |
| TTND (25%) | 0.92 | 0.91, 0.93 | 0.92 | 0.91, 0.93 | <0.0001 | |
| Closeness of Relationship (Ref: Very close) | Close | 0.97 | 0.94, 1.01 | 0.98 | 0.95, 1.02 | 0.341 |
| Distant | 0.96 | 0.93, 0.99 | 0.96 | 0.93, 0.99 | 0.041 | |
| Age Group (Ref: 0–39 year) ‡ | 1.002 | 0.98, 1.02 | 1.03 | 1.01, 1.05 | 0.004 | |
| Cancer Type (Random Intercept) | 1.20 † | - | 1.07 † | - | <0.0001 | |
Abbreviations: RR, rate ratio; TTND, tendency for non-disclosure to relatives; CI, Poisson confidence interval.
†Median incidence rate ratio for cancer type as a random intercept factor.
‡Age groups: 0–39, 40–60, 60+
Familial cancer visibility (FCV)
We collected data on FCV from 27,998 relatives of cancer patients (including 3763 first-degree relatives, 8118 second-degree, 10,201 third-degree, and 5916 affinities). FCV for the total sample was estimated at 86% (95% CI: 83, 89). The rate of FCV for males and females was 83% (95% CI: 77, 88) and 87% (95% CI: 84, 91), respectively (Table 2).
Cancer was more visible in middle-aged patients (FCV = 88%, 95% CI: 84, 92) than younger (FCV = 83%, 95% CI: 76, 89) or older (FCV = 85%, 95% CI: 81, 89) patients. If patients were unaware of their diagnosis, visibility decreased from 89% (95% CI: 86, 92) to 69% (95% CI: 60, 78; Table 2).
Cancer was less visible for parents (FCV = 77%, 95% CI: 71, 83) and more visible for siblings (FCV = 90%, 95% CI: 87, 93), children (FCV = 91%, 95% CI: 88, 94), and spouses (FCV = 90%, 95% CI: 87, 93). FCV was not significantly different between second (FCV = 85%, 95% CI: 82, 89) and third-degree relatives (FCV = 85%, 95% CI: 81, 89) or affinities (FCV = 86%, 95% CI: 83, 89). Data shows that a longer time from diagnosis may lead to more FCV (P = 0.57). Breast (FCV = 88%, 95% CI: 83, 92) and lung (FCV = 83%, 95% CI: 75, 95) cancers were meaningfully more visible than colorectal (FCV = 79%, 95% CI: 66, 93) cancer (Table 2).
FCV was not significantly associated with the relatives’ gender, gender matching of patients and relatives, stage of cancer, tumor metastasis, type of residency (rural or urban), the patient’s monthly income, or type of kinship (affinity or descent) at a 0.3 significance level (results not shown). Therefore, these variables were not included in the multivariate model.
PUAW had an RR of 1.32 (95% CI, 1.27, 1.38) in the final multivariate model. Closeness of relationship (RR = 0.96, 95% CI: 0.93, 0.99), patient’s gender (RR = 0.92, 95% CI: 0.89, 0.95), and age group (RR = 1.03, 95% CI: 1.01, 1.05) as well as TTND (RR = 0.92, 95% CI: 0.91, 0.93) were significantly associated with FCV after being adjusted for other potential determinants (Table 3).
Discussion
In this study we focused on familial cancer visibility (FCV) as a major distal determinant of family cancer history (FCH) sensitivity. Our data showed that the rates of patients’ unawareness about her/his cancer diagnosis (PUAW), Tendency for Non-Disclosure (TTND) to relatives, and FCV were 20% (95% CI: 16, 24), 16% (95% CI: 11.7, 19.4), and 86% (95% CI: 83, 89), respectively. PUAW and TTND were significantly associated with FCV in our dataset (P<0.0001). FCV was also associated with cancer type (P<0.0001), and closeness of relationship (very close vs. distant, P = 0.041), as well as patient’s age (P = 0.004), and gender (P<0.0001).
Our estimated PUAW value was meaningfully less than the values stated in some previous reports, which range from 29% to 60% [31–33], while relatively similar to values found in other reports [34, 35]. The PUAW value found in this study may be affected by our conservative PUAW measurement and the distribution of our sample. Given that PUAW is higher in end-stage and older cancer patients, and that participation in our study was not restricted to terminal or older cancer patients, the PUAW value found in our study may be lower than the values found for older, end-stage patients [32–37].
To our knowledge, this is the first study that has quantitatively assessed non- disclosure of cancer diagnosis to relatives, what we refer to as TTND. Despite using a different methodology, our results are consistent with results of available qualitative studies on TTND [26, 27]. For example, multivariate results showed that women tended to conceal their cancer diagnosis from relatives more than men (RR = 0.87; 95% CI: 0.76, 0.99), which supports qualitative results. However, this contradicts gender stereotypes (i.e., that women are more expressive than men) and may be because of the conventions of the Iranian society and more perceived stigma.
When we compared our estimated values for TTND and PUAW for different cancer types (i.e. breast cancer and gastric cancer (PUAW = 94.4%, 95% CI: 91, 98 and PUAW = 37.5%, 95% CI: 19.9, 55.2, respectively), we showed that the effect of cancer type on PUAW and TTND might be correlated with public attitudes concerning the curability of various cancers. This supports findings from other studies, such as the study by Yoshinaga et al. [38].
Again, as far as we know, this study is the first effort to estimate FCV. Given that people cannot report what they are unaware of, the estimated FCV might be interpreted as maximum sensitivity of FCHs in Kerman.
In Iran, disclosure to cancer patients is not the responsibility of physicians alone. Families and caregivers play central roles for disclosure or non-disclosure to patients and relatives [39]. In light of the central roles that families and caregivers play, the relationship between FCV and PUAW may be because a considerable proportion of families try their best to conceal cancer diagnoses from relatives in order to protect the patient from indirect disclosure. Therefore, in such families, there would be less visibility among the relatives than in families with aware patients.
Though it may be assumed that TTND and FCV are highly correlated, the obtained correlation in this study was -11% (95% Cl: 9, 13%). This weak correlation may be associated with cultural issues in the Iranian society. Information can be transmitted in two ways in Iranian families, including direct and indirect methods. During direct transmission, patients or their caregivers disclose the cancer diagnosis to relatives. Direct transmission occurs for two reasons: one, because of individual inner desires, or two, to attract financial support or other cultural incentives (regardless of inner desire). On the other hand, indirect transmission occurs when information is transmitted from those who are aware to those whom the patients or their caregivers did not intend to disclose the disease. Therefore, the association of FCV and TTND might be explained by direct information transmission.
The results of our study regarding the dependency of FCV on cancer type (MIRR = 1.07, P<0.0001) may be explained by stigmatization. However, although stigmatization may partially explain the relationship, our results are also consistent with previous studies regarding the dependency of FCH sensitivity on cancer type. For example, studies have reported high sensitivity for breast cancer, moderate sensitivity for colorectal cancer, and low sensitivity for prostate and uterine cancers [40, 41]. These findings are consistent with our estimated FCVs.
In addition, even after adjusting for confounders, FCV was lower for males than for females (RR = 0.92, 95% CI: 0.89, 0.95). This may be because men think they should conceal their weaknesses or illnesses to protect themselves and their loved ones [27]. Another possible explanation may be residual confounding caused by lower PUAW in male patients.
We also found a lower FCV in younger patients (RR = 1.03, 95% CI: 1.01, 1.05) that might decrease the accuracy and usability of FCHs. Given that numerous studies have shown that a diagnosis of cancer in younger relatives is more important for FCH than a diagnosis of cancer in older relatives, more precise investigations are needed.
In our study, FCV was highest for first-degree relatives (FDRs) (FCV = 88%, 95% CI: 86, 90). This finding was consistent with the findings for FCH sensitivity. Almost all previous studies have reported a better sensitivity for FCH in FDRs rather than second or third degree relatives [42, 43].
Limitations
The primary limitation of our study was the result of a lower consent rate for men for participation in this study. More female participation led to a higher frequency of female-organ cancers (e.g., breast cancer). Therefore, the estimated FCV for the total sample was possibly affected by the participant sex-ratio (males = 143; females = 272). Accordingly, there is a possibility of FCV overestimation. To account for this possible overestimation, we used sampling weights extracted from Kerman Cancer Registry to weight cancer types. There was no meaningful difference (86% vs. 85.5%) between estimates from analytic or sampling weights; therefore, we presented those provided by analytic weights. In addition, given the abovementioned participant sex ratio, the overall PUAW may be underestimated. Consequently, in this study, the overall PUAW may be underestimated because of a lower PUAW in females.
Furthermore, we did not confirm interviewee claims regarding the awareness (informed or uninformed) of her/his relatives. Although four questions collecting contact information for patient relatives were originally included in the questionnaires, we omitted these questions from the finalized version since it was not feasible to collect relatives’ contact information in a pilot study. However, we used a standardized, validated method that has been used in previous sociological studies.
Finally, given the lack of a robust cancer registry or a complete archive of medical records, it was not possible for us to confirm FCHs. Accordingly, we were unable to directly estimate the effect size of PUAW, FCV, or TTND on FCH sensitivity.
Conclusion
The maximum sensitivity of the self-reported cancer histories and FCHs in the Kermanian population is not likely to be higher than 80% and 86%, respectively. This finding might be generalizable to similar populations, given the values of PUAW and TTND. Our study suggests that improving disclosure to relatives and taking FCHs from close relatives may improve FCH sensitivity.
Supporting Information
(SAV)
Acknowledgments
We would like to thank the study interviewers and all cancer patients who participated in this project. We would also like to thank Maryam Hadipour, Dr. Farzaneh Zolala, and Dr. Behjat Kalantari Khandani, for their valuable comments during data collection and manuscript preparation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The parent study for this work was funded by Kerman University of Medical Sciences. Grant no: 9350 (www.kmu.ac.ir).
References
- 1. Han MA, Oh MG, Choi IJ, Park SR, Ryu KW, Nam BH, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(7):701–8. Epub 2012/01/25. 10.1200/JCO.2011.35.3078 . [DOI] [PubMed] [Google Scholar]
- 2. Chan JA, Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. Jama. 2008;299(21):2515–23. Epub 2008/06/05. 10.1001/jama.299.21.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kleinerman RA, Yu CL, Little MP, Li Y, Abramson D, Seddon J, et al. Variation of second cancer risk by family history of retinoblastoma among long-term survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(9):950–7. Epub 2012/02/23. 10.1200/JCO.2011.37.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(8):824–9. Epub 2014/02/05. 10.1200/JCO.2013.51.4661 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissman SM, Bellcross C, Bittner CC, Freivogel ME, Haidle JL, Kaurah P, et al. Genetic counseling considerations in the evaluation of families for Lynch syndrome—a review. J Genet Counsel. 2011;20(1):5–19. Epub 2010/10/12. 10.1007/s10897-010-9325-x . [DOI] [PubMed] [Google Scholar]
- 6. Hampel H, Sweet K, Westman JA, Offit K, Eng C. Referral for cancer genetics consultation: a review and compilation of risk assessment criteria. J Med Genet. 2004;41(2):81–91. Epub 2004/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berry DA, Iversen ES Jr, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(11):2701–12. Epub 2002/06/01. 10.1200/jco.2002.05.121 . [DOI] [PubMed] [Google Scholar]
- 8. Hemminki K, Fallah M, Hemminki A. Collection and use of family history in oncology clinics. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(29):3344–5. Epub 2014/09/04. 10.1200/JCO.2014.56.4336 . [DOI] [PubMed] [Google Scholar]
- 9. Aitken J, Bain C, Ward M, Siskind V, MacLennan R. How Accurate is Self-Reported Family History of Colorectal Cancer? Am J Epidemiol. 1995;141(9):863–71. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JA, Hawkins J, Watkins DC. Factors Associated with Cancer Family History Communication Between African American Men and Their Relatives. The Journal of Men's Studies. 2013;21(2):97–111. 10.3149/jms.2102.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sie AS, Brunner HG, Hoogerbrugge N. Easy-to-use decision aids for improved cancer family history collection and use among oncology practices. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(29):3343 Epub 2014/09/04. 10.1200/JCO.2014.56.2942 . [DOI] [PubMed] [Google Scholar]
- 12. Ozanne EM, O'Connell A, Bouzan C, Bosinoff P, Rourke T, Dowd D, et al. Bias in the reporting of family history: implications for clinical care. J Genet Counsel. 2012;21(4):547–56. Epub 2012/01/13. 10.1007/s10897-011-9470-x . [DOI] [PubMed] [Google Scholar]
- 13. Daniels MS, Babb SA, King RH, Urbauer DL, Batte BA, Brandt AC, et al. Underestimation of risk of a BRCA1 or BRCA2 mutation in women with high-grade serous ovarian cancer by BRCAPRO: a multi-institution study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(12):1249–55. Epub 2014/03/19. 10.1200/JCO.2013.50.6055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Braun D, Gorfine M, Parmigiani G. Misreported family histories and underestimation of risk. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(32):3682–3. Epub 2014/09/10. 10.1200/JCO.2014.57.4848 . [DOI] [PubMed] [Google Scholar]
- 15. Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The duty to warn a patient's family members about hereditary disease risks. Jama. 2004;292(12):1469–73. [DOI] [PubMed] [Google Scholar]
- 16. Qureshi N, Wilson B, Santaguida P, Little J, Carroll J, Allanson J, et al. Family history and improving health. Evidence report/technology assessment. 2009;(186):1–135. Epub 2009/12/02. . [PMC free article] [PubMed] [Google Scholar]
- 17. Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. Jama. 2004;292(12):1480–9. Epub 2004/09/24. 10.1001/jama.292.12.1480 . [DOI] [PubMed] [Google Scholar]
- 18. Sijmons RH, Boonstra AE, Reefhuis J, Hordijk-Hos JM, de Walle HE, Oosterwijk JC, et al. Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet. 2000;8(3):181–6. Epub 2000/04/26. 10.1038/sj.ejhg.5200441 . [DOI] [PubMed] [Google Scholar]
- 19. H SD, L MS. Agreement between questionnaire data and medical records: the evidence for accuracy of recall Am J Epidemiol. 1989;129(2):233–48. [DOI] [PubMed] [Google Scholar]
- 20. Bolland MJ, Barber A, Doughty RN, Grey A, Gamble G, Reid IR. Differences between self-reported and verified adverse cardiovascular events in a randomised clinical trial. BMJ Open. 2013;3(3). Epub 2013/03/21. 10.1136/bmjopen-2012-002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tolonen H, Koponen P, Mindell JS, Mannisto S, Giampaoli S, Dias CM, et al. Under-estimation of obesity, hypertension and high cholesterol by self-reported data: comparison of self-reported information and objective measures from health examination surveys. Eur J Public Health. 2014;24(6):941–8. Epub 2014/06/08. 10.1093/eurpub/cku074 . [DOI] [PubMed] [Google Scholar]
- 22. Inoue M, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Sasazuki S, et al. Validity of self-reported cancer among a Japanese population: Recent results from a population-based prospective study in Japan (JPHC Study). Cancer Epidemiol. 2011;35(3):250–3. 10.1016/j.canep.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 23. Navarro C, Chirlaque MD, Tormo MJ, Perez-Flores D, Rodriguez-Barranco M, Sanchez-Villegas A, et al. Validity of self reported diagnoses of cancer in a major Spanish prospective cohort study. J Epidemiol Community Health. 2006;60(7):593–9. Epub 2006/06/23. 10.1136/jech.2005.039131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssens A, Henneman L, Detmar SB, Khoury MJ, Steyerberg EW, Eijkemans MJ, et al. Accuracy of self-reported family history is strongly influenced by the accuracy of self-reported personal health status of relatives. J Clin Epidemiol. 2012;65(1):82–9. 10.1016/j.jclinepi.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 25. Kim S, Ko Y, Kwon S, Shin DY, Kim CH, Yang SH, et al. Family caregivers' awareness of illness and attitude toward disclosure during chemotherapy for advanced cancer. Psychooncology. 2014;23(11):1300–6. Epub 2014/05/13. 10.1002/pon.3565 . [DOI] [PubMed] [Google Scholar]
- 26. Thomas KB. Exploring colorectal cancer diagnosis disclosure to first-degree relatives: An African American family case series: University of South Florida; 2010. [Google Scholar]
- 27. Hilton S, Emslie C, Hunt K, Chapple A, Ziebland S. Disclosing a cancer diagnosis to friends and family: a gendered analysis of young men's and women's experiences. Qual Health Res. 2009;19(6):744–54. Epub 2009/04/04. 10.1177/1049732309334737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Killworth PD, McCarty C, Bernard HR, Johnsen EC, Domini J, Shelley GA. Two interpretations of reports of knowledge of subpopulation sizes. Soc Networks. 2003;25(2):141–60. [Google Scholar]
- 29. Salganik MJ, Mello MB, Abdo AH, Bertoni N, Fazito D, Bastos FI. The game of contacts: estimating the social visibility of groups. Soc Networks. 2011;33(1):70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maghsoudi A, Baneshi MR, Neydavoodi M, Haghdoost A. Network scale-up correction factors for population size estimation of people who inject drugs and female sex workers in Iran. PloS one. 2014;9(11):e110917 Epub 2014/11/05. 10.1371/journal.pone.0110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yun YH, Lee MK, Kim SY, Lee WJ, Jung KH, Do YR, et al. Impact of awareness of terminal illness and use of palliative care or intensive care unit on the survival of terminally ill patients with cancer: prospective cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(18):2474–80. [DOI] [PubMed] [Google Scholar]
- 32. Kim SY, Kim JM, Kim SW, Shin IS, Bae KY, Shim HJ, et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psychooncology. 2013;22(10):2206–13. 10.1002/pon.3275 [DOI] [PubMed] [Google Scholar]
- 33. Brokalaki EI, Sotiropoulos GC, Tsaras K, Brokalaki H. Awareness of diagnosis, and information-seeking behavior of hospitalized cancer patients in Greece. Support Care Cancer. 2005;13(11):938–42. Epub 2005/04/01. 10.1007/s00520-005-0794-7 . [DOI] [PubMed] [Google Scholar]
- 34. Hemati M, Hojatollah Shahbazian M, Abdol-Azim Sedighi M, Reza Khodabakhshi M, Ali Taghizadeh M, MD10 FSM, et al. Attitude of Cancer Patients toward Diagnosis Disclosure and their Preference for Clinical Decision-making: A National Survey. Arch Iran Med. 2014;17(4):232 doi: 014174/AIM.004 [PubMed] [Google Scholar]
- 35. Nord C, Mykletun A, Fosså S. Cancer patients' awareness about their diagnosis: a population-based study. J Public Health (Oxf). 2003;25(4):313–7. [DOI] [PubMed] [Google Scholar]
- 36. Caruso A, Di Francesco B, Pugliese P, Cinanni V, Corlito A. Information and awareness of diagnosis and progression of cancer in adult and elderly cancer patients. Tumori. 2000;86(3):199–203. Epub 2000/08/12. . [DOI] [PubMed] [Google Scholar]
- 37. Papadopoulos A, Vrettos I, Kamposioras K, Anagnostopoulos F, Giannopoulos G, Pectasides D, et al. Impact of cancer patients' disease awareness on their family members' health‐related quality of life: a cross‐sectional survey. Psychooncology. 2011;20(3):294–301. 10.1002/pon.1731 [DOI] [PubMed] [Google Scholar]
- 38. Yoshinaga A, Sasaki S, Tsugane S. Sensitivity of self-reports of cancer in a population-based prospective study: JPHC Study Cohort I. J Clin Epidemiol. 2001;54(7):741–6. Epub 2001/07/05. . [DOI] [PubMed] [Google Scholar]
- 39. Mystakidou K, Parpa E, Tsilila E, Katsouda E, Vlahos L. Cancer information disclosure in different cultural contexts. Support Care Cancer. 2004;12(3):147–54. Epub 2004/04/13. 10.1007/s00520-003-0552-7 . [DOI] [PubMed] [Google Scholar]
- 40. Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med. 2003;24(2):190–8. Epub 2003/02/06. 10.1016/S0749-3797(02)00593-7. . [DOI] [PubMed] [Google Scholar]
- 41. Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol. 1997;146(3):244–8. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 42. Bondy ML, Strom SS, Colopy MW, Brown BW, Strong LC. Accuracy of family history of cancer obtained through interviews with relatives of patients with childhood sarcoma. J Clin Epidemiol. 1994;47(1):89–96. [DOI] [PubMed] [Google Scholar]
- 43. Mitchell RJ, Brewster D, Campbell H, Porteous ME, Wyllie AH, Bird CC, et al. Accuracy of reporting of family history of colorectal cancer. Gut. 2004;53(2):291–5. Epub 2004/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


