Abstract
Single ryanodine receptor (RyR) Ca2+ flux amplitude (iCa-RyR) decreases as intra- sarcoplasmic reticulum (SR) Ca2+ levels fall during a cardiac Ca2+ spark. Since iCa-RyR drives the inter-RyR Ca2+-induced Ca2+ release (CICR) that underlies the spark, decreasing iCa-RyR may contribute to spark termination because RyRs that spontaneously close may stay closed. To test this possibility, we simultaneously measured local cytosolic and intra-SR ([Ca2+]cyto & [Ca2+]SR) during Ca2+ sparks in permeabilized rabbit ventricular myocytes. Local cytosolic or intra-SR Ca2+ dynamics were manipulated using Ca2+ buffers. Buffer manipulations applied in cells had no effect on individual RyR channels reconstituted in planar lipid bilayers. Presence of a fast cytosolic Ca2+ buffer (BAPTA) significantly suppressed Ca2+ spark activity and sparks terminated earlier at a higher than usual [Ca2+]SR level (~80% vs. ~62%). When cytosolic Ca2+ buffer power was reduced (i.e. cytosolic EGTA level decreased), sparks terminated later and at a lower than usual [Ca2+]SR level (~45% vs. ~62%). When intra-SR Ca2+ buffer power was increased, sparks also terminated later and at a lower than usual [Ca2+]SR (~48% vs. ~62%). These results suggest that cytosolic local control of inter-RyR CICR by iCa-RyR plays a substantial role during the spark termination process. Thus, alterations in local cytosolic Ca2+ handling dynamics in the dyadic cleft (Ca2+ buffering, extrusion, etc.) likely influence Ca2+ spark termination.
Keywords: Heart, Ca2+ spark, ryanodine receptor, Ca2+-induced Ca2+ release, confocal microscopy
Graphical Abstract
1. INTRODUCTION
In adult ventricular myocytes, the majority of Ca2+ that drives contraction is released from the sarcoplasmic reticulum (SR) via ryanodine receptor (RyR) Ca2+ release channels. Most RyRs release Ca2+ into subcellular microdomains called dyads. At the dyad, RyRs on the SR are arranged in organized clusters [1-3]. L-type Ca2+ channels (LTCCs) on the T-tubule membrane are closely associated with some of the RyRs in the cluster [2]. During an action potential (AP), a small LTCC-mediated Ca2+ influx activates one or more RyRs in the cluster via the process of Ca2+-induced Ca2+ release (CICR) [4]. Once a RyR opens, the Ca2+ released into the dyadic cleft drives inter-RyR CICR, which amplifies the AP-initiated Ca2+ release event. Individual RyRs can also spontaneously open during diastole. These stochastic events may also drive inter-RyR CICR and generate a local non- propagating release event, called a Ca2+ spark [5].
CICR is an inherently self-regenerating process. Once initiated it is expected to continue until intra-SR Ca2+ ([Ca2+]SR) is fully exhausted. However, CICR ends when [Ca2+]SR is only partially depleted [6-8]. This indicates that the inherent positive feedback of CICR is countered by some sort of negative control or termination process. Several CICR termination mechanisms have been proposed (including stochastic attrition, Ca2+-dependent inactivation, adaptation [9-12]), but all of these have either been ruled out or deemed insufficient.
The [Ca2+]SR at which sparks terminate ([Ca2+]SR termination threshold) has been defined by simultaneous recording of sparks and their corresponding blinks (i.e. local drop in local [Ca2+]SR) [6;8;13]. It is commonly thought that local CICR terminates at the [Ca2+]SR termination threshold because Ca2+ unbinds from intra-SR RyR regulatory site(s) and promotes RyR closing [13;14]. This possibility is consistent with a large body of experimental evidence [15-20]. However, falling [Ca2+]SR also reduces the trans-SR Ca2+ driving force and consequently unitary RyR Ca2+ flux (iCa-RyR). Since iCa-RyR drives the inter-RyR CICR within a cluster underlying the spark, the likelihood of inter-RyR CICR decreases as [Ca2+]SR and iCa-RyR falls. At some point, iCa-RyR could become insufficient to drive inter-RyR CICR, RyRs that spontaneously close will remain closed, and this could also contribute to spark termination. Although this mechanism has been embedded into several CICR termination models [21-26], the contribution of falling iCa-RyR in CICR termination has been largely unexplored experimentally. The main limitation is the inability to properly manipulate iCa-RyR and [Ca2+]SR in the cell environment.
Guo et al [27] recently used large RyR permeable cations to differentially manipulate iCa-RyR independent of [Ca2+]SR. They reported that iCa-RyR has a substantial role in Ca2+ spark initiation and termination. Subsequently, the iCa-RyR based termination process has been termed pernicious attrition [28] or alternatively induction decay [29]. It is important to note that existence of iCa-RyR based termination does not mean that other termination mechanisms do not exist. Instead, iCa-RyR based termination is likely one of several termination mechanisms that operate in concert.
Here, we examined how experimentally manipulating iCa-RyR, by applying various Ca2+ buffers influences the [Ca2+]SR termination threshold in permeabilized rabbit ventricular myocytes. The results provide new experimental evidence that iCa-RyR based termination process plays a substantial role in Ca2+ spark local control.
2. METHODS
2.1. Myocyte Isolation
Experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of Loyola University which comply with national regulations. Briefly, ventricular myocytes were isolated from hearts of anaesthetized (sodium pentobarbital; 50 mg/kg I.V.) New Zealand white rabbits (2 kg) following a procedure described previously [30]. Chemicals and reagents were purchased from “Sigma-Aldrich” (St. Louis, MO) unless otherwise stated and experimentation done at room temperature (20-24°C).
2.2. Intracellular Ca2+ Imaging in Permeabilized Myocytes
Sparks (local [Ca2+]cyto changes) were measured using either Flou-4 or Rhod-2 (Molecular Probes/Invitrogen, Carlsbad, CA) as high affinity Ca2+ indicators. Blinks (local [Ca2+]SR changes) were monitored using the low affinity Ca2+ indicator Fluo-5N (Molecular Probes/Invitrogen). The sarcolemma of acutely dissociated ventricular myocytes was permeabilized with saponin as described previously [31]. For blink studies, cardiomyocytes were incubated with Fluo-5N/AM before permeabilization in conditions that promote dye accumulation within the SR [8;30]. Permeabilized myocytes were bathed in an internal solution composed of (in mM): K aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.35; CaCl2 0.12; MgCl2 0.75; phosphocreatine 10; HEPES 10; Rhod-2 tripotassium salt 0.04 (or Fluo-4 pentapotassium salt 0.04 mM; when Ca2+ sparks were measured alone); creatine phosphokinase 5 U/mL; dextran (MW: 40,000) 8%, and pH 7.2 (KOH). Free [Ca2+] and [Mg2+] in this solution were always 150 mM and 1 mM (respectively). To manipulate cytosolic Ca2+ buffering, either BAPTA was added to this internal solution or the internal solution's [EGTA] was reduced. To alter intra-SR Ca2+ buffering, permeabilized cells were incubated for 10 minutes with 5 mM of acetamidoiminodiacetic acid (ADA; Kd for Ca2+ ~ 0.2 mM). After ADA incubation, cytosolic ADA was washed out with ADA-free internal solution. The slow diffusion of ADA out of the SR provided a 10-15 min time period during which sparks/blinks could be measured at greater than normal intra-SR Ca2+ buffer capacity [31;32].
Changes in [Ca2+]cyto and [Ca2+]SR were measured simultaneously using a laser scanning confocal microscope (Radiance 2000 MP, Bio-Rad, UK) equipped with a 40× oil-immersion objective (N.A.=1.3). Fluo-5N was excited at 488 nm and its emitted fluorescence recorded at 515±15 nm. Rhod-2 was excited at 543 nm and its emitted fluorescence recorded at wavelengths >600 nm. Images were acquired in linescan mode (3 ms per scan; pixel size 0.12 μm). Sparks were detected and analyzed using SparkMaster [33]. Maximum SR Ca2+ release flux during a spark was calculated from the peak of the first derivative of the cytosolic fluorescence intensity and expressed as d(ΔF/F0)/ms [31]. Sparks and blink parameters were defined as previously described [8]. The Fluo-5N signal was corrected for its Ca2+-independent component as assessed by completely depleting SR of Ca2+ using 10 mM caffeine [34].
2.3. Single RyR channel recordings
Regulation of cardiac intracellular Ca2+ homeostasis is species-specific [35], but single mammalian cardiac RyR function is not [36]. As single cardiac RyR function in bilayers is species-independent, heavy SR microsomes were prepared from rat ventricular muscle using a standard cellular sub-fractionation process [37]. Planar lipid bilayers were formed across an 100 μm diameter hole in a 12 μm thick Teflon partition. This partition separated two 1 ml compartments. One (cis) was virtually grounded and initially filled with a HEPES-Tris solution (250 mM HEPES, 120 mM Tris, pH 7.4). This chamber was always facing the cytosolic side of the channel. The other (trans) chamber was initially filled with HEPES-Ca solution (250 mM HEPES, 53 mM Ca(OH)2, pH 7.4) and faced the luminal side of the channel. Exposure to this high luminal [Ca] assured that no calsequestrin (Casq) was associated with the channels tested here [38].
Planar bilayers were formed from a 5:4:1 mixture (50 mg/ml total lipids in decane) of bovine brain phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine. Then, 500 mM CsCl and heavy SR microsomes were added to the cis chamber. Once ion channels were incorporated into the bilayer, solutions in the cis and trans chambers were replaced by cell-like recording solutions. The trans (luminal) cell-like recording solution contained 1 mM free Ca2+, 200 mM Cs-Methansulfonate and 10 mM Hepes (pH 7.4). The cis (cytosolic) cell-like recording solution contained 10 μM free Ca2+, 0.5 mM EGTA, 10 mM HEPES (pH 7.4), 200 mM Cs-Methansulfonate and MgATP (1 mM free Mg2+, 5 mM total ATP). For some studies, BAPTA was added to the cis recording solution as well as sufficient CaCl2 to keep the free Ca2+ concentration constant. Free [Ca2+] was verified using a Ca-selective electrode. Single RyR channel recordings were sampled at 100 μs/pt and filtered (8-pole Bessel) at 1 kHz. Single channel analysis was done using pCLAMP9 software (Axon Instruments/Molecular Devices, Sunnyvale, CA). Single channel recordings were idealized using the half-amplitude threshold method. Unit current, mean open time, mean closed times and open probability (Po) were determined from idealized traces.
2.4. Statistics
Data are presented as mean ± SEM of n measured cells, Ca2+ release events or channels. Statistical comparisons between groups were performed by the Student's t test. Differences were considered statistically significant at P<0.05.
3. RESULTS
3.1. Action of Fast Cytosolic Ca2+ Buffering
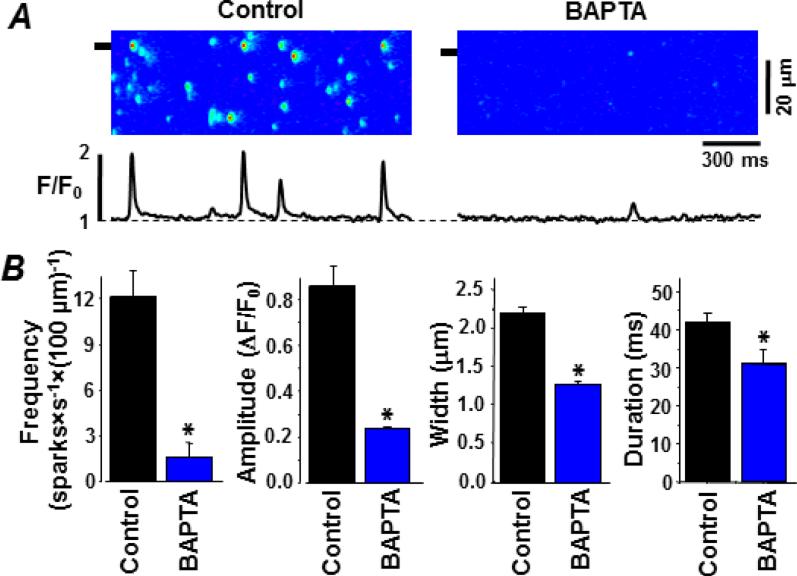
Figure 1A shows representative line-scan Fluo-4 images of sparks recorded in permeabilized myocytes before (control) and after cytosolic BAPTA (0.7 mM) was added. In both conditions, cytosolic EGTA (0.35 mM) was present and the free [Ca2+]cyto was 150 nM. In the control condition, sparks occurred at a stable frequency of 12.0±2.1 sparks×s−1×(100 μm)−1 (Fig. 1B; n=8 cells). With the cytosolic BAPTA present, spark frequency was significantly lower (>7 fold) at 1.6±1.2 sparks×s−1×(100 μm)−1. The added cytosolic BAPTA also significantly reduced spark amplitude, width and duration (Fig. 1B). Moreover, BAPTA decreased the Time-to-Peak of Ca2+ sparks by 68%: from 36.3±0.6 ms (n=208 events) in control conditions to 11.6±0.9 ms (P<0.05; n=52 events) in the presence of BAPTA. These changes in spark properties persisted as long as the cytosolic BAPTA was present (~5 min) and were fully reversed when the cytosolic BAPTA was removed. The substantial spark attenuation by 0.7 mM cytosolic BAPTA may impose a methodological limitation, the possible failure to detect the small sparks. Thus, subsequent studies were done using a lower cytosolic BAPTA concentration.
Figure 1. Fast cytosolic buffering attenuates sparks in permeabilized ventricular myocytes.
Sparks were detected as local changes in cytosolic Fluo-4 fluorescence. A. Representative sparks in the control and BAPTA-containing cytosolic solutions. The control cytosolic solution had 0.36 mM EGTA and a free [Ca2+] of 150 nM. The BAPTA cytosolic solution had 0.36 mM EGTA, 0.7 mM BAPTA and a free [Ca2+] of 150 nM. Line scan images are shown at top. Shown at the bottom is fluo-4 fluorescence profile at the point marked on the line scan image (see left margin). B. Summary results of various measured spark parameters in the two tested conditions.
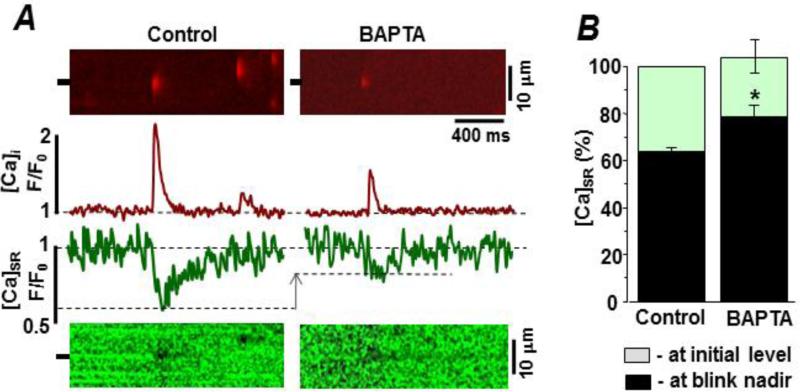
Measurement of spark-blink pairs provides information about the local rise in [Ca2+]cyto and the corresponding local decline in intra-SR [Ca2+]SR, respectively. The latter does not depend on the fate of the released Ca2+ (e.g. diffuse out of the permeabilized cell, bind to cytosolic proteins, bind to cytosolic buffers, etc). Figure 2A shows simultaneously measured Rhod-2 (top) and Fluo-5N (bottom) line-scan images of spark-blink pairs before (control) and after addition of cytosolic BAPTA (0.36 mM). Cytosolic free [Ca2+] and [EGTA] was the same (150 nM & 0.35 mM, respectively) in both test conditions. With the cytosolic BAPTA present, spark frequency was 5.9±1.1 sparks×s−1×(100 μm)−1 (n=10 cells). Also, the blink nadir occurred at a higher [Ca2+]SR level (compared to control). Figure 2B compares the pre-spark [Ca2+]SR levels and blink nadirs before and after addition of the cytosolic BAPTA. In control conditions, the blink nadir occurred when the [Ca2+]SR reached 61.3±4.6% (n=19 events) of its initial level. With cytosolic BAPTA present, the blink nadir occurred when the [Ca2+]SR reached just 79.5±7.1% (P<0.05; n=9 events). The added cytosolic BAPTA did not change the initial [Ca2+]SR level, which is proportional to resting SR Ca2+ load.
Figure 2. Action of fast cytosolic buffering on spark-blink pairs in permeabilized ventricular myocytes.
Sparks were detected as changes in cytosolic Rhod-2 fluorescence. Blinks were detected as changes in intra-SR Fluo-5N fluorescence. A. Representative spark-blink pairs in the control and BAPTA-containing cytosolic recording solutions. The control cytosolic solution had 0.36 mM EGTA (150 nM free [Ca2+]). The BAPTA cytosolic solution here had 0.36 mM EGTA, 0.36 mM BAPTA (150 nM free [Ca2+]). Line scan images are shown at top (spark) and bottom (blink). The fluorescence profiles at the marked point on the line scan image are shown in the center. B. Summary [Ca2+]SR results in the 2 test conditions. Grey bars reflect the average initial [Ca2+]SR level before the spark. Black bars reflect the average [Ca2+]SR level at which the blink nadir occurred.
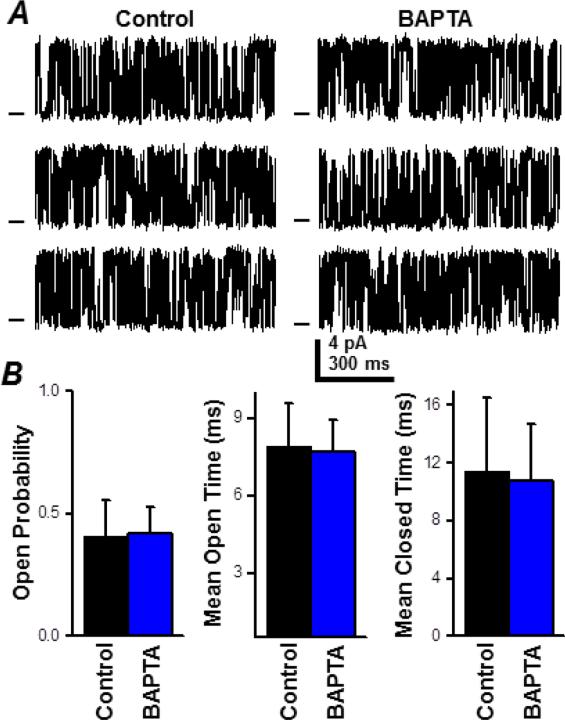
Individual cardiac RyR channels were reconstituted in lipid bilayers and their function was defined in cell-like recording solutions with and without cytosolic BAPTA (0.36 mM) present. Cytosolic EGTA (0.35 mM) was present in both cases. Single RyR Po was very low at submicromolar diastole-like levels of [Ca2+]cyto (Supplemental Fig. S1A). Therefore, single RyR function was defined with 10 μM cytosolic free Ca2+ present. This level was chosen because local [Ca2+]cyto levels within the dyad are likely near 10 μM when a spark terminates [21]. Figure 3A shows sample single RyR recordings before (control) and after the cytosolic BAPTA was added. The cytosolic BAPTA did not alter the RyR's unitary current (or slope conductance; Supplemental Fig S1B). Figure 3B compares single RyR open probability, mean open time and mean closed time before and after addition of the cytosolic BAPTA. There was no significant change in any of these parameters. Thus, addition of 0.36 mM cytosolic BAPTA did not alter single RyR function.
Figure 3. Fast cytosolic buffer action on single cardiac RyR function.
Single channel recordings were done at +40 mV in cell-like solutions. A. Representative single RyR recordings in control and BAPTA-containing cytosolic solutions. The control cytosolic solution had 0.36 mM EGTA (10 μM free [Ca2+]). The BAPTA cytosolic solution here had 0.36 mM EGTA, 0.36 mM BAPTA (10 μM free [Ca2+]). Open events are upward deflections from the marked zero current level. B. Summary results (open probability, mean open time and mean closed time) in the 2 test conditions. No significant difference in single RyR function was detected after the cytosolic BAPTA addition.
3.2. Action of Low Cytosolic Ca2+ Buffer Capacity
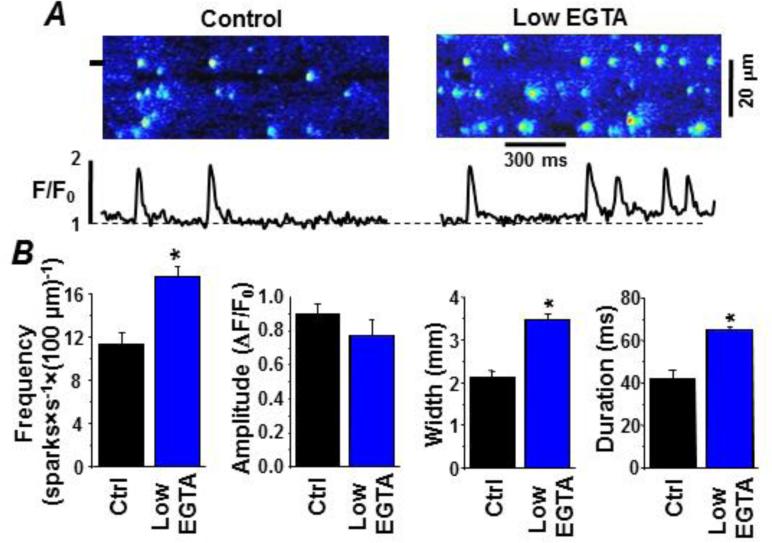
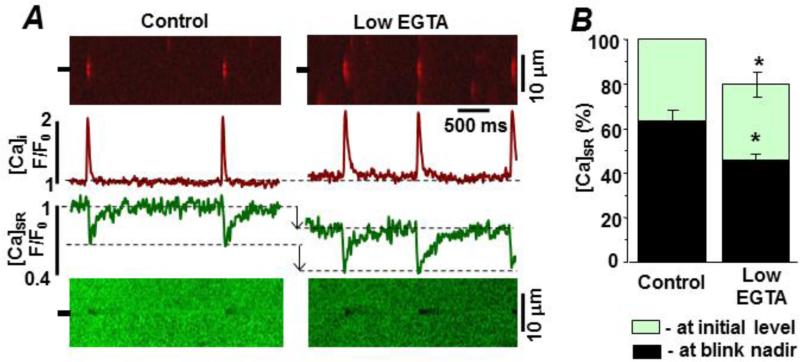
Figure 4A shows representative Fluo-4 line-scan images of Ca2+ sparks recorded with 0.36 mM (control) and 0.12 mM cytosolic EGTA present (low EGTA). The free [Ca2+]cyto in both cases was 150 nM. Figure 4B compares spark frequency, amplitude, width and duration at the two cytosolic EGTA levels. Average spark frequency, width and duration were significantly greater at the lower [EGTA]. The low [EGTA] also increased the Time-to-Peak of sparks by 31%: from 34.9±0.7 ms (n=177 events) in control conditions to 45.7±0.7 ms (P<0.05; n=212 events) in the presence of low [EGTA]. There was also a small elevation in basal Fluo-4 fluorescence that was likely the consequence of the increased spark activity (i.e. the in- and out-of-focus events). Figure 5A shows spark-blink pairs in control and at the lower cytosolic [EGTA]. Recurring sparks at the same release site (Fig 5A) were more frequently observed at the lower cytosolic Ca2+ buffer capacity. Consecutive sparks at the same release site consistently generated blinks whose nadir's occurred at the same [Ca2+]SR level. The reduction in cytosolic buffer power (from 0.36 to 0.12 mM EGTA) altered the [Ca2+]SR level at which the blink nadir occurred. Figure 5B compares initial and blink nadir [Ca2+]SR levels at the two cytosolic [EGTA]. Both initial and blink nadir [Ca2+]SR were significantly decreased at the lower cytosolic [EGTA]. The initial [Ca2+]SR decreased by 20±6% (P<0.05; n=6). The [Ca2+]SR at which the blink nadir decreased from 62.4±5.1% (n=12 events) to 46.4±7.1% (n=16 events, P<0.05). This indicates that at the lower cytosolic Ca2+ buffer capacity, spark termination occurs after [Ca2+]SR drops to a significantly lower level. Interestingly, the low cytosolic EGTA results are nearly identical (see supplemental Fig. S2) to the initial and blink nadir [Ca2+]SR level changes we have previously reported in a pressure/volume overload rabbit heart failure (HF) model [30].
Figure 4. Low cytosolic buffer power enhances spark activity in permeabilized ventricular myocytes.
Sparks were detected as local changes in cytosolic Fluo-4 fluorescence. A. Representative sparks in the control and BAPTA-containing cytosolic recording solutions. The control cytosolic solution had 0.36 mM EGTA (150 nM free [Ca2+]). The low EGTA cytosolic solution had 0.12 mM EGTA (150 nM free [Ca2+]). Line scan images are shown at top. At the bottom are fluorescence profiles at the point marked on the line scan images (see left margin). B. Summary results of various measured spark parameters in the control and low EGTA test conditions.
Figure 5. Low cytosolic buffer power action on spark-blink pairs in permeabilized ventricular myocytes.
Sparks and blinks were measured simultaneously. A. Representative spark-blink pairs in the control and low EGTA recording solutions. The control cytosolic solution had 0.36 mM EGTA (150 nM free [Ca2+]) and the low EGTA cytosolic solution had 0.12 mM EGTA (150 nM free [Ca2+]). Line scan images are shown at top (spark) and bottom (blink). Corresponding fluorescence profiles are shown in the center. Changes in the initial and blink nadir [Ca2+]SR are indicated by arrows. B. Summary [Ca2+]SR results in the 2 test conditions. The average initial and blink nadir [Ca2+]SR levels were significantly lower in the low EGTA solution.
3.3. Action of Higher Intra-SR Ca2+ Buffer Capacity
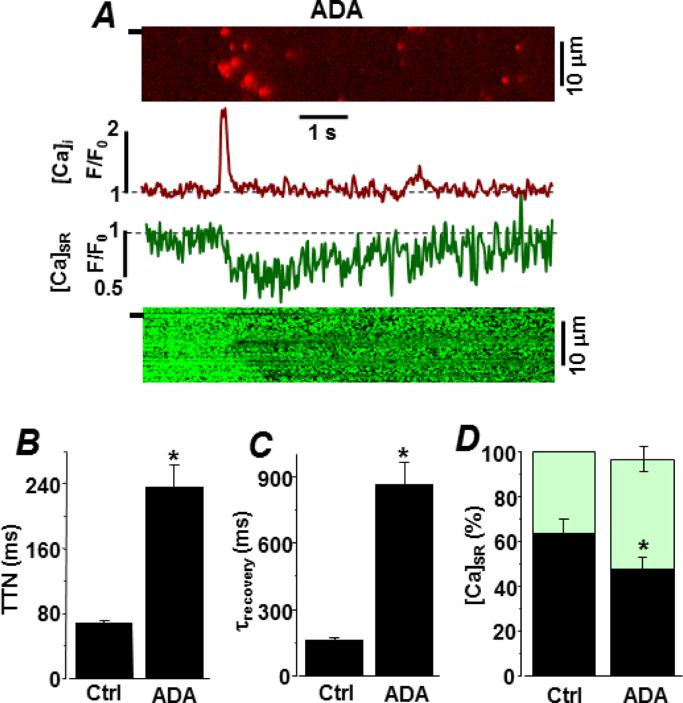
To increase the intra-SR Ca2+ buffer capacity, permeabilized myocytes were incubated in a cytosolic solution containing the low affinity Ca2+ buffer, N-(2-acetamido)iminodiacetic acid (ADA; 5 mM), which accumulates in the SR over time. We showed in a previous study [31] that incubation of permeabilized cells in 5 mM ADA increases total SR Ca2+ content by ~40%. Figure 6A shows representative spark-blink pairs after ADA loading. The cytosolic solution contained 0.36 mM EGTA (150 nM free Ca2+). Spark frequency was not altered by the presence of ADA in the SR. However, it did significantly increase the spark amplitude (0.83±0.09 to 1.22±0.11 ΔF/F0; P<0.05), Ca2+ release flux (0.052±0.007 to 0.103±0.012 d(ΔF/F0)/dt; P<0.05) and spark duration (38.3±7.9 to 112.2±35.6 ms; P<0.05). Figure 6B compares the time-to-nadir (TTN) of blinks with and without ADA in the SR. With ADA present, TTN increased more than three times (69±8 vs. 236±1 ms; P<0.05). Figure 6C compares the average time constant of local SR Ca2+ refilling following a spark. With ADA present, the time constant of SR refilling was increased from 161±19 to 856±122 ms (P<0.05). Figure 6D compares the initial and blink nadir [Ca2+]SR levels without and with ADA in the SR. With ADA present, the [Ca2+]SR at which the blink nadir occurs decreased from 63±7% (n=21 events) to 48±7% (n=10 events, P<0.05).
Figure 6. High intra-SR buffer power action on spark-blink pairs in permeabilized ventricular myocytes.
Sparks and blinks were measured simultaneously after the SR was loaded with the low affinity Ca2+ buffer ADA. A. Representative spark-blink pair is shown. The cytosolic solution had 0.36 mM EGTA (150 nM free [Ca2+]). Line scan images are shown at top (spark) and bottom (blink). Corresponding fluorescence profiles are shown in the center. B. Comparison of blink time-to-nadir (TTN) with and without intra-SR ADA present. C. Time constant of [Ca2+]SR recovery after a spark is compared with and without intra-SR ADA present. D. Comparison of average initial and blink nadir [Ca2+]SR levels with and without intra-SR ADA present.
4. DISCUSSION
Diastolic Ca2+ sparks are spontaneous bouts of localized inter-RyR CICR that are likely triggered by a rare stochastic opening of a single RyR channel. A spark occurs if the iCa-RyR mediated by that rare opening of the channel is sufficient to drive inter-RyR CICR. During a spark, local [Ca2+]SR falls and inter-RyR CICR terminates when [Ca2+]SR reaches a critical [Ca2+]SR level, known as the termination threshold [8]. Here, we report that the spark [Ca2+]SR termination threshold depends on cytosolic and intra-SR Ca2+ buffering. In control conditions, sparks terminated when [Ca2+]SR fell to ~62% of its initial value. When a fast cytosolic Ca2+ buffer was present, sparks terminated when [Ca2+]SR fell to ~80% of its initial value. At the reduced Ca2+ buffer power, sparks terminated when [Ca2+]SR fell to ~45% of its initial value. Increasing intra-SR Ca2+ buffer power delayed termination which occurred when [Ca2+]SR fell to ~50% of its initial value. Pre-spark [Ca2+]SR was not significantly different than control (except in the reduced cytosolic Ca2+ buffer power case). The action of Ca2+ buffers on Ca2+ spark-blink pairs are difficult to explain if local CICR termination was solely due to Ca2+ unbinding from luminal-SR RyR regulatory sites as previously suggested [13;39].
4.1. Intra-SR Ca2+ Control of Spark Termination
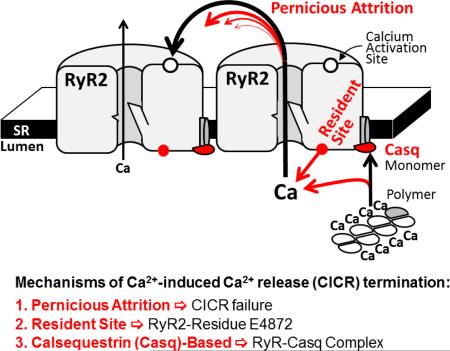
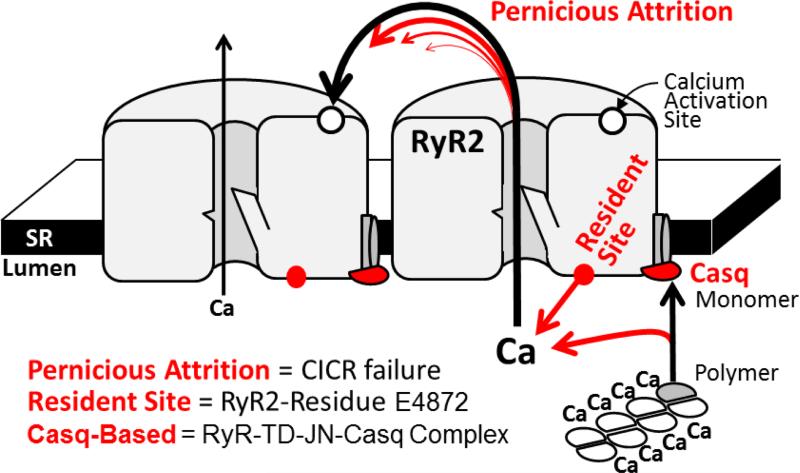
Figure 7 (see also Graphical Abstract) depicts three ways by which falling [Ca2+]SR could promote spark termination. The first involves Ca2+-dependent intra-SR calsequestrin (Casq) polymerization [40]. As [Ca2+]SR falls, more Casq monomers become available to associate with the RyR-junctin-triadin complex and this association inhibits RyR activity [38;41]. Recently, Chen et al. [42] identified an intra-SR Ca2+ sensing domain on the RyR itself. As [Ca2+]SR falls, Ca2+ occupation at this intra-SR RyR-resident site decreases and this could also inhibit RyR activity. Guo et al [27] provided experimental evidence indicating pernicious attrition [28;29] as a contributor to spark termination as well. Pernicious attrition (or induction decay [29]) describes the failure of local inter-RyR CICR as unitary iCa-RyR amplitude decreases during a spark. The iCa-RyR amplitude decreases because the trans-SR Ca2+ driving force decreases during local [Ca2+]SR depletion (or a blink). The idea is that at some point iCa-RyR will become insufficient to sustain inter-RyR CICR. At that point, RyRs that stochastically close will remain closed and thus contribute to termination. Note that pernicious attrition is not stochastic attrition [9], which describes the possibility that all open RyRs could simultaneously close. In pernicious attrition, RyR closing is innately asynchronous. It is described as pernicious because declining iCa-RyR halts inter-RyR CICR in a way that is not easily seen or noticed. We propose that the concerted operation of the three mechanisms depicted in Figure 7 is what terminates Ca2+ sparks and produces the experimentally observed [Ca2+]SR spark termination threshold.
Figure 7. Intra-SR Ca2+ dependent termination mechanisms.
The pernicious attrition, RyR-resident and Casq-based processes are illustrated (not to scale).
4.2. Cytosolic Ca2+ Buffering and the [Ca2+]SR Spark Termination Threshold
If spark termination was solely due to intra-SR Ca2+ acting on intra-SR sites (Casq-based or RyR-resident site), the spark [Ca2+]SR termination threshold should be independent of the cytosolic fate of released Ca2+. In other words, the Ca2+ interaction with the salient intra-SR Ca2+ sites would not depend on whether the released Ca2+ binds to a cytosolic buffer or simply diffuses away. However, the contribution of pernicious attrition to termination should be sensitive to cytosolic buffering, which determines the cytosolic spatiotemporal Ca2+ signal generated by an open RyR. In our study, the spark [Ca2+]SR termination threshold changed predictably when cytosolic buffering changed. Fast cytosolic Ca2+ buffers increased the [Ca2+]SR termination threshold while reduced cytosolic Ca2+ buffer capacity decreased it. Since the fast buffer narrows the cytosolic spatiotemporal spread of iCa-RyR, one would predict that a smaller drop in [Ca2+]SR (i.e. iCa-RyR) would be needed for pernicious attrition to occur. Since lower cytosolic buffer widens the cytosolic spatiotemporal spread of iCa-RyR, a larger drop in [Ca2+]SR would be needed for pernicious attrition to occur. Thus, the [Ca2+]SR termination threshold sensitivity to cytosolic Ca2+ buffering is consistent with pernicious attrition contributing to termination.
4.3. Intra-SR Ca2+ Buffer Capacity and Spark Termination
In agreement with previous studies [43;44], we found that higher SR Ca2+ buffer capacity significantly increased Ca2+ spark amplitude, time to blink nadir and time constant of local SR Ca2+ refilling. The higher buffer capacity slows the fall in local [Ca2+]SR and iCa-RyR amplitude, delaying termination. Prolonged local release explains the larger sparks and time to blink nadir. Refilling takes longer, because the SR has a larger Ca2+ buffer capacity to be replenished. Interestingly, higher SR Ca2+ buffer capacity significantly lowered the [Ca2+]SR at which sparks terminate (~45%). This suggests that pernicious attrition is not the only factor responsible for termination because intra-SR Ca buffering should not change the effectiveness of the unitary iCa-RyR to drive inter-RyR CICR. Thus, higher SR buffer capacity should not change the [Ca2+]SR termination threshold if termination were only due to pernicious attrition. One possible explanation of the lower [Ca2+]SR termination threshold at the higher SR buffer capacity is that intra-SR ADA somehow promotes RyR opening either directly or by altering the intra-SR Ca2+ interaction with the Casq-based and/or RyR-resident mechanisms. Another possibility is that the ADA may alter intra-SR Mg2+ levels or dynamics. Since Ca2+ and Mg2+ compete for occupancy of the open RyR pore, intra-SR Mg2+ changes would alter unitary iCa-RyR amplitude [45]. If so, then this could also explain the lower [Ca2+]SR spark termination threshold at the higher SR buffer capacity. Yet, another possibility is that the prolonged Ca2+ release at the higher SR buffer capacity results in a larger than normal number of locally active RyRs. It would be inherently more difficult to terminate release from a larger group of active channels.
4.4. Physiological Implications
This study revealed that the [Ca2+]SR spark termination threshold in permeabilized ventricular cardiomyocytes depends on cytosolic Ca2+ buffer speed and capacity. This indicates that that pernicious attrition likely contributes to CICR termination. Dependence of termination on local cytosolic Ca2+ dynamics has some interesting physiological ramifications. Shorter RyR open time would make unitary iCa-RyR less effective at driving inter-RyR CICR, promoting pernicious attrition and consequently preventing Ca2+ waves. Ischemia is associated with cellular acidification which could alter local cytosolic Ca2+ buffering and thus local CICR termination. The similar actions of HF and low Ca2+ buffer capacity on the [Ca2+]SR spark termination threshold (see supplemental Fig. S2) suggests that HF alters pernicious attrition in some way. One possibility is that RyR's cytosolic Ca2+ sensitivity is heighted in HF so that smaller than normal unitary iCa-RyR can drive inter-RyR CICR, explaining the lower [Ca2+]SR termination threshold observed in HF [17;30]. It is also possible that any diastolic Ca2+ handling dysfunction in the dyad (e.g. abnormal Na+-Ca2+ exchange; NCX) could influence local cytosolic Ca2+ dynamics and thus termination. Indeed, we recently found that in healthy myocytes local Ca2+ extrusion from the dyadic cleft by NCX limited inter-RyR CICR, suppressing Ca2+ sparks during diastole [46]. In HF, this mechanism can be impaired due to a mismatch in the organization of the t-tubular system and the SR network [47]. This may explain why HF myocytes are prone to arrhythmogenic SR Ca2+ release events during stress. Dependence of CICR termination on local cytosolic Ca2+ dynamics in the dyadic cleft could thus have some interesting pathogenic ramifications in many disease phenotypes.
Supplementary Material
Highlights.
Cardiac Ca sparks terminate when [Ca]SR falls to a certain critical level
Local [Ca]SR depletion can terminate a spark by reducing the unitary RyR current, decreasing cytosolic [Ca] around a RyR cluster and thus breaking the positive feedback of local CICR
We conclude that multiple [Ca]SR-dependent termination mechanisms likely co-exist to ensure a stability of cardiac Ca cycling
ACKNOWLEDGEMENTS
The authors would like to acknowledge the single-channel recording efforts of Ms. Alma Nani.
5. SOURSES OF FUNDING.
This work was supported by National Institutes of Health grants R01HL057832 and R01AR054098 (to M.F.) and The Research Career Development Award from The Schweppe Foundation and the RFC pilot grant from Loyola University Chicago (to A.V.Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. AUTHOR CONTRIBUTIONS
All authors (E.B., S.R.M., M.F. and A.V.Z.) contributed to design of the study, performing the experimental work, analyzing results and writing of the manuscript. All authors have approved the version to be published.
8. CONFLICT OF INTEREST STATEMENT
None.
References
- 1.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi T, Martone ME, Yu Z, et al. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci. 2009;122:1005–1013. doi: 10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 6.Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 8.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyorke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- 11.Lukyanenko V, Wiesner TF, Gyorke S. Termination of Ca2+ release during Ca2+ sparks in rat ventricular myocytes. J Physiol. 1998;507(Pt 3):667–677. doi: 10.1111/j.1469-7793.1998.667bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sham JS, Song LS, Chen Y, et al. Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1998;95:15096–15101. doi: 10.1073/pnas.95.25.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terentyev D, Kubalova Z, Valle G, et al. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+. J Membr Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- 16.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin J, Valle G, Nani A, et al. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Xiao J, Jiang D, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Tian X, Wang R, Fill M, Chen SR. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ Res. 2012;110:968–977. doi: 10.1161/CIRCRESAHA.111.256560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato D, Bers DM. How does stochastic ryanodine receptor-mediated Ca leak fail to initiate a Ca spark? Biophys J. 2011;101:2370–2379. doi: 10.1016/j.bpj.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams GS, Chikando AC, Tuan HT, Sobie EA, Lederer WJ, Jafri MS. Dynamics of calcium sparks and calcium leak in the heart. Biophys J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice JJ, Jafri MS, Winslow RL. Modeling gain and gradedness of Ca2+ release in the functional unit of the cardiac diadic space. Biophys J. 1999;77:1871–1884. doi: 10.1016/s0006-3495(99)77030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinch R. A mathematical analysis of the generation and termination of calcium sparks. Biophys J. 2004;86:1293–1307. doi: 10.1016/S0006-3495(04)74203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramay HR, Liu OZ, Sobie EA. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc Res. 2011;91:598–605. doi: 10.1093/cvr/cvr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobie EA, Dilly KW, dos Santos CJ, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T, Gillespie D, Fill M. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res. 2012;111:28–36. doi: 10.1161/CIRCRESAHA.112.265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillespie D, Fill M. Pernicious attrition and inter-RyR2 CICR current control in cardiac muscle. J Mol Cell Cardiol. 2013;58:53–58. doi: 10.1016/j.yjmcc.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannell MB, Kong CH, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J. 2013;104:2149–2159. doi: 10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol. 2009;587:5197–5209. doi: 10.1113/jphysiol.2009.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum ca release evokes long-lasting ca release events in ventricular myocytes: role of luminal ca in termination of ca release. Biophys J. 2008;94:1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 33.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau E, Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989;256:H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- 35.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers; Dordrecht: 2001. [Google Scholar]
- 36.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 37.Tate CA, Bick RJ, Chu A, Van Winkle WB, Entman ML. Nucleotide specificity of cardiac sarcoplasmic reticulum. GTP-induced calcium accumulation and GTPase activity. J Biol Chem. 1985;260:9618–9623. [PubMed] [Google Scholar]
- 38.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang D, Wang R, Xiao B, et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 40.Kim E, Tam M, Siems WF, Kang C. Effects of drugs with muscle-related side effects and affinity for calsequestrin on the calcium regulatory function of sarcoplasmic reticulum microsomes. Mol Pharmacol. 2005;68:1708–1715. doi: 10.1124/mol.105.016253. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Valle G, Furlan S, et al. Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J Gen Physiol. 2013;142:127–136. doi: 10.1085/jgp.201311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W, Wang R, Chen B, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20:184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 44.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillespie D, Chen H, Fill M. Is ryanodine receptor a calcium or magnesium channel? Roles of K+ and Mg2+ during Ca2+ release. Cell Calcium. 2012;51:427–433. doi: 10.1016/j.ceca.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bovo E, de Tombe PP, Zima AV. The role of dyadic organization in regulation of sarcoplasmic reticulum Ca(2+) handling during rest in rabbit ventricular myocytes. Biophys J. 2014;106:1902–1909. doi: 10.1016/j.bpj.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D. Ca handling during excitation-contraction coupling in heart failure. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.