Abstract
Exploration is important for animals to be able to gather information about features of their environment that may directly or indirectly influence survival and reproduction. Closely related to exploration is neophobia, which may reduce exposure to danger, but also constrain explorative behaviour. Here we investigated the effects of social relationships on neophobia and exploration in wolves, Canis lupus, and dogs, Canis familiaris. Eleven pack-living wolves reared by human foster parents and 13 identically raised and kept dogs were tested in a novel object test under three different conditions: (1) alone, (2) paired with a pack mate and (3) together with the entire pack. Dogs were less neophobic than wolves and interacted faster with the novel objects. However, the dogs showed overall less interest in the novel objects than wolves, which investigated the objects for longer than the dogs. Both wolves and dogs manipulated objects for longer when paired or in the pack than when alone. While kinship facilitated the investigation of novel objects in the pair condition in both wolves and dogs, rank distance had opposite effects. Our results suggest that the presence of conspecifics supported the exploration of novel objects in both wolves and dogs, particularly within kin and that this may be interpreted as risk sharing. The reduced latency to approach objects and less time spent exploring objects in dogs compared to wolves may be interpreted as an effect of domestication.
Keywords: dog, domestication, exploration, neophobia, relationship, wolf
Highlights
-
•
We tested neophobia and exploration in dogs and wolves.
-
•
Dogs were quicker to approach, but showed less interest in, novel objects.
-
•
Presence of conspecifics enhanced approaches to, and exploration of, novel objects.
-
•
Our findings suggest risk sharing mediates cooperation in wolves and dogs.
-
•
We assume the differences between wolves and dogs to be an effect of domestication.
Exploration is important for animals to be able to gather information about features of their environment that may directly or indirectly influence survival and reproduction. Exploring animals may collect information about food distribution and abundance, shelters, predators, escape routes or potential mates (Dall, Giraldeau, Olsson, McNamara, & Stephens, 2005; Heinrich, 1995; Mettke-Hofmann, Winkler, & Leisler, 2002; Renner, 1988; Schwagmeyer, 1995). To acquire such knowledge, an individual may assess its environment alone (Day, Kyriazakis, & Rogers, 1998), by social learning or by using public information (Swaney, Kendal, Capon, Brown, & Laland, 2001; Valone & Templeton, 2002; Visalberghi & Adessi, 2001; Visalberghi & Fragaszy, 1995).
Closely related to exploration is neophobia with highly neophilic animals being quick to approach and explore a novel object, while highly neophobic animals are slow to do so (Day, Coe, Kendal, & Laland, 2003). Neophobia is linked to exploration because individuals only explore if they are interested in an object and the same is true for active avoidance. Thus objects can be neither explored nor avoided out of sheer disinterest/lack of perceived relevance. Accordingly, neophobia has been defined as ‘the avoidance of an object or other aspect of the environment solely because it has never been experienced and is dissimilar from what has been experienced in the individual's past’ (Stöwe, Bugnyar, Heinrich, & Kotrschal, 2006, p. 1079). Neophobic responses can therefore reduce exposure to danger but they can also constrain explorative behaviour and thus opportunities for learning and innovating (Stöwe, Bugnyar, Heinrich, et al., 2006; Stöwe, Bugnyar, Loretto, et al., 2006).
Depending on a species' ecology and the animal's motivation, individuals approach and investigate changes in their familiar environment with different latencies and for variable periods (Day et al., 2003; Mettke-Hofmann, Wink, Winkler, & Leisler, 2005; Mettke-Hofmann et al., 2002; Stöwe, Bugnyar, Heinrich, et al., 2006). This may also be affected by social context. For example, the presence or action (handling or food intake) of a conspecific facilitated the acceptance of novel food in gerbils, Meriones unguiculatus (Forkman, 1991), zebra finches, Taeniopygia guttata (Coleman & Mellgren, 1994), capuchin monkeys, Cebus apella (Visalberghi & Fragaszy, 1995; Visalberghi & Addessi, 2000), rats, Rattus norvegicus (Galef, 1996; Galef & Whiskin, 2000), keas, Nestor notabilis (Huber, Rechbergen, & Taborsky, 2001) and house mice, Mus musculus domesticus (Valsecchi, Bosellini, Sabatini, Mainardi, & Fiorito, 2002). In contrast, delay and inhibition of approach/acceptance of novel food in a social context have been observed in chum salmon, Oncorhynchus keta (Ryer & Olla, 1991), Atlantic salmon, Salmo salar (Brown & Laland, 2001, 2002) and great tits, Parus major (van Oers, Klunder, & Drent, 2005). It is not unlikely that the delay/inhibition reported in these studies was caused by dominance rank differences (and associated risk of agonistic interaction) between the participating individuals (Brown & Laland, 2001, 2002; van Oers et al., 2005; Ryer & Olla, 1991). Individual ravens, Corvus corax, for example, approached a novel object faster when tested alone than when paired with a conspecific, but they spent more time close to, and manipulating the novel object in dyads or in groups (Stöwe, Bugnyar, Loretto, et al., 2006). This study showed that social relationships mattered: ravens approached a novel object faster when paired with siblings than nonsiblings and dominant males approached the novel object first when in a dyad with a female, but not when with a male (Stöwe, Bugnyar, Loretto, et al., 2006).
Wolves, Canis lupus, are cooperative, group-hunting animals that provide communal care for the pups in a kind of helper system supporting the exclusive reproduction of the dominant pair (Mech & Boitani, 2003). Moreover, wolves also defend their territories (Mech & Boitani, 2003, 2004) and kills (Kaczensky, Hayes, & Promberger, 2005) together. A pack usually consists of the reproductive pair and their offspring of 1 or more years; however, many variations of this theme have been observed (Packard, 2003). The pack is structured according to a sex–age graded hierarchy that reflects the composition of the family group (Packard, 2003). Domestic dogs, Canis familiaris, although phylogenetically closely related to wolves (Pang et al., 2009; Savolainen, Zhang, Luo, Lundeberg & Leitner, 2002; Scott & Fuller, 1965), differ fundamentally not just genetically (Axelsson et al., 2013) in regard to their closeness to humans, but also in their breeding system and, possibly, other cooperative interactions (Boitani & Ciucci, 1995; Butler, du Toit & Bingham, 2004; but see Bonanni, Valsecchi, & Natoli, 2010). Similar to wolves, free-ranging dogs may form stable social groups (Cafazzo, Valsecchi, Bonanni, & Natoli, 2010) consisting of several unrelated males and females. Feral dogs form a relatively steep, sex–age graded dominance hierarchy (Cafazzo et al., 2010). Particularly during feeding on dumps or on carcasses, aggression tends to be high (Boitani, Francisci, Ciucci, & Andreoli, 1995; Macdonald & Carr, 1995), which may make it less costly for them to explore a new source of food alone rather than in a group. Moreover, while free-ranging dogs, similar to wolves, defend their territories together (Boitani et al., 1995; Macdonald & Carr, 1995), they usually do not raise pups cooperatively (Boitani et al., 1995; Daniels & Bekoff, 1989; but see Pal, 2005), nor is it clear how closely they cooperate during hunting (Boitani et al., 1995; Macdonald & Carr, 1995).
If dogs are indeed less cooperative than wolves within groups of conspecifics, it may be predicted that also with novel objects, potentially perceived as a source of danger, wolves might rely more on support from conspecifics than dogs. For example if the social context mediates the expression of an individual's personality by either synchronizing its behaviours to the behaviour of its partner or by increasing individual differences between the partners (King, Williams, & Mettke-Hofmann, 2015), wolves could be more prone to synchronize than dogs because, in general, cooperativeness with conspecifics is more important for their daily survival than for dogs. On the other hand, in social mammals the presence of a familiar conspecific has been shown to be more effective for social buffering, namely in alleviating acute stress responses, compared to the presence of an unfamiliar conspecific (Kiyokawa, Honda, Takeuchi & Mori, 2014). Therefore in potentially stressful situations, as when confronted with a novel object, the presence of a conspecific might be a valuable resource reducing the potential stress, which might be the same for dogs and wolves.
While wolves have experienced various degrees of persecution and exploitation from humans during the last centuries, potentially selecting for greater neophobia (Fritts, Stephenson, Hayes, & Boitani, 2003), dogs have undergone the opposite selection through the domestication process (Clutton-Brock, 1995; Hare & Tomasello, 2005; Thorne, 1995). It has been argued that neophilia is an adaptive consequence of selection by living in association with humans (Kaulfuβ & Mills, 2008), suggesting that dogs should be inherently less neophobic than wolves, which may also decrease the dependency on a group in their approach of novelty, as compared to wolves. Still, wolves may be more strongly interested in novelty than dogs, because the potential costs or benefits of contact with novelty may be greater in the former than in the latter because of their reliance on prey rather than relatively stable food resources.
In this study, we compared the responses of identically raised and kept pack-living wolves and dogs to novel objects presented in three different conditions: alone, as a pair with a pack mate and with the entire pack. The aim was to investigate how the social context and relationship between pack members influenced their neophobic responses and explorative behaviour. For reasons discussed above, we predicted that wolves would be overall more neophobic than dogs towards human-related objects (Clutton-Brock, 1995; Fritts et al., 2003; Hare & Tomasello, 2005; Thorne, 1995), approaching the objects slower than dogs, but possibly exploring novel objects more thoroughly than dogs as novelty may lead to potential benefits or costs that are greater for wolves than for dogs. Moreover, owing to the inherently higher cooperativeness of wolves towards conspecifics (Boitani et al., 1995; Kaczensky et al., 2005; Mech & Boitani, 2003, 2004; Pal, 2005; Range & Virányi, 2015), we expected a greater facilitating influence of the presence of conspecifics on the exploratory and neophobic behaviour of wolves than dogs, that is, wolves would approach the novel objects faster and explore the objects for longer when tested with a pack member or the entire pack. We also expected that when tested alone this effect would be larger in wolves than dogs; that is, there would be no or little influence of the presence of a pack member in dogs.
Methods
Ethical Note
No special permission for use of animals (wolves and dogs) in such sociocognitive studies is required in Austria (Tierversuchsgesetz 2012 – TVG 2012). The relevant committee that allows running research without special permissions regarding animals is Tierversuchskommission am Bundesministerium für Wissenschaft und Forschung (Austria).
Subjects
We tested 11 wolves and 13 dogs raised and kept the same way at the Wolf Science Center, Austria (for details see Table 1). All animals were hand-raised after being separated from their mother at approximately 10 days after birth. During the first 5 months of their lives, the puppies had 24 h contact with their human hand-raisers. At the age of 2–3 months, the puppies were gradually socialized with the older animals (dogs with dogs and wolves with wolves) and at the age of 5 months they were integrated into their respective packs. Individual packs were not natural families, as older individuals were not the parents of the younger ones. In all packs but one (pack 5), at least one pair of siblings was present. Both wolves and dogs lived in three packs (Table 1) in 2000–8000 m2 enclosures with natural vegetation such as trees and bushes. Each enclosure contained two or three shelters and the dogs also had access to indoor shelters year-long. Water was available ad libitum. Wolves were fed two or three times a week, dogs on a daily basis, wolves mainly with carcasses of rabbits and deer, dogs mainly with dry food, owing to the different specific dietary requirements of wolves and dogs (Axelsson et al., 2013).
Table 1.
Individual data for all the wolves and dogs housed at the Wolf Science Center (Austria)
| Species | Subject | Sex | Birth date | Puppy origin | Sibling | Pack no. |
|---|---|---|---|---|---|---|
| Wolf | Apache | Male | 19 May 2009 | Zoo Basel, Switzerland | Cherokee | 1 |
| Aragorn | Male | 4 May 2008 | Game park Herberstein, Austria | Shima | 1 | |
| Cherokee | Male | 19 May 2009 | Zoo Basel , Switzerland | Apache | 1 | |
| Geronimo | Male | 2 May 2009 | Triple D Farm, Montana, U.S.A. | Yukon | 2 | |
| Kaspar | Male | 4 May 2008 | Game park Herberstein, Austria | – | 1 | |
| Kenai | Male | 1 Apr 2010 | Quebec, Canada | Wapi | 3 | |
| Nanuk | Male | 28 Apr 2009 | Triple D Farm, Montana, U.S.A. | – | 2 | |
| Shima | Female | 4 May 2008 | Game park Herberstein, Austria | Aragorn | 1 | |
| Tatonga | Female | 21 Apr 2009 | Triple D Farm, Montana, U.S.A. | – | 2 | |
| Wapi | Male | 1 Apr 2010 | Quebec, Canada | Kenai | 3 | |
| Yukon | Female | 2 May 2009 | Triple D Farm, Montana, U.S.A. | Geronimo | 2 | |
| Dog | Asali | Male | 13 Sept 2010 | Szeged, Hungary | Binti | 4 |
| Bashira | Female | 13 Sept 2010 | Paks, Hungary | Hakima | 5 | |
| Binti | Female | 13 Sept 2010 | Szeged, Hungary | Asali | 4 | |
| Bora | Female | 2 Aug 2011 | Györ, Hungary | Layla | 6 | |
| Hakima | Male | 13 Sept 2010 | Paks, Hungary | Bashira | 4 | |
| Kilio | Male | 18 Dec 2009 | Paks, Hungary | Maisha | 5 | |
| Layla | Female | 2 Aug 2011 | Györ, Hungary | Bora | 6 | |
| Maisha | Male | 18 Dec 2009 | Paks, Hungary | Kilio | 4 | |
| Meru | Male | 1 Oct 2010 | Velence, Hungary | – | 5 | |
| Nia | Female | 21 July 2011 | Paks, Hungary | – | 5 | |
| Nuru | Male | 24 June 2011 | Paks, Hungary | Zuri | 6 | |
| Rafiki | Male | 30 Nov 2009 | Tengelic, Hungary | – | 4 | |
| Zuri | Female | 24 June 2011 | Paks, Hungary | Nuru | 6 |
From puppyhood on, all animals were regularly trained and participated in different behavioural tests. During the training sessions, they were usually separated from the rest of the pack. Wolves and dogs were exposed to the same type of experiences and the same behavioural tests at the same age from puppyhood on. At the age of 3–10 weeks, all subjects participated in several tests where they were confronted with novel objects in a room. It is unlikely that these early tests influenced the current tests in any specific way since, first, all animals (dogs and wolves) were exposed to the same objects; second, they were tested in a very different context (inside versus outside; human handler present in puppy tests versus absent in the current setting) and, finally, the time elapsed since these early tests (>2 years).
Behavioural Observations
To assess the individuals' dominance relationships behavioural data were collected using continuous focal animal sampling (Altmann, 1974) with the help of the Pocket Observer program installed on a hand-held device (version 3.1, Noldus Information Technology, Wageningen, The Netherlands; for ethogram see Appendix Table A1, for details on dominance rank assessment see data analysis below). Data from pack 1 and pack 2 were collected by M.H. from February to November 2010. The data from the remaining wolf and dog packs were collected by L.M. (wolf pack 3: October to November 2011; dog packs 4, 5 and 6: February to May 2012). Data collection periods were distributed equally during daylight hours from 0800 to 2100 hours. Ten minute focal sampling periods covered a total of 93 h of observation for pack 1 and pack 2 (each animal was observed 62 times); a total of 45 h and 20 min of observation were recorded for the other packs (pack 3 was observed 15 times per individual, pack 4 14 times, pack 5 15 times and pack 6 28 times). Each animal was sampled only once per day, all the pack members were present during every sampling sessions and no familiar human interacted with the animals during the data collection. The observer positioned herself outside the enclosure with a good overview of the main part of it.
Experiments
Experimental conditions
Each animal was presented with various novel objects (all unknown to the individual) to test their neophobic reactions in three different test conditions: alone, with a partner and with the entire pack. All tests were conducted in an outside enclosure with which the animals were familiar to ensure that they were investigating the novel object, but not a potentially novel environment. (1) In the alone condition each animal was tested alone twice with two different objects in separate sessions. (2) In the pair condition each animal was tested twice in different sessions with novel objects in the presence of the same pack member. All possible pair combinations were tested for each pack, resulting in a total of 10 pairs in pack 1, six pairs in pack 2, one pair in pack 3, 10 pairs in pack 4, six pairs in pack 5 and six pairs in pack 6. (3) In the pack condition each pack was tested twice in different sessions with every pack member being present.
Each test lasted 15 min starting when the animal(s) was/were released into the test enclosure. Tests were at least 2 days apart and each animal was tested at least once every 2 weeks but no more than three times per week.
The first alone condition test was arranged at the beginning of data collection, the second after the animals had participated in the first series of pair tests and one pack test condition. For the pack condition, each pack was tested twice: once in the middle of the pair conditions and once at the very end. The data were collected and analysed for each member of the pair and of the pack participating in each test.
The alone conditions were conducted in the beginning and the middle of testing. Pair conditions and pack conditions were pseudorandomized between and after these alone conditions so that each dyad was tested once before the second alone condition and once after. Pack conditions were conducted when half of the pairs per pack were tested.
Objects
A total of 38 objects were used (e.g. bicycle, book, teddy bear, balloons, helmets, baby pushchair, garden gnome). The objects were made of different materials such as plastic, metal, wood, straw and fabrics. Their size ranged from 16 to 140 cm high, from 11 to 130 cm long and from 4 to 60 cm wide (Fig. 1). Each object was used only once for each animal. We used the same two objects for all animals in the alone condition. In the pair condition, we used 10 objects for pack 1 and pack 4, six objects for pack 2, pack 5 and pack 6 and two objects for pack 3. Finally in the pack condition we used the same two objects for each pack. All objects were stored away from food and handled with clean hands to avoid the influence of familiar smells as much as possible. Also, all objects were cleaned after being used with each pack.
Figure 1.
Example of objects used in the novel object test.
Experimental set-up
Wolf pack 1 and pack 2 were tested in one of their enclosures. The tests with wolf pack 3 were conducted in a test enclosure, where they regularly spent long periods. The tests with the dogs were conducted in their own enclosure or in parts of it.
Next to each enclosure was a small holding pen, where animals could be kept for a short period. This area was connected to the ‘test’ enclosure through a wire-mesh tunnel system with sliding doors at each side (‘slides’). During the test trials, the respective test enclosure and tunnel were covered with curtains to prevent other pack members from observing the tests and subject(s) being distracted. Curtains were also placed on the fence between the tunnel and the test enclosure to prevent the subject(s) from seeing the object before the start of the test.
In all the tests, the object was placed on the ground or was hung from a tree 15 m in front of the slide from where the animals entered the test enclosure out of sight of the subjects. This was done to prevent social facilitation by familiar persons, i.e. that dogs or wolves would learn that an object placed by a known person is not dangerous, consequently decreasing their neophobia in subsequent tests. All tests were recorded with two video cameras held by persons outside the test enclosure positioned at its two opposite corners.
Procedure
Before the start of a test trial, the curtains were put in place. Then the subject(s) were shuttled from the enclosure into a holding pen where they waited their turn, a procedure with which they were very well acquainted and they cooperated well. Then the object was carried inside the enclosure, covered with a sheet to prevent the animals seeing it, put into position, and the covering sheet removed. Then the first animal, pair or entire pack was let into the tunnel and once the camera operators were ready, the slide between the tunnel and the enclosure was opened so that the animal(s) could enter the test area. The slide was closed immediately after the animal(s) left the tunnel. At the end of the test the animal(s) was called back into the tunnel and then shuttled back into the holding pen where the other animals waited. If during the test, the object was displaced from its position, it was returned to its original place before testing the next animal.
Data Analysis
Behavioural observations
Dominance ranks for individuals in each pack were calculated based on the outcomes of their agonistic interactions with other pack members using the David's score with the formula:
| DS = w + w2 − l − l2 |
where w represents the sum of i's Pij values, w2 represents the summed w values (weighted by the appropriate Pij values) of those individuals with which i interacted, l represents the sum of i's Pji values and l2 represents the summed l values (weighted by the appropriate Pji values) of those individuals with which i interacted. Pij is the proportion of wins by individual i in its interactions with another individual j, that is the number of times that i defeats j divided by the total number of interactions between i and j. The proportion of losses by i in interaction with j is Pji = 1 − Pij (Gammell, De Vries, Jennings, Carlin, & Hayden, 2003).
The David's score was calculated for each animal in every pack and the rank position number (1 being the highest rank position) was assigned accordingly. In pack 4, two animals had the same David's score so the same rank value was assigned to both of them and it was calculated as the mean value between the rank of the animal ranking immediately above and below them.
Experiments
The videos of the tests were coded using The Observer XT 10.5 program (Noldus Information Technology). Data related to interaction, exploration and avoidance of the objects were extracted. First, we coded a binomial variable of the animal's likelihood of approaching the object, regardless of the distance to the object, during each test. One active approach was scored for each test where the animal approached or investigated the object (looked or sniffed at it) even from a distance; a zero was scored for each test in which the animal never showed any interest in the object. The approach latency was calculated as the elapsed time from the beginning of the test until the subject reached the 1 m periphery of the object for the first time. The contact latency was the time a subject needed to touch the object for the first time starting after it reached the 1 m periphery of the object for the first time. The total time spent investigating the object was calculated as the sum of all times the animal spent sniffing or looking at the object from a distance excluding all instances when there was direct contact with the object. The total time spent manipulating the object was calculated as the sum of times the animal spent touching the object with the nose, the mouth or the paws and interacting with it in different ways (chewing, manipulating with either mouth or paws, carrying it around). The occurrence of fleeing, defined as walking, running or jumping away from the object, usually with tail tucked in and body ducked, was recorded and calculated as a frequency.
To confirm scoring consistency, 20% of videos were cross coded between L.M. and M.H. Spearman rank correlation was perfect for likelihood of approaching the object (rS = 1) and very good for the other variables: approach latency: rS = 0.99; contact latency: rS = 0.94; time spent investigating the object: rS = 0.99; time spent manipulating the object: rS = 0.91; flee: rS = 0.97.
Statistical data analyses were carried out with the software R (version 2.15.2, The R Foundation for Statistical Computing, Vienna, Austria). First we calculated a general linear mixed-effect model (GLMM) to investigate whether species and test conditions had an influence on the likelihood of approaching the object and on the frequency of fleeing. Subject, test order (assigned to each animal from first to last test: 1, 2, 3, etc) and object identity were added in the model as random factors. The influence of species and test conditions on approach and contact latencies, time spent investigating and manipulating the object were analysed using linear mixed-effect models (LME) with subject, object identity and test order as random factors. Since the residuals of the models were not normally distributed, we used reciprocal transformation for the approach latency and for the frequency of fleeing. The inverse square root was used with the contact latency, while the square root was used with the investigation time and the natural logarithm was used for the manipulation time. We also used an LME to investigate the effect of age (in months) and rank of the focal animal on the approach and contact latencies, on the time spent investigating and manipulating the object and on the flee frequency, with a GLMM for each test condition (alone, pair, pack) separately. Rank distance and relatedness were taken into account in the pair condition comparisons. Interactions between species and all other factors were also tested. The subject, the object identity and the test sequence were added in all models as random factors. Additionally, for the pair condition, the partner was added as a random factor, while in the pack condition, the subject within pack was added as a random factor. To fit the residuals of our model to a normal distribution we transformed all our variables as follows: for the approach latency we used reciprocal transformation when analysing the data of the alone and pair conditions and the inverse square root for the pack condition data. We applied an inverse square root to the contact latency in all analyses, and a logarithm transformation to the investigation time of the alone condition; for all other transformations of the investigation time we used the square root transformation. Finally, the manipulation time data were normalized using the natural logarithm except in the pair condition where we used the square root transformation. When analysing latencies and durations, we excluded all tests in which an individual did not show the relevant behaviour from the relevant tests (excluded tests: approach latency: 26/252; contact latency and time spent manipulating the object: 56/252; time spent investigating the object: 27/252). All full model results are provided in the Appendix (Tables A2–A22).
Results
Influence of Species and Test Conditions
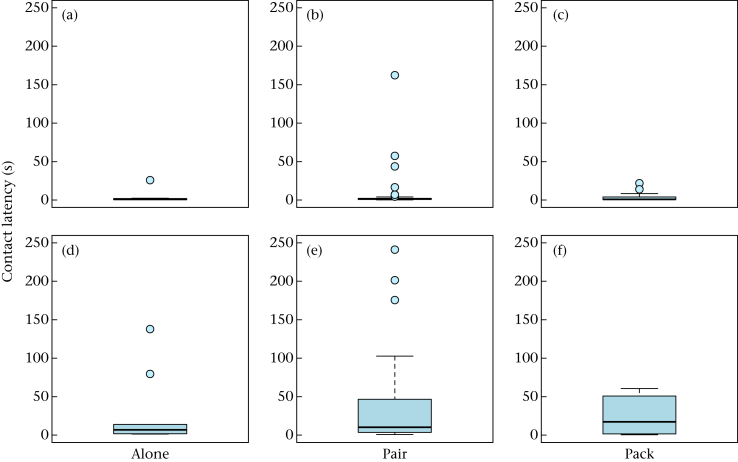
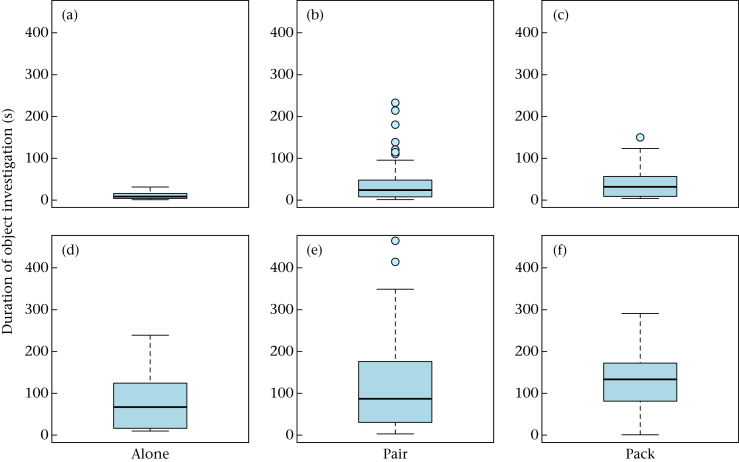
Six of the 13 dogs did not approach the object in at least one of the tests (total 9.3% of tests), while the wolves always approached; hence, the likelihood of approaching the object differed significantly between wolves and dogs (Table A2). No difference between wolves and dogs was found in regard to the approach latency (Table A3). However, wolves were slower in making contact with the object and fled from it more often than dogs (Fig. 2, Table A4, A5). Test conditions had no influence on any of the parameters (Table A3, A4, A5). While test condition did not influence the time spent investigating the object (Table A6), wolves investigated the objects for longer than the dogs (Fig. 3, Table A6). Both wolves and dogs manipulated the object for longer in the pair and in the pack conditions than in the alone condition (Table A7) and wolves tended to manipulate the object for longer than dogs (Table A7).
Figure 2.
Latency (s) of making contact with the object for the first time for (a, b, c) dogs and (d, e, f) wolves in the (a, d) alone, (b, e) pair and (c, f) pack conditions. Box plots show the median and the interquartile range from the 25th to the 75th percentile. Whiskers indicate the 1.5 interquartile range of the data. Circles represent outliers. (For statistical results of the separate test conditions see Tables A9, A14, A19.)
Figure 3.
Duration (s) of object investigation for (a, b, c) dogs and (d, e, f) wolves in the (a, d) alone, (b, e) pair and (c, f) pack conditions. Box plots show the median and the interquartile range from the 25th to the 75th percentile. Whiskers indicate the 1.5 interquartile range of the data. Circles represent outliers. (For statistical results of the separate test conditions see Tables A10, A15, A20.)
Alone Condition
Older animals, wolves as well as dogs, approached the object more quickly than younger individuals (Table A8). In contrast, no influence of age was found on contact latency (Table A9). Also, while rank influenced the approach latency (Table A8) with higher-ranking animals being quicker to approach than lower-ranking animals, no influence of rank was found for the latency to make contact with the object (Table A9).
Furthermore, we found an interaction of wolf/dog and rank with regard to object investigation (Table A10). Accordingly, we analysed dogs and wolves separately and found that in dogs the higher-ranking animals spent significantly more time investigating the objects than the lower-ranking ones, while in wolves no such difference appeared (Table A10). In contrast, rank had no influence on manipulating the object (Table A11) or the frequency of fleeing from the object (Table A12). Age had no effect on any of these three behaviours (Tables A10, A11, A12; see Table 2 for a summary of all results).
Table 2.
Summary of the main results
| Alone |
Pair |
Pack |
||||
|---|---|---|---|---|---|---|
| Wolves | Dogs | Wolves | Dogs | Wolves | Dogs | |
| Approach latency | ||||||
| Age | Older=decrease ↓ | NS | Older=decrease ↓ | |||
| Rank | Higher=decrease ↓ | NS | NS | |||
| Siblings or not | NA | Yes=increase ↑ | NA | |||
| Contact latency | ||||||
| Age | NS | NS | NS | |||
| Rank | NS | NS | NS | |||
| Siblings or not | NA | NS | NA | |||
| Investigation | ||||||
| Age | NS | NS | NS | |||
| Smaller | ||||||
| Higher= | rank | |||||
| Rank | NS | increase | distance | NS | NS | |
| ↑ | =increase | |||||
| ↑ | ||||||
| Siblings or not | NA | Yes=increase ↑ | NA | |||
| Manipulation | ||||||
| Age | NS | NS | NS | |||
| Rank | NS | NS | NS | |||
| Siblings or not | NA | NS | NA | |||
| Flee | ||||||
| Older= | Older= | |||||
| Age | NS | increase | NS | increase | NS | |
| ↑ | ↑ | |||||
| Rank | NS | NS | NS | |||
| Siblings or not | NA | NS | NA | |||
NA = not available.
Pair Condition
In the pair condition, the relationship between animals influenced their behaviour towards the objects. Animals approached the object faster when tested with an unrelated partner rather than with a sibling (Table A13), however, relatedness did not influence contact latency (Table A14). Rank distance did not influence approach latency (Table A13) or contact latency (Table A14).
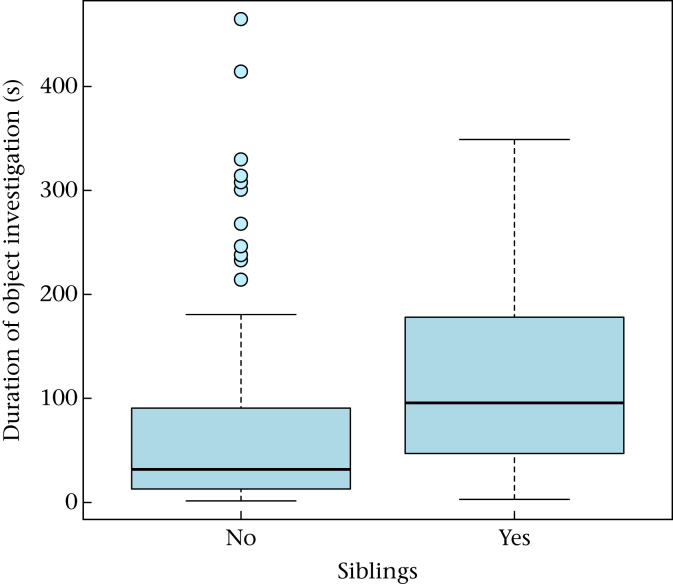
In addition, we found that in both wolves and dogs, sibling pairs investigated the object for longer than nonsiblings (Fig. 4, Table A15), but did not manipulate it for longer (Table A16). Moreover, we found a significant interaction between wolf/dog and rank distance between the partners on the time the focal animal spent investigating the object (Table A15). The smaller the rank distance was in the wolves, the longer they investigated the object (Table A15). No significant difference according to rank distance was found in the dogs (Table A15). Rank distance had no influence on the time the animals manipulated the object (Table A16).
Figure 4.
Duration (s) of object investigation in wolves and dogs when tested with a sibling or with an unrelated animal in the pair condition tests. Box plots show the median and the interquartile range from the 25th to the 75th percentile. Whiskers indicate the 1.5 interquartile range of the data. Circles represent outliers.
Fleeing was not influenced by rank distance or relatedness (Table A17). In contrast to the alone condition, the interaction between species and age had a significant effect on the flee frequency with older wolves fleeing more often than younger ones, while no difference was found for dogs (Table A17). No significant effect on any of the other variables was found for age in the pair condition (Tables A13, A14, A15, A16; see Table 2 for a summary of all results).
Pack Condition
We found a significant interaction of age on the approach latency in the pack condition (Table A18) with older animals approaching the object faster than younger ones. No influence of rank was found for the approach latency (Table A18). No influence of rank or age was found for the latencies to contact the object, the durations of investigating or manipulating in the pack condition (Tables A19, A20, A21).
Also here we found a significant effect of the interaction between species and age on the flee frequency with older wolves fleeing more often than younger ones, while no difference was found for dogs (Table A22). No influence of rank was found for the frequency of fleeing (Table A22; see Table 2 for a summary of all results).
Discussion
Our results indicate that in wolves and dogs, the presence of pack mates facilitated the manipulation of novel objects in a similar way with both manipulating longer in the pair and pack than in the alone condition. However, they differed in a few informative ways. Although wolves and dogs did not differ in their approach latencies, we still found differences, which may be interpreted as domestication effects: wolves jumped back more often, made contact with the object more slowly and investigated the objects for longer than dogs. These results indicate a greater interest of wolves in novelty, but also greater neophobia than dogs. Hence, the fact that all wolves but not all dogs approached the novel objects, even though wolves showed more fearful reactions than dogs, seems to be an indication of less interest by the dogs rather than neophobia.
More so than in dogs, unknown objects in the wolves' environment may be potentially dangerous; in fact, a wolf must be sufficiently careful to avoid harm, yet bold enough to attack (Peterson & Ciucci, 2003). In contrast, dogs living in a human environment should tolerate artefacts of human origin and even, potentially, tolerate novelty in general, as a result of selection during domestication towards neophilia (Kaulfuβ & Mills, 2008). However, this does not automatically mean a selection for a greater interest in novel objects as compared to wolves. Interestingly, while both higher-ranking dogs and wolves approached the novel objects quicker than lower-ranking animals, higher-ranking dogs investigated the object for longer than lower-ranking ones, while in wolves no such difference was found, again suggesting that in wolves interest in the environment is generally higher, even if lower-ranking wolves might not be as bold as higher-ranking ones, approaching novel objects more slowly. Actually, selection for dogs' problem-solving abilities may have relaxed due to the buffering effect of humans who take care of them (‘information processing hypothesis’; Frank, 1980); our present results indicate that this may lead to less caution and, generally, to less interest in their environment than wolves. Moreover, since domestication began, even free-ranging dogs consume food of human origin either actively provided to the dogs or in the form of rubbish dumps (Boitani et al., 1995; Bonanni, Natoli, Cafazzo, & Valsecchi, 2011; Cafazzo et al., 2010). Thus, the combination of competition over food with other pack members and reduced neophobia might have selected them to be less careful in contacting novel objects and thus be individually quicker than wolves to exploit novel food sources.
In this study we found that older individuals were quicker to approach the novel objects than younger ones, but in wolves, older animals also fled from the object more often than younger ones when in the presence of a pack mate but not when alone. This seemingly contradicts results in ravens, in which juvenile individuals were found to be less neophobic than adults and individuals fled from the object more often when alone than when paired with a mate (Stöwe, Bugnyar, Heinrich et al., 2006). The reason could be a different mode of adaptive maturation of neophobia in ravens than in our canids. Young ravens are usually in the company of their parents or are embedded in nonbreeder groups in which they learn to forage by exploring a wide range of choices that are then narrowed with experience and by copying social role models (Heinrich, 1995). In wolves, juvenile animals often remain with the pack until adulthood and probably learn from older members, generally their parents, how to hunt. Therefore younger wolves may rely more on older pack members than do ravens in acquiring information on the safety of a novel situation, thus showing higher neophobic responses when facing a novel object alone, and taking longer to approach than the older pack mates when the pack faces a novel object together. On the other hand, they might be more relaxed once an object has been approached in the presence of a pack mate, not fleeing as often. A similar mechanism may be true also for dogs when the youngest start following the pack to the feeding sites.
As in other mammals (Forkman, 1991; Galef, 1996; Galef & Whiskin, 2000; Valsecchi et al., 2002) and birds (Coleman & Mellgren, 1994; Huber et al., 2001), the presence of a conspecific in wolves and dogs in a novel object situation had some facilitating effects. For example, both wolves and dogs manipulated the object more in the pair and pack situations than when alone. It is possible that this effect is due to an exposure-type social buffering where the opportunity to interact with a conspecific during exposure to a novel environment attenuated subjects' stress responses (Kiyokawa, Kodama, Takeuchi & Mori, 2013). The strong cooperative tendencies in wolves and, still, in free-ranging dogs (Boitani et al., 1995; Bonanni et al., 2010; Mech & Boitani, 2003), for whom risk sharing in an uncertain situation might be a form of cooperation, could facilitate the exploration of novel objects. This was also supported by the facts that, first, co-exploration occurred for longer in wolves than in dogs in the pair condition and, second, wolves investigated for longer the smaller the rank distance, while dogs showed no such difference. This could potentially be explained by the tendency of wolves to explore together, whereas dogs rather tend to monopolize potential resources. Apart from a greater tolerance of wolves towards each other in such situations (Range, Ritter, & Virányi, 2015), wolves are also much better than dogs in imitation learning from each other (Range & Virányi, 2014). Hence, the ability to co-explore novel objects is also likely to facilitate the spread of skills that were developed individually and, hence, would also promote tradition forming in wolves, but not in dogs.
For the focal animal, it mattered not only if a partner was present but also who was present. While being with a sibling did not lower the latencies to approach or interact with the object, individuals investigated the objects for longer if paired with a sibling than with nonsiblings, which could be due to a facilitation effect caused by a more relaxed situation or a social conformity effect where subjects tend to synchronize their behaviour (King et al., 2015). Interestingly, the behaviour of the canines does not match raven behaviour, where the number of objects was the same as the number of individuals (Stöwe, Bugnyar, Loretto, et al., 2006). In these ravens, the presence of a sibling did not influence the time they spent close to, or manipulating, the novel object, but it seemingly had an enhancing effect on neophilia with siblings approaching faster than nonsiblings (Stöwe, Bugnyar, Loretto, et al., 2006).
We conclude that wolves were more neophobic than dogs, but probably less so than previously thought (Hare & Tomasello, 2005; Kaulfuβ & Mills, 2008). As predicted, we found significant facilitation effects for the manipulation of the objects in the pair and pack conditions as compared to animals tested alone. This suggests that risk sharing may be an important form of cooperation in both wolves and dogs. Still, wolves seemed generally more interested and explorative as well as more cooperative than dogs, where co-exploration may be more constrained by hierarchy issues than in wolves.
Acknowledgments
The Wolf Science Center was established by Zsofia Virányi, Friederike Range and Kurt Kotrschal and we thank all our helpers who made this possible and thus indirectly supported this research. We thank Marianne Heberlein for helping with the statistical analyses and two anonymous referees and the editor for comments on the manuscript. The project was financially supported by Austrian Science Fund (FWF) project P21244-B17. Writing has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement n. [311870]' to F.R. We further thank many private sponsors including Royal Canin for financial support and the Game Park Ernstbrunn for hosting the Wolf Science Center.
MS. number: 14-00950R
Appendix.
Table A1.
Definitions of behaviours recorded
| Behaviour | Definition |
|---|---|
| Dominant behaviours | |
| Dominant approach | To go forward within 2 m to another subject with the tail perpendicularly or above the plane of the back, the ears erect and pointed forwards and head held high |
| Head on | The animal approaches another and often puts its head on the other's shoulder. Formation looks like a capital T |
| Mark | To urinate with the hind legs lifted up in the air, mostly near or on bushes, trees or other objects |
| Mark over | To deliberately mark beside or on top of the urine mark of another animal |
| Muzzle bite | To grab the muzzle of another animal; it can be soft or with enough pressure to make the grabbed animal whimper |
| Ride up | To mount another animal from behind or from the side, also often seen during the breeding season |
| Stand over | To stand over opponent's body, or place the forepaws on the opponent and stand tall over him |
| Stand tall | Drawing up to full height and appearing as large as possible. May include raised hackles, ears erect and tail perpendicularly or above the back |
| T-position | An animal moves in front of another to stop it or make it change direction. The animal that blocks has tail up and may have hackles up and ears in front |
| Submissive behaviours | |
| Active submission | To be in a crouched position, tail tucked between the legs, sometimes wagging it. May attempt to paw or to lick the side of aggressor's muzzle |
| Crouch | To lower the head, bend the legs, the back often arched and the tail between the legs. The animal looks hunched and smaller |
| Flee | To walk or run away from another animal with tail tucked up and body ducked |
| Passive submission | To lie on the back, show the stomach, the tail between the legs. The ears directed backwards and close to the head and inguinal presentation |
Table A2.
The influence of species and test condition on the likelihood of approaching the object during the novel object tests
| Model terms | Estimate | SE | z | Pr (>|z|) | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2,224 | 1.63 | 0.20 | ||||||
| Species | 1,22 | 5.360 | 0.030 | ||||||
| Dogs vs wolves | −17.153 | 2043.781 | −0.008 | 0.993 | |||||
| Test condition | 2,112 | 0.66 | 0.52 | ||||||
| Alone vs Pair | −1.792 | 1.143 | −1.568 | 0.117 | |||||
| Pack vs Pair | 0.838 | 1.550 | 0.540 | 0.589 | |||||
| Subject (random) | 0.258 | 0.508 | |||||||
| Object (random) | 4.028 | 2.007 | |||||||
| Test order (random) | 2.730 | 1.652 |
GLMM statistics. Significant P value in bold.
Table A3.
The influence of species and test condition on the approach latency
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2,149 | 0.49 | 0.60 | |||||
| Species | 1,24 | 1.04 | 0.30 | |||||
| Dogs vs Wolves | −0.023 | 0.023 | −1.019 | |||||
| Test condition | 2,17 | 2.03 | 0.20 | |||||
| Alone vs Pair | −0.048 | 0.025 | −1.95 | |||||
| Pack vs Pair | −0.017 | 0.02 | −0.857 | |||||
| Subject (random) | 0.002 | 0.048 | ||||||
| Object (random) | 0.001 | 0.031 | ||||||
| Test order (random) | 0.000 | 0.010 | ||||||
| Residuals (random) | 0.005 | 0.074 |
LME statistic: model transformation to fit normal distribution of residuals: 1/x; Shapiro–Wilk test: W = 0.99, P = 0.35.
Table A4.
The influence of species and test condition on the contact latency
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2,114 | 1.01 | 0.40 | |||||
| Species | 1,21 | 42.760 | <0.001 | |||||
| Dogs vs Wolves | 0.432 | 0.066 | 6.539 | |||||
| Test condition | 2,19 | 0.42 | 0.70 | |||||
| Alone vs Pair | 0.089 | 0.096 | 0.920 | |||||
| Pack vs Pair | 0.017 | 0.080 | 0.208 | |||||
| Subject (random) | 0.009 | 0.099 | ||||||
| Object (random) | 0.013 | 0.115 | ||||||
| Test order (random) | 0.009 | 0.099 | ||||||
| Residuals (random) | 0.094 | 0.307 |
LME statistic: model transformation to fit normal distribution of residuals: 1/sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.48. Significant P and t values in bold.
Table A5.
The influence of species and test condition on the flee frequency
| Model terms | Estimate | SE | z | Pr(>|z|) | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2, 200 | 0.59 | 0.55 | ||||||
| Species | 1,26 | 4.290 | 0.050 | ||||||
| Dogs vs wolves | −1.432 | 0.398 | −3.601 | <0.001 | |||||
| Test condition | 2,22 | 0.96 | 0.40 | ||||||
| Alone vs Pair | −0.205 | 0.897 | −0.228 | 0.819 | |||||
| Pack vs Pair | −1.658 | 0.598 | −2.772 | 0.005 | |||||
| Subject (random) | 0.591 | 0.769 | |||||||
| Object (random) | 1.645 | 1.283 | |||||||
| Test order (random) | 0.353 | 0.594 |
GLMM statistic. Significant P value in bold.
Table A6.
The influence of species and test condition on the investigation time
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2,178 | 2.01 | 0.10 | |||||
| Species | 1,29 | 30.150 | <0.001 | |||||
| Dogs vs Wolves | −3.892 | 0.709 | −5.491 | |||||
| Test condition | 2,27 | 0.83 | 0.40 | |||||
| Alone vs Pair | −1.646 | 1.495 | −1.101 | |||||
| Pack vs Pair | 0.521 | 1.090 | 0.478 | |||||
| Subject (random) | 1.623 | 1.274 | ||||||
| Object (random) | 5.466 | 2.338 | ||||||
| Test order (random) | 1.187 | 1.089 | ||||||
| Residuals (random) | 7.857 | 2.803 |
LME statistic: model transformation to fit normal distribution of residuals: sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.37. Significant P and t values in bold.
Table A7.
The influence of species and test condition on the manipulation time
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species*Test condition | 2,137 | 1.01 | 0.37 | |||||
| Species | 1,27 | 3.43 | 0.07 | |||||
| Dogs vs Wolves | −0.676 | 0.365 | −1.851 | |||||
| Test condition | 2,25 | 5.330 | 0.010 | |||||
| Alone vs Pair | −1.449 | 0.496 | −2.920 | |||||
| Pack vs Pair | 0.351 | 0.389 | 0.902 | |||||
| Subject (random) | 0.510 | 0.714 | ||||||
| Object (random) | 0.495 | 0.703 | ||||||
| Test order (random) | 0.085 | 0.292 | ||||||
| Residuals (random) | 1.584 | 1.259 |
LME statistic: model transformation to fit normal distribution of residuals: log(x); Shapiro–Wilk test: W = 0.99, P = 0.06. Significant P and t values in bold.
Table A8.
Factors influencing the approach latency in the alone condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,30 | 0.05 | 0.83 | |||||
| Dogs vs Wolves | 0.008 | 0.037 | 0.219 | |||||
| Species*Rank | 1,15 | 0.70 | 0.42 | |||||
| Rank | −0.029 | 0.011 | −2.639 | 1,16 | 6.960 | 0.020 | ||
| Species*Age | 1,19 | 0.64 | 0.43 | |||||
| Age | 0.009 | 0.002 | 3.890 | 1,20 | 15.570 | <0.001 | ||
| Subject (random) | 0.002 | 0.039 | ||||||
| Object (random) | 0.000 | 0.011 | ||||||
| Test order (random) | 0.002 | 0.045 | ||||||
| Residuals (random) | 0.003 | 0.058 |
LME statistic: model transformation to fit normal distribution of residuals: 1/x; Shapiro–Wilk test: W = 0.97, P = 0.33. Significant P and t values in bold.
Table A9.
Factors influencing the contact latency in the alone condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,17 | 11.450 | 0.003 | |||||
| Dogs vs Wolves | 0.471 | 0.139 | 3.383 | |||||
| Species*Rank | 1,23 | 2.24 | 0.15 | |||||
| Rank | −0.009 | 0.046 | −0.212 | 1,27 | 0.04 | 0.83 | ||
| Species*Age | 1,25 | 0.00 | 1.00 | |||||
| Age | 0.014 | 0.008 | 1.618 | 1,28 | 2.62 | 0.12 | ||
| Subject (random) | 9.761e−12 | 3.124e−06 | ||||||
| Object (random) | 0.004 | 0.065 | ||||||
| Test order (random) | 0.043 | 0.206 | ||||||
| Residuals (random) | 0.067 | 0.259 |
LME statistic: model transformation to fit normal distribution of residuals: 1/sqrt(x); Shapiro–Wilk test: W = 0.97, P = 0.43. Significant P and t values in bold.
Table A10.
Factors influencing the investigation time in the alone condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,31 | 22.700 | <0.001 | |||||
| Dogs vs Wolves | −2.359 | 0.495 | −4.769 | |||||
| Species*Rank | 1,14 | 7.090 | 0.020 | |||||
| Dogs' rank | −0.456 | 0.128 | −3.571 | 1,13 | 12.800 | 0.003 | ||
| Wolves' rank | 0.213 | 0.194 | 1.095 | 1, 7 | 1.20 | 0.30 | ||
| Rank | −0.109 | 0.141 | −0.776 | 1,14 | 0.60 | 0.50 | ||
| Species*Age | 1,18 | 1.98 | 0.18 | |||||
| Age | −0.005 | 0.033 | −0.149 | 1,19 | 0.02 | 0.88 | ||
| Subject (random) | 0.391 | 0.626 | ||||||
| Object (random) | 0.275 | 0.525 | ||||||
| Test order (random) | 0.384 | 0.619 | ||||||
| Residuals (random) | 0.240 | 0.489 |
LME statistic: model transformation to fit normal distribution of residuals: log(x); Shapiro–Wilk test: W = 0.99, P = 0.91. Significant P and t values in bold.
Table A11.
Factors influencing the manipulation time in the alone condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,20 | 2.63 | 0.10 | |||||
| Dogs vs Wolves | −0.927 | 0.587 | −1.579 | |||||
| Species*Rank | 1,23 | 2.75 | 0.10 | |||||
| Rank | −0.225 | 0.212 | −1.064 | 1,17 | 1.07 | 0.30 | ||
| Species*Age | 1,26 | 0.28 | 0.60 | |||||
| Age | 0.022 | 0.053 | 0.416 | 1,21 | 0.17 | 0.70 | ||
| Subject (random) | 0.273 | 0.522 | ||||||
| Object (random) | 0.000 | 0.000 | ||||||
| Test order (random) | 0.000 | 0.000 | ||||||
| Residuals (random) | 2.089 | 1.445 |
LME statistic: model transformation to fit normal distribution of residuals: log(x); Shapiro–Wilk test: W = 0.98, P = 0.77.
Table A12.
Factors influencing the flee frequency in the alone condition tests
| Model terms | Estimate | SE | z | Pr(>|z|) | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|---|
| Species | 1,30 | 0.99 | 0.30 | ||||||
| Dogs vs Wolves | −1.038 | 0.953 | −1.090 | 0.276 | |||||
| Species*Rank | 1,39 | 0.58 | 0.50 | ||||||
| Rank | −0.347 | 0.280 | −1.238 | 0.216 | 1,43 | 0.28 | 0.60 | ||
| Species*Age | −0.607 | 0.391 | −1.551 | 0.121 | |||||
| Age | −0.043 | 0.072 | −0.594 | 0.552 | 1,41 | 1.46 | 0.20 | ||
| Subject (random) | 0.738 | 0.859 | |||||||
| Object (random) | 2.887e−12 | 1.699e−06 | |||||||
| Test order (random) | 5.912 | 2.431 |
GLMM statistic.
Table A13.
Factors influencing the approach latency in the pair condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,24 | 2.76 | 0.11 | |||||
| Dogs vs Wolves | −0.039 | 0.024 | −1.660 | |||||
| Species*Rank distance | 1,130 | 1.06 | 0.31 | |||||
| Rank distance | 0.007 | 0.009 | 0.774 | 1,129 | 0.60 | 0.44 | ||
| Species*Age | 1,18 | 1.22 | 0.28 | |||||
| Age | 0.001 | 0.002 | 0.326 | 1,20 | 0.11 | 0.75 | ||
| Species*Siblings | 1,117 | 0.00 | 0.99 | |||||
| Siblings | 1,133 | 7.740 | 0.006 | |||||
| No vs Yes | 0.056 | 0.019 | 2.782 | |||||
| Subject (random) | 2.016e−03 | 4.490e−02 | ||||||
| Partner (random) | 1.399e−15 | 3.742e−08 | ||||||
| Object (random) | 7.848e−04 | 2.801e−02 | ||||||
| Test order (random) | 7.632e−16 | 2.763e−08 | ||||||
| Residuals (random) | 6.014e−03 | 7.755e−02 |
LME statistic: model transformation to fit normal distribution of residuals: 1/x; Shapiro–Wilk test: W = 0.988, P = 0.256. Significant P and t values in bold.
Table A14.
Factors influencing the contact latency in the pair condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,19 | 37.540 | <0.001 | |||||
| Dogs vs Wolves | 0.493 | 0.080 | 6.127 | |||||
| Species*Rank distance | 1,94 | 1.05 | 0.31 | |||||
| Rank distance | 0.013 | 0.036 | 0.346 | 1,106 | 0.12 | 0.70 | ||
| Species*Age | 1,16 | 1.30 | 0.30 | |||||
| Age | 0.007 | 0.006 | 1.239 | 1,18 | 1.54 | 0.20 | ||
| Species*Siblings | 1,113 | 0.40 | 0.50 | |||||
| Siblings | 1,116 | 1.16 | 0.30 | |||||
| No vs Yes | −0.091 | 0.084 | −1.079 | |||||
| Subject (random) | 0.013 | 0.112 | ||||||
| Partner (random) | 0.003 | 0.052 | ||||||
| Object (random) | 0.013 | 0.113 | ||||||
| Test order (random) | 0.012 | 0.109 | ||||||
| Residuals (random) | 0.084 | 0.289 |
LME statistic: model transformation to fit normal distribution of residuals: 1/sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.64. Significant P and t values in bold.
Table A15.
Factors influencing the investigation time in the pair condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,32 | 15.480 | <0.001 | |||||
| Dogs vs Wolves | −3.249 | 0.839 | −3.871 | |||||
| Species*Rank distance | 1,126 | 6.120 | 0.010 | |||||
| Dogs' rank distance | 0.337 | 0.349 | 0.964 | 1,68 | 0.92 | 0.30 | ||
| Wolves' rank distance | −1.255 | 0.496 | −2.529 | 1,59 | 6.400 | 0.010 | ||
| Rank distance | −0.635 | 0.314 | −2.022 | 1,130 | 3.950 | 0.050 | ||
| Species*Age | 1,19 | 0.54 | 0.47 | |||||
| Age | −0.068 | 0.061 | −1.16 | 1,19 | 1.25 | 0.28 | ||
| Species*Siblings | 1,123 | 1.20 | 0.28 | |||||
| Siblings | 1,115 | 6.480 | 0.010 | |||||
| No vs Yes | −2.069 | 0.813 | −2.546 | |||||
| Subject (random) | 2.089 | 1.445 | ||||||
| Partner (random) | 0.022 | 0.148 | ||||||
| Object (random) | 6.226 | 2.495 | ||||||
| Test order (random) | 1.303 | 1.142 | ||||||
| Residuals (random) | 7.295 | 2.701 |
LME statistic: model transformation to fit normal distribution of residuals: sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.41. Significant P and t values in bold.
Table A16.
Factors influencing the manipulation time in the pair condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,22 | 1.75 | 0.20 | |||||
| Dogs vs Wolves | −2.422 | 1.972 | 5.831 | |||||
| Species*Rank distance | 1,99 | 3.61 | 0.06 | |||||
| Rank distance | −0.707 | 0.612 | −1.156 | 1,101 | 1.34 | 0.30 | ||
| Species*Age | 1,15 | 1.56 | 0.23 | |||||
| Age | −0.087 | 0.136 | −0.631 | 1,17 | 0.40 | 0.54 | ||
| Species*Siblings | 1,91 | 1.28 | 0.26 | |||||
| Siblings | 1,77 | 1.28 | 0.26 | |||||
| No vs Yes | 1.782 | 1.573 | 1.133 | |||||
| Subject (random) | 10.135 | 3.184 | ||||||
| Partner (random) | 5.762 | 2.401 | ||||||
| Object (random) | 11.132 | 3.337 | ||||||
| Test order (random) | 1.722 | 1.312 | ||||||
| Residuals (random) | 19.315 | 4.395 |
LME statistic: model transformation to fit normal distribution of residuals: sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.73.
Table A17.
Factors influencing the flee frequency in the pair condition tests
| Model terms | Estimate | SE | z | Pr(>|z|) | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|---|
| Species | 1,35 | 2.99 | 0.09 | ||||||
| Dogs vs Wolves | −1.407 | 0.457 | −3.078 | 0.002 | |||||
| Species*Rank distance | 1,136 | 2.29 | 0.13 | ||||||
| Rank distance | −0.217 | 0.164 | −1.327 | 0.185 | 1,131 | 1.18 | 0.28 | ||
| Species*Age | 1,24 | 5.520 | 0.030 | ||||||
| Dogs' Age | −0.004 | 0.038 | −0.113 | 0.910 | 1,71 | 0.09 | 0.80 | ||
| Wolves' Age | 0.080 | 0.040 | 2.011 | 0.044 | 1, 9 | 3.60 | 0.09 | ||
| Age | 0.026 | 0.029 | 0.867 | 0.386 | 1,25 | 1.73 | 0.20 | ||
| Species*Siblings | 1,146 | 2.22 | 0.14 | ||||||
| Siblings | 1,144 | 1.47 | 0.23 | ||||||
| No vs Yes | 0.139 | 0.324 | 0.428 | 0.669 | |||||
| Subject (random) | 0.274 | 0.524 | |||||||
| Partner (random) | 0.189 | 0.436 | |||||||
| Object (random) | 1.265 | 1.125 | |||||||
| Test order (random) | 0.039 | 0.197 |
GLMM statistic. Significant P values in bold.
Table A18.
Factors influencing the approach latency in the pack condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,20 | 0.92 | 0.35 | |||||
| Dogs vs Wolves | −0.043 | 0.045 | −0.960 | |||||
| Species*Rank | 1,14 | 0.34 | 0.60 | |||||
| Rank | 0.002 | 0.018 | 0.102 | 1,12 | 0.01 | 0.92 | ||
| Species*Age | 1,13 | 1.46 | 0.20 | |||||
| Age | 0.007 | 0.003 | 2.123 | 1,13 | 4.510 | 0.050 | ||
| Subject (random) | 0.004 | 0.065 | ||||||
| Pack:Subject (random) | 0.004 | 0.065 | ||||||
| Object (random) | 0.002 | 0.044 | ||||||
| Test order (random) | 0.015 | 0.121 | ||||||
| Residuals (random) | 0.003 | 0.054 |
LME statistic: model transformation to fit normal distribution of residuals: 1/sqrt(x); Shapiro–Wilk test: W = 0.97, P = 0.36. Significant P and t values in bold.
Table A19.
Factors influencing the contact latency in the pack condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,24 | 4.220 | 0.050 | |||||
| Dogs vs Wolves | 0.387 | 0.128 | 3.029 | |||||
| Species*Rank | 1,34 | 0.74 | 0.40 | |||||
| Rank | −0.048 | 0.051 | −0.925 | 1,35 | 0.99 | 0.33 | ||
| Species*Age | 1,36 | 4.08 | 0.05 | |||||
| Age | 0.012 | 0.009 | 1.253 | 1,37 | 1.32 | 0.26 | ||
| Subject (random) | 0.00 | 0.00 | ||||||
| Pack:Subject (random) | 0.00 | 0.00 | ||||||
| Object (random) | 1.30e−48 | 1.14e−24 | ||||||
| Test order (random) | 0.032 | 0.18 | ||||||
| Residuals (random) | 0.136 | 0.368 |
LME statistic: model transformation to fit normal distribution of residuals: 1/sqrt(x); Shapiro–Wilk test: W = 0.98, P = 0.64. Significant P and t values in bold.
Table A20.
Factors influencing the investigation time in the pack condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,21 | 13.740 | 0.001 | |||||
| Dogs vs Wolves | −4.402 | 1.188 | −3.706 | |||||
| Species*Rank | −1.636 | 0.863 | −1.896 | |||||
| Rank | −0.034 | 0.412 | −0.083 | 1,15 | 0.01 | 0.94 | ||
| Species*Age | −0.307 | 0.192 | −1.597 | |||||
| Age | −0.002 | 0.087 | −0.021 | 1,18 | 0.00 | 0.98 | ||
| Subject (random) | 1.769 | 1.330 | ||||||
| Pack:Subject (random) | 1.769 | 1.330 | ||||||
| Object (random) | 2.523 | 1.588 | ||||||
| Test order (random) | 2.744 | 1.657 | ||||||
| Residuals (random) | 3.977 | 1.994 |
LME statistic: model transformation to fit normal distribution of residuals: sqrt(x); Shapiro–Wilk test: W = 0.96, P = 0.17. Significant P and t values in bold.
Table A21.
Factors influencing the manipulation time in the pack condition tests
| Model terms | Estimate | SE | t | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|
| Species | 1,23 | 0.29 | 0.60 | |||||
| Dogs vs Wolves | −1.431 | 2.673 | −0.535 | |||||
| Species*Rank | 1,20 | 0.04 | 0.85 | |||||
| Rank | −1.274 | 0.981 | −1.298 | 1,19 | 1.69 | 0.20 | ||
| Species*Age | 1,20 | 0.45 | 0.51 | |||||
| Age | −0.309 | 0.197 | −1.568 | |||||
| Subject (random) | 9.555 | 3.091 | ||||||
| Pack:Subject (random) | 9.555 | 3.091 | ||||||
| Object (random) | 0.000 | 0.000 | ||||||
| Test order (random) | 13.818 | 3.717 | ||||||
| Residuals (random) | 17.766 | 4.215 |
LME statistic: model transformation to fit normal distribution of residuals: sqrt(x); Shapiro–Wilk test: W = 0.99, P = 0.99.
Table A22.
Factors influencing the flee frequency in the pack condition tests
| Model terms | Estimate | SE | z | Pr(>|z|) | df | F | P | Variance | SD |
|---|---|---|---|---|---|---|---|---|---|
| Species | 1,27 | 1.31 | 0.26 | ||||||
| Dogs vs Wolves | −0.343 | 0.692 | −0.496 | 0.619 | |||||
| Species*Rank | 1,18 | 1.75 | 0.20 | ||||||
| Rank | −0.086 | 0.325 | −0.264 | 0.792 | 1,20 | 0.41 | 0.50 | ||
| Species*Age | −0.307 | 0.133 | −2.300 | 0.021 | |||||
| Dogs' Age | −0.073 | 0.091 | −0.801 | 0.423 | |||||
| Wolves' Age | 0.279 | 0.109 | 2.574 | 0.010 | |||||
| Age | 0.113 | 0.057 | 1.986 | 0.047 | 1,22 | 4.39 | 0.05 | ||
| Subject (random) | 0.174 | 0.417 | |||||||
| Pack:Subject (random) | 0.174 | 0.417 | |||||||
| Object (random) | 0.796 | 0.892 | |||||||
| Test order (random) | 0.000 | 0.000 |
GLMM statistic. Significant P values in bold.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49(3,4):227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Axelsson E., Ratnakumar A., Arendt M., Maqbool K., Webster M.T., Perloski M. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- Boitani L., Ciucci P. Comparative social ecology of feral dogs and wolves. Ethology Ecology & Evolution. 1995;7:49–72. [Google Scholar]
- Boitani L., Francisci F., Ciucci P., Andreoli G. Population biology and ecology of feral dogs in central Italy. In: Serpell J., editor. The domestic dog: its evolution, behaviour and interactions with people. Cambridge University Press; Cambridge, U.K.: 1995. pp. 217–244. [Google Scholar]
- Bonanni R., Natoli E., Cafazzo S., Valsecchi P. Free-ranging dogs assess the quantity of opponents in intergroup conflicts. Animal Cognition. 2011;14:103–115. doi: 10.1007/s10071-010-0348-3. [DOI] [PubMed] [Google Scholar]
- Bonanni R., Valsecchi P., Natoli E. Pattern of individual participation and cheating in conflicts between groups of free-ranging dogs. Animal Behaviour. 2010;79:957–968. [Google Scholar]
- Brown C., Laland K. Social learning and life skills training for hatchery reared fish. Journal of Fish Biology. 2001;59:471–493. [Google Scholar]
- Brown C., Laland K. Social enhancement and social inhibition of foraging behaviour in hatchery reared Atlantic salmon. Journal of Fish Biology. 2002;61:987–998. [Google Scholar]
- Butler J.R.A., du Toit J.T., Bingham J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: threats of competition and disease to large wild carnivores. Biological Conservation. 2004;115:369–378. [Google Scholar]
- Cafazzo S., Valsecchi P., Bonanni R., Natoli E. Dominance in relation to age, sex and competitive contexts in a group of free-ranging domestic dogs. Behavioral Ecology. 2010;21:443–455. [Google Scholar]
- Clutton-Brock J. Origins of the dog: domestication and early history. In: Serpell J., editor. The domestic dogs its evolution, behaviour and interactions with people. Cambridge University Press; 1995. pp. 8–20. [Google Scholar]
- Coleman S.L., Mellgren R.L. Neophobia when feeding alone or in flocks in zebra finches, Taeniopygia guttata. Animal Behaviour. 1994;48:903–907. [Google Scholar]
- Dall S.R.X., Giraldeau L., Olsson O., McNamara J.M., Stephens D.W. Information and its use by animals in evolutionary ecology. Trends in Ecology & Evolution. 2005;20(4):187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Daniels T.J., Bekoff M. Population and social biology of free-ranging dogs, Canis familiaris. Journal of Mammalogy. 1989;70:754–762. [Google Scholar]
- Day R.L., Coe R.L., Kendal J.R., Laland K.N. Neophilia, innovation and social learning: a study of intergeneric differences in callitrichid monkeys. Animal Behaviour. 2003;65:559–571. [Google Scholar]
- Day J.E.L., Kyriazakis I., Rogers P.J. Food choice and intake: towards a unifying framework of learning and feeding motivation. Nutrition Research Reviews. 1998;11:25–43. doi: 10.1079/NRR19980004. [DOI] [PubMed] [Google Scholar]
- Forkman B. Social facilitation is shown by gerbils when presented with novel but not with familiar food. Animal Behaviour. 1991;42:860–861. [Google Scholar]
- Frank H. Evolution of canine information. Processing under conditions of natural and artificial selection. Zeitschrift für Tierpsychologie. 1980;53(4):389–399. doi: 10.1111/j.1439-0310.1980.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Fritts S.H., Stephenson R.O., Hayes R.D., Boitani L. Wolves and humans. In: Mech L.D., Boitani L., editors. Wolves: Behavior, ecology, and conservation. The University of Chicago Press; Chicago, IL: 2003. pp. 289–316. [Google Scholar]
- Galef B.G., Jr. Social enhancement of food preferences in Norway rats: a brief review. In: Heyes C.M., Galef B.G. Jr., editors. Social learning in Animals, the Roots of Culture. Academic Press; San Diego, CA: 1996. pp. 49–64. [Google Scholar]
- Galef B.G., Jr., Whiskin E.E. Social exploitation of intermittently available foods and the social reinstatement of food preference. Animal Behaviour. 2000;60:611–615. doi: 10.1006/anbe.2000.1521. [DOI] [PubMed] [Google Scholar]
- Gammell M.P., De Vries H., Jennings D.J., Carlin C.M., Hayden T.J. David's score: a more appropriate dominance ranking method than Clutton-Brock et al.'s index. Animal Behaviour. 2003;66:601–605. [Google Scholar]
- Hare B., Tomasello M. The emotional reactivity hypothesis and cognitive evolution. Trends in Cognitive Sciences. 2005;9:464–465. [Google Scholar]
- Heinrich B. Neophilia and exploration in juvenile common ravens, Corvus corax. Animal Behaviour. 1995;50:695–704. [Google Scholar]
- Huber L., Rechbergen S., Taborsky M. Social learning affects object exploitation and manipulation in keas, Nestor notabilis. Animal Behaviour. 2001;62:945–954. [Google Scholar]
- Kaczensky P., Hayes R.D., Promberger C. Effect of raven Corvus corax scavenging on the kill rates of wolf Canis lupus packs. Wildlife Biology. 2005;11:101–108. [Google Scholar]
- Kaulfuβ P., Mills D.S. Neophilia in domestic dogs (Canis familiaris) and its implication for studies of dog cognition. Animal Cognition. 2008;11:553–556. doi: 10.1007/s10071-007-0128-x. [DOI] [PubMed] [Google Scholar]
- King A.J., Williams L.J., Mettke-Hofmann C. The effects of social conformity on Gouldian finch personality. Animal Behaviour. 2015;99:25–31. [Google Scholar]
- Kiyokawa Y., Honda A., Takeuchi Y., Mori Y. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behavioural Brain Research. 2014;267:189–193. doi: 10.1016/j.bbr.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y., Kodama Y., Takeuchi Y., Mori Y. Physical interaction is not necessary for the induction of housing-type social buffering of conditioned hyperthermia in male rats. Behavioural Brain Research. 2013;256:414–419. doi: 10.1016/j.bbr.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Macdonald D.W., Carr G.M. Variation in dog society: between resource dispersion and social flux. In: Serpell J., editor. The domestic dog: its evolution, behaviour and interactions with people. Cambridge University Press; Cambridge, U.K.: 1995. pp. 199–216. [Google Scholar]
- Mech L.D., Boitani L. Wolf social ecology. In: Mech L.D., Boitani L., editors. Wolves: Behavior, ecology, and conservation. The University of Chicago Press; Chicago, IL: 2003. pp. 1–34. [Google Scholar]
- Mech L.D., Boitani L. Grey wolf Canis lupus. In: Sillero-Zubiri C., Hoffmann M., Macdonald D.W., editors. Canids: Foxes, wolves, jackals and dogs. Status survey and conservation action plan. IUCN/SSC Canid Specialist Group; Gland, Switzerland and Cambridge, U.K.: 2004. pp. 124–129. [Google Scholar]
- Mettke-Hofmann C., Winkler H., Leisler B. The significance of ecological factors for exploration and neophobia in parrots. Ethology. 2002;108:249–272. [Google Scholar]
- Mettke-Hofmann C., Wink M., Winkler H., Leisler B. Exploration of environmental changes relates to lifestyle. Behavioral Ecology. 2005;16(1):247–254. [Google Scholar]
- van Oers K., Klunder M., Drent P.J. Context dependence of personalities: risk-taking behavior in a social and non-social situation. Behavioural Ecology. 2005;16:716–723. [Google Scholar]
- Packard J.M. Wolf behavior: reproductive, social, and intelligent. In: Mech L.D., Boitani L., editors. Wolves: Behavior, ecology, and conservation. The University of Chicago Press; Chicago, IL: 2003. pp. 35–65. [Google Scholar]
- Pal S.K. Parental care in free-ranging dos, Canis familiaris. Applied Animal Behaviour Science. 2005;90:31–47. [Google Scholar]
- Pang J.-F., Kluetsch C., Zou X.-J., Zhang A.-B., Luo L.-Y., Angleby H. mtDNA data indicate a single origin for dogs south of Yangtze River, less than 16,300 years ago, from numerous wolves. Molecular Biology and Evolution. 2009;26:2849–2864. doi: 10.1093/molbev/msp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.O., Ciucci P. The wolf as a carnivore. In: Mech L.D., Boitani L., editors. Wolves: Behavior, ecology, and conservation. The University of Chicago Press; Chicago, IL: 2003. pp. 104–130. [Google Scholar]
- Range F., Ritter C., Virányi Z. Testing the myth: tolerant dogs and aggressive wolves. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20150220. doi: 10.1098/rspb.2015.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F., Virányi Z. Wolves are better imitators of conspecifics than dogs. PLoS One. 2014;9:e86559. doi: 10.1371/journal.pone.0086559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F., Virányi Z. Tracking the evolutionary origins of dog-human cooperation: the “Canine Cooperation Hypothesis”. Frontiers in Psychology. 2015;5:1582. doi: 10.3389/fpsyg.2014.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M.J. Learning during exploration: the role of behavioural topography during exploration in determining subsequent adaptive behavior. International Journal of Comparative Psychology. 1988;2:43–56. [Google Scholar]
- Ryer C.H., Olla B.L. Information transfer and the facilitation and inhibition of feeding in a schooling fish. Environmental Biology of Fishes. 1991;3:317–323. [Google Scholar]
- Savolainen P., Zhang Y.P., Luo J., Lundeberg J., Leitner T. Genetic evidence for an East Asian origin of domestic dogs. Science. 2002;298:1610–1613. doi: 10.1126/science.1073906. [DOI] [PubMed] [Google Scholar]
- Schwagmeyer P.L. Searching today for tomorrow's mates. Animal Behaviour. 1995;50:759–767. [Google Scholar]
- Scott J.P., Fuller J.L. The University of Chicago Press; Chicago, IL: 1965. Genetics and the social behavior of the dog. [Google Scholar]
- Stöwe M., Bugnyar T., Heinrich B., Kotrschal K. Effects of group size on approach to novel objects in ravens (Corvus corax) Ethology. 2006;112:1079–1088. [Google Scholar]
- Stöwe M., Bugnyar T., Loretto M.C., Schloegl C., Range F., Kotrschal K. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behavioural Processes. 2006;73(1):68–75. doi: 10.1016/j.beproc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Swaney W., Kendal J., Capon H., Brown C., Laland K.N. Familiarity facilitates social learning of foraging behaviour in the guppy. Animal Behaviour. 2001;62:591–598. [Google Scholar]
- Thorne C. Feeding behaviour of domestic dogs and the role of experience. In: Serpell J., editor. The domestic dogs its evolution, behaviour and interactions with people. Cambridge University Press; Cambridge, U.K.: 1995. pp. 104–114. [Google Scholar]
- Valone T.J., Templeton J.J. Public information for the assessment of quality: a widespread social phenomenon. Philosophical Transactions of the Royal Society of London B. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi P., Bosellini H., Sabatini F., Mainardi M., Fiorito G. Behavioural analysis of social effects on problem-solving ability in the house mouse. Ethology. 2002;108:1115–1134. [Google Scholar]
- Visalberghi E., Fragaszy D. The behaviour of capuchin monkeys, Cebus paella, with novel food: the role of social context. Animal Behaviour. 1995;49:1089–1095. [Google Scholar]
- Visalberghi E., Addessi E. Seeing group members eating a familiar food enhances the acceptance of novel foods in capuchin monkeys. Animal Behaviour. 2000;60:69–76. doi: 10.1006/anbe.2000.1425. [DOI] [PubMed] [Google Scholar]
- Visalberghi E., Adessi E. Acceptance of novel foods in capuchin monkeys: do specific social facilitation and visual stimulus enhancement play a role? Animal Behaviour. 2001;62:567–576. [Google Scholar]