Abstract
Background
Elevated circulating concentrations of phosphate and fibroblast growth factor 23 (FGF23) contribute to the pathogenesis of cardiovascular disease in chronic kidney disease (CKD). Retinopathy is a common manifestation of microvascular disease in CKD, but its associations with phosphate and FGF23 have not been studied. We tested the hypothesis that higher serum phosphate is associated with more severe retinopathy in individuals with CKD, independent of FGF23 and known risk factors for retinopathy.
Methods
We tested the associations of serum phosphate and plasma FGF23 with retinopathy in a cross-sectional analysis of 1800 participants in the Chronic Renal Insufficiency Cohort Study who underwent fundus photography. Retinopathy severity was graded according to the Early Treatment of Diabetic Retinopathy Severity score, and retinal venous and arterial diameters were measured.
Results
Mean estimated glomerular filtration rate (eGFR) was 46.5 ± 15.4 mL/min/1.73 m2, mean serum phosphate was 3.7 ± 0.6 mg/dl and median plasma C-terminal FGF23 was 133 RU/mL (interquartile range 87.2, 217.8 RU/mL). In multivariable ordinal logistic regression models, higher serum phosphate was associated with greater retinopathy severity independent of hypertension, diabetes, CKD severity and FGF23 [adjusted odds ratio of being in one higher category of retinopathy severity: 1.19 per 1 standard deviation increase; 95% confidence interval (CI) 1.05, 1.36; P = 0.007]. Presence of diabetes or hypertension did not modify the results. Higher serum phosphate was also independently associated with greater retinal venous diameter (multivariable-adjusted 1.70 µm increase per 1 standard deviation increase in phosphate; 95% CI 0.46, 2.93; P = 0.007). FGF23 levels were not independently associated with retinopathy severity or retinal venous diameter, and neither FGF23 nor phosphate was associated with retinal arterial diameter.
Conclusions
Among individuals with moderate-to-severe CKD, higher serum phosphate but not FGF23 was independently associated with more severe retinopathy and microvascular retinal venous dilatation.
Keywords: CKD, FGF-23, phosphate, retinopathy, vascular disease
INTRODUCTION
Abnormalities of phosphate homeostasis contribute to the high burden of cardiovascular disease in patients with chronic kidney disease (CKD). Elevated circulating levels of phosphate and the phosphate-regulating hormone, fibroblast growth factor 23 (FGF23), are each independently associated with increased risk of cardiovascular events and mortality in patients with end-stage renal disease undergoing hemodialysis, those with earlier stages of CKD, and in the community [1–12]. Underlying these associations, higher serum phosphate concentrations contribute directly to the pathogenesis of medium and large arterial calcification, which increases risk of cardiovascular disease and death in CKD [8–11]. In contrast, elevated FGF23 levels are not consistently associated with arterial calcification but may increase the risk of cardiovascular events and death through direct effects on cardiac myocytes that culminate in left ventricular hypertrophy, atrial fibrillation and increased risk of congestive heart failure [11–16].
These results suggest that elevated phosphate and FGF23 levels may induce distinct and perhaps synergistic cardiovascular toxicities in patients with CKD, but few studies have directly compared the relationships of phosphate and FGF23 to vascular disease [17]. Furthermore, data on the effects of phosphate and FGF23 on microvascular disease are scarce. The retina is an ideal vascular bed to investigate microvascular disease in humans because it can be easily visualized in vivo using noninvasive techniques. Furthermore, given the similarities between the glomerular and retinal vasculature, understanding mechanisms of fundus pathology may provide novel insight into mechanisms of microvascular disease in the kidney [18]. Previously, we showed that higher serum phosphate was associated with coronary artery calcification in moderate-to-severe CKD, independently of FGF23 [11]. In this study, we tested the hypothesis that higher serum phosphate is similarly associated with more severe retinopathy and with retinal vascular disease in moderate-to-severe CKD, independent of FGF23 and established risk factors for retinopathy.
MATERIALS AND METHODS
Study population
We evaluated the associations of circulating levels of phosphate and FGF23 with retinopathy in individuals with CKD who participated in the Chronic Renal Insufficiency Cohort (CRIC) Study and its ancillary Retinopathy in CRIC Study (RCRIC). The CRIC study is a prospective observational cohort study that enrolled 3612 adults aged 21–74 years with an estimated glomerular filtration rate (eGFR) between 20 and 70 mL/min/1.73 m2 [19, 20]. Enrollment occurred between June 2003 and August 2008 at seven main clinical centers across the USA. Exclusion criteria included pregnancy, New York Heart Association class III–IV heart failure, cirrhosis, human immunodeficiency virus infection, myeloma, renal cancer, polycystic kidney disease, recent chemotherapy or immunosuppressive therapy, institutionalization, prior treatment with dialysis for at least 1 month, organ transplantation, enrollment in other studies or inability to consent.
A primary goal of the CRIC Study is to evaluate risk factors for cardiovascular disease in patients with moderate-to-severe CKD. The goals of the RCRIC ancillary study are to investigate risk factors for retinopathy and its association with CKD progression and cardiovascular disease [21]. All participants from six of the seven CRIC clinical centers were offered inclusion into the RCRIC ancillary study. Between 2006 and 2008, 1936 of 2605 (74%) participants agreed to undergo ocular photography. Of these 1936, 1820 (94%) had photographs that were of sufficient quality to support grading of retinopathy severity in at least one or both eyes [21–23]. The final population for the current study included the 1800 of these 1820 participants who also had blood samples available to measure serum phosphate and plasma FGF23. The study adhered to the Declaration of Helsinki, was approved by the institutional review boards of the participating institutions, and all participants provided written informed consent.
Retinal photography
Participants were seated for 5 minutes in a darkened room to induce physiologic dilatation of the pupils without use of pharmacologic mydriatic compounds. A set of two 45° digital color fundus photographs were taken from each eye by trained personnel using a Canon CR-DGI, Non-Mydriatic Retinal Camera (Canon Inc., Tokyo, Japan). One image was centered on the optic disc and the other was centered on the macula. Digital fundus photographs were mailed to the University of Pennsylvania's central RCRIC Fundus Photograph Reading Center where they were evaluated by a trained grader and a retinal specialist who were masked to all participant information. The graders assessed fundus pathology, and measured the diameter of the major retinal vessels in each photograph [21, 22].
Retinopathy score
The primary outcome of this study was the retinopathy severity score. The Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol was used to assess retinopathy severity score, as described previously for diabetic and non-diabetic populations [24]. Among individuals with assessable images in both eyes, retinopathy severity was graded in both, and the eye with the more severe score was assigned as participants' overall score. Among individuals with assessable images from only one eye, the score of that eye was assigned as participants' overall score. Retinopathy features that contributed to the severity scoring included photocoagulation scars, microaneurysm and retinal hemorrhage counts, retinal hemorrhage type (flame or blot), hard and soft exudates, intraretinal microvascular abnormality, neovascularization and fibrous proliferation. Participants' overall retinopathy scores were classified on an ordered categorical scale as none (score <14), mild non-proliferative retinopathy (14–20), moderate non-proliferative retinopathy (35–53) or proliferative retinopathy (≥60), as has been done previously [22, 23]. Intergrader and intragrader reliability for retinopathy scoring was assessed in 200 eyes of 100 participants. The weighted kappa for participants' ETDRS score was 0.80 (95% CI 0.69, 0.91) for intergrader agreement and 0.77 (95% CI 0.67, 0.88) for intragrader agreement, consistent with the standard of reproducibility reported by ETDRS [22].
Retinal vessel diameter
The secondary outcomes of the study were mean retinal arterial and venous diameters. Among the 1800 participants with retinopathy severity scores, 1599 also had measurements of retinal arterial and venous caliber, which was assessed by the Atherosclerotic Risk in Communities (ARIC) protocol [25]. Distance from the optic nerve was established by overlaying a grid centered on the optic disc, and vessels were measured within an annulus of 0.5–1 disc diameter from the edge of the disc [22]. Measuring at this distance from the disc reduces potential bias that could be introduced by the presence of optic nerve atrophy. Based on the vessels' sharpness and straightness, graders chose up to six major veins and six major arteries in each eye for measurement [22]. The individual measurements were combined into summary measures that reflected the average diameters of the veins and arteries of each eye, and the averages across both eyes served as individual participants' overall venous and arterial diameters, as has been done previously [25]. The intragrader and intergrader reliability for measuring retinal vessel caliber was assessed in 98 eyes of 50 participants. The intraclass correlation coefficient for intragrader agreement was 0.99 (95% CI 0.98, 0.99) for venous diameters and 0.96 (95% CI 0.93, 0.98) for arterial diameters. The intraclass correlation coefficient for intergrader agreement was 0.97 (95% CI 0.95, 0.98) for venous diameters and 0.89 (95% CI 0.80, 0.94) for arterial diameters [22, 23].
Exposures
The primary exposures were serum phosphate and plasma FGF23 levels, which were measured after a single thaw of samples that were collected and stored at the annual CRIC study visit most proximate to when fundus photography was performed. In 97% of CRIC participants, fasting samples were collected. The second-generation C-terminus assay (Immutopics, San Clemente, CA) was used to measure FGF23 in duplicate with a mean intra-assay coefficient of variation of <5%. Plasma parathyroid hormone (PTH) was measured using a total intact assay which captures the 1–84 and 7–84 PTH peptides with a mean coefficient of variation <5% (Scantibodies, Santee, CA). Hemoglobin A1C, total cholesterol, serum creatinine, albumin, calcium and phosphate, and urinary albumin and creatinine were measured using standard assays. All assays were performed by the CRIC Study central laboratory at the University of Pennsylvania [4]. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [26].
Statistical analysis
We used standard descriptive statistics to summarize and compare demographics and clinical characteristics of the study population according to ascending categories of retinopathy severity. To test the associations between phosphate, FGF23 and retinopathy severity, we used ordinal logistic regression, which estimated the odds of being in one higher category of retinopathy severity (none to mild to moderate to proliferative) per 1 standard deviation increase in phosphate or natural log-transformed FGF23 (transformed to approximate a normal distribution). We fit separate models for phosphate and FGF23, and hierarchically adjusted for demographics (age, sex, race, ethnicity), traditional retinopathy risk factors [presence of diabetes, presence of hypertension, history of cardiovascular disease, body mass index (BMI), smoking, hemoglobin A1C and systolic blood pressure], CKD-specific factors (eGFR, urinary albumin-to-creatinine ratio) and other laboratory covariates (parathyroid hormone, albumin and total cholesterol). In the final models, we further adjusted the full phosphate model for FGF23, and the full FGF23 model for phosphate, to test the independent associations of each. As a sensitivity analysis, we also adjusted for the time between the retinal assessments and when blood was collected for phosphate and FGF23 testing. For ease of interpretation, we repeated these analyses using quartiles of phosphate and FGF23 instead of their continuous measures, and tested the statistical significance of the linear trend across ascending phosphate and FGF23 quartiles in the full multivariable-adjusted ordinal logistic regression models.
We used linear regression to analyze the associations of phosphate and FGF23 with retinal vascular diameters as the outcome. Separate sets of models were fit for venous and arterial diameters. We identified factors that were significantly associated (P < 0.05) with retinal vascular diameter in univariable regression models and carried these factors forward into the multivariable models.
We assessed whether the associations of phosphate with retinopathy severity and retinal venous diameter were modified by sex, race, diabetes, hypertension, smoking, prior history of cardiovascular disease and eGFR by testing the significance of the interaction terms of phosphate with each of these candidate effect modifiers. All analyses were performed using Intercooled Stata 11. P values < 0.05 were considered statistically significant.
RESULTS
In the overall study population of 1800 participants, mean eGFR was 46.5 ± 15.4 mL/min/1.73 m2, mean serum phosphate was 3.7 ± 0.6 mg/dl, median FGF23 was 133 RU/mL (interquartile range 87.2, 217.8). Sixty-nine percent of participants (n = 1236) demonstrated no retinopathy, 8% (n = 141) demonstrated mild non-proliferative retinopathy, 13% (n = 240) demonstrated moderate non-proliferative retinopathy and 10% (n = 183) demonstrated proliferative retinopathy. Clinical and demographic characteristics of study participants are presented in Table 1 according to retinopathy severity. In addition to higher levels of phosphate and FGF23, other characteristics that were associated with greater retinopathy severity included black race, Hispanic ethnicity, hypertension, diabetes, lower eGFR, higher urinary albumin-to-creatinine ratio, higher levels of PTH and lower levels of serum albumin and calcium (Table 1). There were no differences in age or sex according to retinopathy severity. Participants with more severe retinopathy had larger retinal venous diameter, but retinal arterial diameter did not vary by retinopathy severity (Table 1).
Table 1.
Characteristics according to retinopathy severity
| No retinopathy (N = 1236) | Mild non-proliferative retinopathy (N = 141) | Moderate non-proliferative retinopathy (N = 240) | Proliferative retinopathy (N = 183) | Pa | |
|---|---|---|---|---|---|
| Age, years | 57.8 ± 11.0 | 57.2 ± 10.3 | 57.7 ± 10.9 | 56.1 ± 10.7 | 0.27 |
| Sex, % female | 47.1 | 40.4 | 40.8 | 40.4 | 0.09 |
| Race-ethnicity, % | |||||
| Non-Hispanic White | 53.2 | 36.9 | 32.1 | 31.7 | <0.001 |
| Non-Hispanic Black | 38.1 | 52.5 | 53.8 | 53.0 | |
| Hispanic | 4.4 | 2.1 | 8.3 | 10.9 | |
| Other | 4.3 | 8.5 | 5.8 | 4.4 | |
| Hypertension, % | 79.4 | 92.2 | 92.1 | 95.6 | <0.001 |
| Systolic BP, mmHg | 122.9 ± 19.0 | 129.5 ± 22.1 | 135.2 ± 23.0 | 138.4 ± 22.5 | <0.001 |
| Diastolic BP, mmHg | 71.5 ± 11.8 | 75.4 ± 14.3 | 71.1 ± 13.9 | 71.6 ± 14.4 | 0.004 |
| Diabetes mellitus, % | 27.6 | 42.6 | 86.7 | 93.4 | <0.001 |
| Hemoglobin A1c, % | 6.1 ± 1.1 | 6.5 ± 1.3 | 7.5 ± 1.7 | 7.9 ± 1.6 | <0.001 |
| Body mass index kg/m2 | 31.1 ± 7.3 | 32.9 ± 8.6 | 32.6 ± 7.8 | 32.5 ± 6.8 | 0.002 |
| Current smoking, % | 11.0 | 15.6 | 16.7 | 10.9 | 0.05 |
| History of CVD, % | 24.1 | 39.0 | 37.5 | 42.6 | <0.001 |
| Medicationsb | |||||
| ACE inhibitor or ARB, % | 64.6 | 68.6 | 77.7 | 78.1 | <0.001 |
| Diuretic, % | 49.9 | 60.7 | 73.5 | 72.1 | <0.001 |
| Beta blocker, % | 42.2 | 57.9 | 56.7 | 56.8 | <0.001 |
| Calcium channel blocker, % | 33.6 | 47.1 | 46.2 | 54.1 | <0.001 |
| Statin, % | 49.7 | 56.4 | 62.2 | 77.1 | <0.001 |
| Laboratory and renal parameters | |||||
| eGFR, mL/min/1.73m2c | 48.4 ± 15.5 | 45.5 ± 15.7 | 41.8 ± 14.1 | 40.5 ± 12.8 | <0.001 |
| Urinary albumin–creatinine ratio, mg/g | 17.3 (5.5–160.5) | 44.5 (9.5–341.9) | 190.8 (23.9–892.2) | 326.8 (61.7–1392.5) | <0.001 |
| Serum albumin, mg/dl | 4.1 ± 0.4 | 3.9 ± 0.4 | 3.8 ± 0.5 | 3.7 ± 0.5 | <0.001 |
| Serum calcium, mg/dl | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.0 ± 0.5 | <0.001 |
| PTH, pg/mL | 45.1 (31.7–72.5) | 58.7 (39.0–102.0) | 61.0 (38.0–106.0) | 70.0 (39.0–125.1) | <0.001 |
| Total cholesterol, mg/dl | 184.3 ± 39.4 | 179.5 ± 41.7 | 178.3 ± 47.0 | 172.1 ± 53.2 | 0.001 |
| Serum phosphate, mg/dl | 3.6 ± 0.6 | 3.7 ± 0.8 | 3.8 ± 0.7 | 4.0 ± 0.7 | <0.001 |
| Plasma FGF23, RU/mL | 118.7 (81.0–192.7) | 137.0 (94.4–220.2) | 172.7 (108.4–282.8) | 194.1 (119.5–315.2) | <0.001 |
| Retinal vascular diameters | |||||
| Venous diameter, µm | 218.2 ± 22.6 | 220.1 ± 23.3 | 226.8 ± 25.7 | 231.0 ± 32.6 | <0.001 |
| Arterial diameter, µm | 149.0 ± 14.4 | 148.6 ± 15.0 | 151.2 ± 14.2 | 145.3 ± 15.0 | 0.05 |
Results are reported as mean ± standard deviation, medians (interquartile range) or proportions.
BP, blood pressure; BMI, body mass index; CVD, cardiovascular disease; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23
aP for overall differences between groups.
bValues based on data available from 1792 of the total 1800 individuals.
cBy CKD-EPI equation.
Retinopathy score
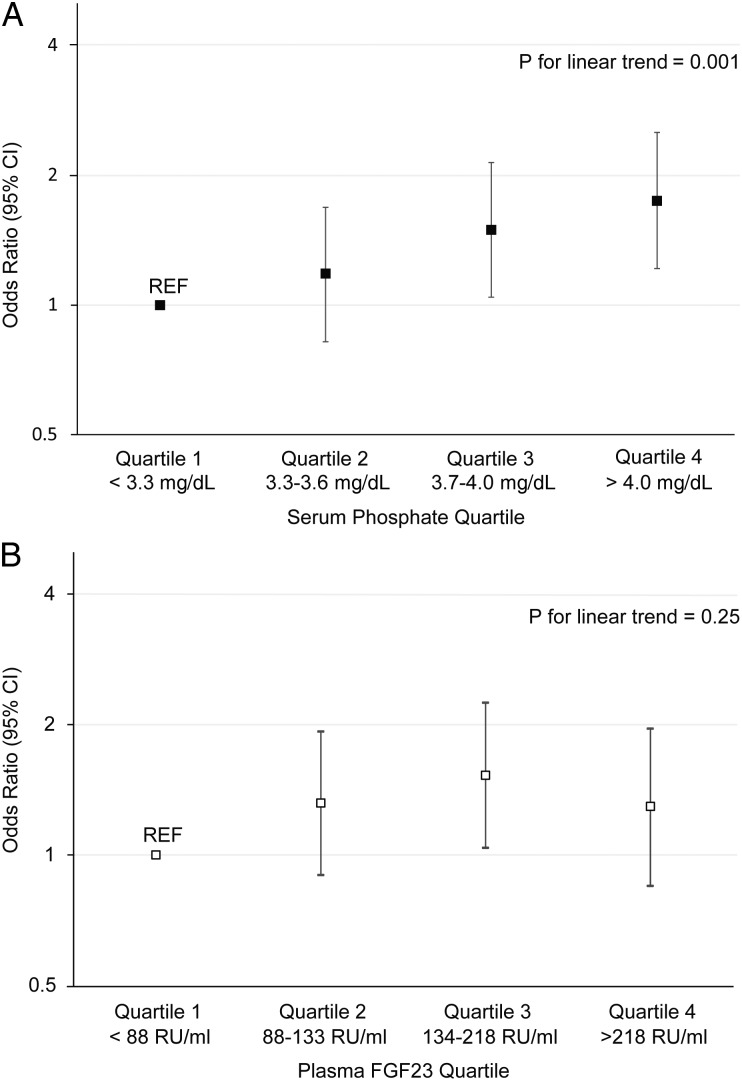
In univariable analysis, higher serum phosphate was associated with greater severity of retinopathy (Table 2). This association remained significant after adjustment for age, gender, race and ethnicity, and was only partially attenuated with further hierarchical adjustments for other known risk factors for retinopathy including the presence of diabetes and hypertension, history of cardiovascular disease, BMI, smoking, hemoglobin A1C and systolic blood pressure, and for eGFR, urinary albumin-to-creatinine ratio and other laboratory covariates. The association of serum phosphate with more severe retinopathy remained significant after adjusting for plasma FGF23 levels and when we further adjusted for the time between the retinal assessments and when serum was collected for phosphate testing (data not shown). The multivariable-adjusted odds ratio for greater retinopathy severity increased in a stepwise manner with ascending quartiles of serum phosphate (Figure 1A). There was no significant modification of the association of higher serum phosphate with more severe retinopathy across subgroups of sex, race, diabetes, hypertension, smoking, history of cardiovascular disease or eGFR (P for interactions all >0.3).
Table 2.
Unadjusted and adjusted associations of serum phosphate and plasma FGF23 levels with retinopathy severity
| Hierarchical models | Phosphate odds ratio (95% CI) | P | FGF23 odds ratio (95% CI) | P |
|---|---|---|---|---|
| Univariable | 1.59 (1.43, 1.76) | <0.001 | 1.55 (1.40, 1.71) | <0.001 |
| + age, gender, race | 1.57 (1.42, 1.74) | <0.001 | 1.53 (1.38, 1.69) | <0.001 |
| + CVD, DM, A1c, HTN, SBP, BMI, smoking | 1.27 (1.13, 1.43) | <0.001 | 1.28 (1.14, 1.43) | <0.001 |
| + eGFR, ACR | 1.15 (1.02, 1.30) | 0.024 | 1.08 (0.95, 1.23) | 0.22 |
| + PTH, albumin, TC | 1.20 (1.05, 1.36) | 0.006 | 1.04 (0.92, 1.19) | 0.53 |
| + FGF23 or phosphate | 1.19 (1.05, 1.36) | 0.007 | 1.01 (0.89, 1.16) | 0.83 |
Results are reported as odds ratios per 1 standard deviation increase in serum phosphate and natural log-transformed FGF23.
CVD, cardiovascular disease; DM, diabetes mellitus; A1c; hemoglobin A1c; HTN, hypertension; SBP, systolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; ACR, albumin/creatinine ratio; PTH, parathyroid hormone; TC, total cholesterol; FGF23, fibroblast growth factor 23; CI, confidence interval.
FIGURE 1:
Multivariable-adjusted odds ratio for greater retinopathy severity. (A) Quartiles of serum phosphate; (B) Quartiles of plasma FGF23. For each analysis, quartile 1 served as the referent group (REF). P values represent the tests of linear trend of the ascending quartiles in the full multivariable-adjusted ordinal logistic regression models.
Plasma FGF23 levels were associated with greater severity of retinopathy on univariable analysis, but the effect was attenuated after adjusting for known retinopathy risk factors and for eGFR and urinary albumin-to-creatinine ratio (Table 2). The results were unchanged when further adjusted for serum phosphate. Similarly, higher FGF23 quartiles were not independently associated with more severe retinopathy (Figure 1B).
Retinal vessel diameter
In univariable analysis, higher serum phosphate was associated with a graded increase in retinal venous diameter. After adjusting for other associated factors, higher serum phosphate remained independently associated with greater retinal venous diameter (Table 3). Forcing FGF23 into the multivariable model did not alter the results (Table 3). The association of higher serum phosphate with greater retinal venous diameter was unchanged when we further adjusted for the time between the retinal assessments and when blood was collected for phosphate testing (data not shown), and did not differ across subgroups of sex, race, diabetes, hypertension, smoking or prior history of cardiovascular disease (P for interactions all >0.3). FGF23 levels were not associated with retinal venous diameter in any analysis (Table 3), and neither FGF23 nor phosphate was associated with retinal arterial diameter (data not shown).
Table 3.
Unadjusted and adjusted associations of demographic and clinical factors associated with mean retinal venous diameter
| Univariable β (95% CI) | P | Multivariable β (95% CI) | P | Multivariable including FGF23 β (95% CI) | P | |
|---|---|---|---|---|---|---|
| Age, per 10 year increase | −4.49 (−5.56, −3.43) | <0.001 | −3.63 (−4.81, −2.45) | <0.001 | −3.63 (−4.81, −2.54) | <0.001 |
| Sex, female versus male | 0.10 (−2.29, 2.49) | 0.94 | — | — | ||
| Race-ethnicity | ||||||
| Non-Hispanic white | Reference | Reference | Reference | |||
| Non-Hispanic black | 15.74 (13.38, 18.11) | <0.001 | 14.63 (12.21, 17.06) | <0.001 | 14.63 (12.21, 17.06) | <0.001 |
| Hispanic | 16.87 (11.47, 22.28) | <0.001 | 12.61 (7.11, 18.11) | <0.001 | 12.59 (7.09, 18.10) | <0.001 |
| Other | 13.28 (7.90, 18.65) | <0.001 | 10.92 (5.46, 16.37) | <0.001 | 10.90 (5.44, 16.36) | <0.001 |
| Diabetes mellitus, present versus absent | 4.86 (2.42, 7.30) | <0.001 | 3.65 (1.15, 6.14) | 0.004 | 3.66 (1.16, 6.15) | 0.004 |
| SBP, per 10 mmHg | 0.20 (−0.38, 0.78) | 0.50 | — | — | ||
| eGFR, per 10 mL/min/1.73m2 increase | 0.81 (0.04, 1.57) | 0.04 | 1.11 (0.25, 1.97) | 0.012 | 1.08 (0.18, 1.98) | 0.019 |
| Ln ACR, per 1 SD increase | 2.78 (1.56, 4.00) | <0.001 | 0.68 (−0.65, 2.01) | 0.316 | 0.71 (−0.64, 2.07) | 0.303 |
| Calcium, per 1 SD increase | −0.92 (−2.13, 0.28) | 0.13 | — | — | ||
| Phosphate, per 1 SD increase | 2.82 (1.61, 4.04) | <0.001 | 1.70 (0.46, 2.93) | 0.007 | 1.72 (0.47, 2.97) | 0.007 |
| Ln FGF23, per 1 SD increase | 0.52 (−0.66, 1.70) | 0.39 | — | −0.16 (−1.45, 1.13) | 0.809 | |
| Ln PTH, per 1 SD increase | 0.23 (−0.99, 1.45) | 0.71 | — | — | ||
Results (β coefficients) refer to the mean µm increase in venous diameter. Factors that were significant in univariable analyses were included in the multivariable model.
SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; ACR, albumin/creatinine ratio; FGF23, fibroblast growth factor 23, PTH, parathyroid hormone; SD, standard deviation; CI, confidence interval; Ln, natural log; vs, versus. Phosphate and Ln FGF23 are bolded as they are the primary exposures in the current study.
DISCUSSION
Retinopathy is a common end-organ microvascular complication of CKD [21, 22] that is associated with more rapid progression of CKD and higher risk of cardiovascular events [27–31]. In a large cohort of individuals with moderate-to-severe CKD, we observed that higher serum phosphate was associated with more severe retinopathy independent of traditional risk factors for retinopathy, including diabetes and hypertension. Corroborating our primary findings, higher serum phosphate, along with other classic cardiovascular risk factors, was also independently associated with greater retinal venous diameter, which itself is linked to higher risk of cardiovascular events [32–34]. In each of these concordant analyses, the associations of phosphate with retinopathy were independent of FGF23, which was not associated with retinopathy or retinal venous diameter. In aggregate, these findings suggest that the retina may be a novel end organ that is susceptible to injury from higher serum phosphate concentrations. More broadly, these results offer new evidence in support of a role of phosphate excess in the pathogenesis of microvascular complications of CKD independent of FGF23.
Although serum phosphate levels were within the normal range in the majority of participants in the CRIC Study, we observed a monotonic, linear increase in retinopathy severity across the range of serum phosphate levels. This finding is consistent with previous reports of strong independent associations between higher serum phosphate, within the normal range and vascular and valvular calcification, cardiovascular events and death [9, 35, 36]. Higher phosphate concentrations can induce endothelial dysfunction, osteochondrogenic transformation of vascular smooth muscle cells and arterial calcification that contribute to a pattern of accelerated vascular aging [37–41]. It is possible that similar mechanisms also promote ocular pathology in CKD by inducing retinal vascular injury. It is, however, important to acknowledge that the definition of retinopathy included several pathological components, some of which are less likely to be plausibly related to higher serum phosphate. Although it is possible that the direct toxic effects of phosphate on retinal endothelial cells that promote capillary leak, ischemia and release of vasoactive cytokines could contribute to formation of microaneurysms, hemorrhages and other vascular changes of retinopathy, further studies are needed to investigate these possibilities [42].
Most prior studies that investigated the relationship between phosphate and cardiovascular disease focused on the arterial system. Thus, an especially novel finding of the current study is that higher serum phosphate was independently associated with greater retinal venous diameter. These results suggest that phosphate-associated vascular toxicity may extend beyond the arterial system to perhaps also include the venous vasculature. Ocular venous toxicity of phosphate is indirectly supported by a previous report that linked higher serum phosphate to significantly increased incidence of retinal vein occlusion [43], and by reports of eye disease in familial tumoral calcinosis, a rare disease in which deficiency of biologically active FGF23 or α-klotho results in hyperphosphatemia due to impaired renal phosphate excretion [44, 45]. Collectively, these data raise the possibility that certain aspects of the vascular toxicity of phosphate occur independently of pressure and shear, which are much lower in veins [46], and that phosphate may specifically injure endothelial cells that are common to arteries and veins. This could result in not only pathological structural changes that are characteristic of retinopathy, but also microcirculatory changes that culminate in retinal venous dilatation. Alternatively, retinal venous dilatation may be an indirect consequence of phosphate-induced arterial toxicity that results in retinal ischemia and subsequent release of vasodilatory cytokines such as nitric oxide, interleukin 1 and tumor necrosis factor α, that induce secondary venous dilatation [47]. Factors such as optic atrophy, venous congestion and vascular wall thinning are other possible alternative mechanisms of retinal venous dilatation. Additional research is needed to investigate these possibilities.
In contrast to serum phosphate, the level of FGF23 was not independently associated with retinopathy severity or retinal venous caliber. FGF23 regulates serum phosphate by stimulating urinary phosphate excretion and modulating renal production and degradation of 1,25-dihydroxyvitamin D [15]. As glomerular filtration of phosphate gradually declines with CKD progression, rising FGF23 levels maintain normal serum phosphate levels. However, chronic elevation of FGF23 may be ultimately maladaptive as shown by its association with increased risk for left ventricular hypertrophy, congestive heart failure and death [3, 12–15]. In contrast, higher FGF23 levels have not been consistently associated with increased risk of atherosclerotic events or coronary artery calcification, and, unlike phosphate, in vitro studies confirmed no direct pro-calcification effects of FGF23 on vascular smooth muscle cells or explanted aortic rings [11, 48]. This suggests distinct pathways whereby phosphate promotes cardiovascular disease through vascular toxicity whereas FGF23 contributes via cardiac toxicity [17]. While preclinical data suggest that FGF23 and phosphate may each have adverse effects on microcirculatory function [49, 50], the current study provides further support for the emerging concept of distinct cardiovascular toxicities of phosphate and FGF23.
The large sample size, detailed covariate data, rigorous adjudication of retinopathy severity and novel results are strengths of the current study. We also acknowledge certain limitations. The study design precludes us from determining whether higher serum phosphate predates or follows the development of retinopathy. However, given the lack of prior studies to investigate disordered phosphate homeostasis and microvascular disease of the eye, the current results provide the impetus to extend this line of investigation into prospective studies with repeated, longitudinal evaluations of the fundus. Single measurements of serum creatinine, phosphate and FGF23, and single sets of fundus photographs, occasionally with only one evaluable eye, may have led to misclassification of the severity of retinal disease, the severity of CKD or its associated alterations in mineral metabolites. However, if such misclassification was random, as expected, it would have biased our results towards the null hypothesis of no associations. Another potential limitation is that although retinopathy is a leading cause of vision loss in diabetes, the clinical implications of the quantitative measurements of retinal arterial and venous caliber are currently less clear. Finally, although we adjusted for a wide range of potentially confounding clinical covariates, since we were unable to adjust for vitamin D levels, which have been associated with retinopathy [51], we cannot exclude the possibility of residual confounding.
In conclusion, higher serum phosphate but not FGF23 was independently associated with more severe retinopathy and greater retinal venous diameter independent of diabetes, hypertension and other cardiovascular risk factors. Since phosphate excess is also associated with cardiovascular events and accelerated progression of CKD, perhaps it activates common mechanisms of microvascular injury in the kidney, retina and elsewhere. Additional human studies are needed to validate our results in both CKD and non-CKD populations, and if confirmed, further investigation of potential molecular mechanisms of phosphate-associated retinopathy and retinal venous disease should be pursued. Given the likelihood of shared mechanistic pathways underlying microvascular disease in different organs, such studies could yield novel insights into vascular pathobiology and mechanisms of CKD progression.
CONFLICT OF INTEREST STATEMENT
T.I. has received honoraria from Bayer. M.W. has received research support, honoraria or consultant fees from Amgen, Bayer, Genzyme, Keryx, Luitpold, Opko, Pfizer and Shire. The results presented in this paper have not been published previously in whole or part, except in abstract form.
ACKNOWLEDGEMENTS
CRIC Study Investigators include: Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Jiang He, MD, PhD, Mahboob Rahman, MD, Raymond R. Townsend, MD.
This study was supported by grants R01DK081374 (M.W.), K24DK093723 (M.W.), K23DK081673 (T.I.) and DK074151 (J.G.) from the National Institutes of Health. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963 and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award, NIH/NCATS UL1TR000003; Johns Hopkins University, UL1 TR-000424; University of Maryland, GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433; University of Illinois at Chicago, CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology, P30GM103337; Kaiser Permanente NIH/NCRR UCSF-CTSI, UL1 RR-024131, Vivian S. Lasko Research Fund; Nina C. Mackall Trust and Research to Prevent Blindness.
Contributor Information
Collaborators: the CRIC Study Investigators, Lawrence J. Appel, Harold I. Feldman, Jiang He, Mahboob Rahman, and Raymond R. Townsend
REFERENCES
- 1.Gutierrez OM, Mannstadt M, Isakova T et al. . Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Sachs MC, Hoofnagle AN et al. . Fibroblast growth factor-23 and cardiovascular disease in the general population: the multi-ethnic study of atherosclerosis. Circ Heart Fail 2014; 7: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ix JH, Katz R, Kestenbaum BR et al. . Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 2012; 60: 200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W et al. . Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnlov J, Carlsson AC, Sundstrom J et al. . Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 2013; 83: 160–166 [DOI] [PubMed] [Google Scholar]
- 6.Kendrick J, Cheung AK, Kaufman JS et al. . FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker BD, Schurgers LJ, Brandenburg VM et al. . The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 2010; 152: 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hage FG, Venkataraman R, Zoghbi GJ et al. . The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol 2009; 53: 2129–2140 [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M et al. . Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112: 2627–2633 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW et al. . Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 11.Scialla JJ, Lau WL, Reilly MP et al. . Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 2013; 83: 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scialla JJ, Xie H, Rahman M et al. . Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 2014; 25: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faul C, Amaral AP, Oskouei B et al. . FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew JS, Sachs MC, Katz R et al. . Fibroblast growth factor-23 and incident atrial fibrillation: the multi-ethnic study of atherosclerosis (MESA) and the cardiovascular health study (CHS). Circulation 2014; 130: 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez OM, Januzzi JL, Isakova T et al. . Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014; 10: 268–278 [DOI] [PubMed] [Google Scholar]
- 18.Wong CW, Wong TY, Cheng CY et al. . Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int 2014; 85: 1290–1302 [DOI] [PubMed] [Google Scholar]
- 19.Feldman HI. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol 2003; 14: 148S–1153 [DOI] [PubMed] [Google Scholar]
- 20.Lash JP, Go AS, Appel LJ et al. . Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009; 4: 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunwald JE, Alexander J, Maguire M et al. . Prevalence of ocular fundus pathology in patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunwald JE, Alexander J, Ying GS et al. . Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch Ophthalmol 2012; 130: 1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunwald JE, Ying GS, Maguire M et al. . Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC] Study). Am J Cardiol 2012; 110: 246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98: 786–806 [PubMed] [Google Scholar]
- 25.Hubbard LD, Brothers RJ, King WN et al. . Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999; 106: 2269–2280 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deva R, Alias MA, Colville D et al. . Vision-threatening retinal abnormalities in chronic kidney disease stages 3 to 5. Clin J Am Soc Nephrol 2011; 6: 1866–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricardo AC, Grunwald JE, Parvathaneni S et al. . Retinopathy and CKD as predictors of all-cause and cardiovascular mortality: National Health and Nutrition Examination Survey (NHANES) 1988–1994. Am J Kidney Dis 2014; 64: 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunwald JE, Pistilli M, Ying GS et al. . Retinopathy and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 2014; 9: 1217–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards MS, Wilson DB, Craven TE et al. . Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis 2005; 46: 214–224 [DOI] [PubMed] [Google Scholar]
- 31.Wong TY, Coresh J, Klein R et al. . Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 2004; 15: 2469–2476 [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Moss SE et al. . Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 2007; 114: 1884–1892 [DOI] [PubMed] [Google Scholar]
- 33.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol 2011; 129: 8–15 [DOI] [PubMed] [Google Scholar]
- 34.Wong TY, Kamineni A, Klein R et al. . Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med 2006; 166: 2388–2394 [DOI] [PubMed] [Google Scholar]
- 35.Dhingra R, Gona P, Benjamin EJ et al. . Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail 2010; 12: 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhingra R, Sullivan LM, Fox CS et al. . Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167: 879–885 [DOI] [PubMed] [Google Scholar]
- 37.Jono S, McKee MD, Murry CE et al. . Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000; 87: E10–E17 [DOI] [PubMed] [Google Scholar]
- 38.Shroff RC, McNair R, Skepper JN et al. . Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanahan CM, Crouthamel MH, Kapustin A et al. . Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011; 109: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009; 75: 890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuro OM. A phosphate-centric paradigm for pathophysiology and therapy of chronic kidney disease. Kidney Int Suppl (2011) 2013; 3: 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chew EY. Diabetes Mellitus: A Fundamental and Clinical Text. 3rd edn Philadelphia, PA: JB Lippincott Co [Google Scholar]
- 43.Klein R, Moss SE, Meuer SM et al. . The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol 2008; 126: 513–518 [DOI] [PubMed] [Google Scholar]
- 44.Ghanchi F, Ramsay A, Coupland S et al. . Ocular tumoral calcinosis. A clinicopathologic study. Arch Ophthalmol 1996; 114: 341–345 [DOI] [PubMed] [Google Scholar]
- 45.McGrath E, Harney F, Kinsella F. An ocular presentation of familial tumoral calcinosis. BMJ Case Rep 2010; doi:10.1136/bcr.05.2010.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schubert A, Cattaruzza M, Hecker M et al. . Shear stress-dependent regulation of the human beta-tubulin folding cofactor D gene. Circ Res 2000; 87: 1188–1194 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol Metab 2006; 17: 262–268 [DOI] [PubMed] [Google Scholar]
- 48.Garimella PS, Ix JH, Katz R et al. . Fibroblast growth factor 23, the ankle-brachial index, and incident peripheral artery disease in the Cardiovascular Health Study. Atherosclerosis 2014; 233: 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silswal N, Touchberry CD, Daniel DR et al. . FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 2014; 307: E426–E436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van TV, Watari E, Taketani Y et al. . Dietary phosphate restriction ameliorates endothelial dysfunction in adenine-induced kidney disease rats. J Clin Biochem Nutr 2012; 51: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patrick PA, Visintainer PF, Shi Q et al. . Vitamin D and retinopathy in adults with diabetes mellitus. Arch Ophthalmol 2012; 130: 756–760 [DOI] [PubMed] [Google Scholar]