Abstract
Objectives
To investigate the concordance of blood count indices measured locally and at a central laboratory.
Design and Methods
In a multi-center clinical trial of hydroxyurea therapy in infants with sickle cell anemia (BABY HUG), the concordance between blood count indices measured locally and at a central laboratory was investigated.
Results
Local laboratory measurements of neutrophil and monocyte counts were significantly higher (44% and 37%, respectively) compared to the central measurements (p<0.0001), and mean corpuscular volume (MCV) was higher centrally.
Conclusion
Overnight shipping with processing delay causes spurious reductions in absolute neutrophil count (ANC) and absolute monocyte count (AMC) that may result in incorrect monitoring decisions in multicenter clinical trials.
Keywords: Neutrophil, shipping, transportation, complete blood count, clinical trial
Introduction
Multi-center clinical research studies frequently utilize central laboratories rather than local laboratories to ensure study assays are performed in a standardized manner. However, there is little information about the effects of storage, shipping and time delay in processing these samples on common laboratory parameters1,2. The Pediatric Hydroxyurea Phase III Clinical Trial (BABY HUG) (NCT00006400) was an NHLBI-NICHD-sponsored double-blinded controlled trial in infants with sickle cell anemia (SCA) to ascertain the efficacy of hydroxyurea (HU) in preventing damage to the spleen and kidney3. Complete blood counts (CBC) were utilized to assess the dose limiting toxicity of HU, primarily reduction of absolute neutrophil count (ANC). In an effort to avoid adding center-to-center variability in HU dosing due to differences in ANC measurements at multiple local center laboratories, all CBCs were performed initially in a central lab at the Georgia Health Sciences University, Augusta, GA4.
Though no dose escalations were allowed (per protocol) beyond the starting dosage of 20 mg/kg/day, subjects’ CBCs were monitored every 2 weeks initially, then monthly to detect hematological toxicity. Study medication was held if neutrophil counts were less than 1250/mm3 until the ANC increased above this value. Persistent or recurrent drug stoppage mandated dosage reduction4.
Unexpectedly a number of centrally measured toxicities led to the issuance of toxicity alerts, but counts were not validated locally. This led us to compare central to local CBC measurements taken on the same blood samples collected at 13 BABY HUG clinical sites. Specifically analyzed were ANC, white blood cell count (WBC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), red blood cell count (RBC), absolute reticulocyte count, hemoglobin concentration (Hb), platelet count, and mean corpuscular volume (MCV).
Materials and Methods
Infants with known sickle cell anemia (SCA) ages 9 to 17 months, were enrolled as previously described to the BABY HUG trial4. Approval for this study was granted by Institutional Review Boards at all participating local sites where subjects (guardian) signed the informed consent. CBCs drawn during screening and at baseline (before treatment) and performed in both the local and central laboratories were used for this analysis. Whole blood samples were collected in potassium-EDTA and various automated hematology analyzers were used as per local CLIA-approved laboratory standards. A simultaneously drawn specimen was shipped via overnight priority service (FedEx) in a plastic container with foam inserts to hold the tubes in an upright position with frozen ice packs in a foam cushioned box (EXAKT PAK) to a central laboratory (the Georgia Health Sciences University, Augusta, GA). A CBC was done at the central laboratory using a Beckman Coulter LH750 20–30 hours after collection.
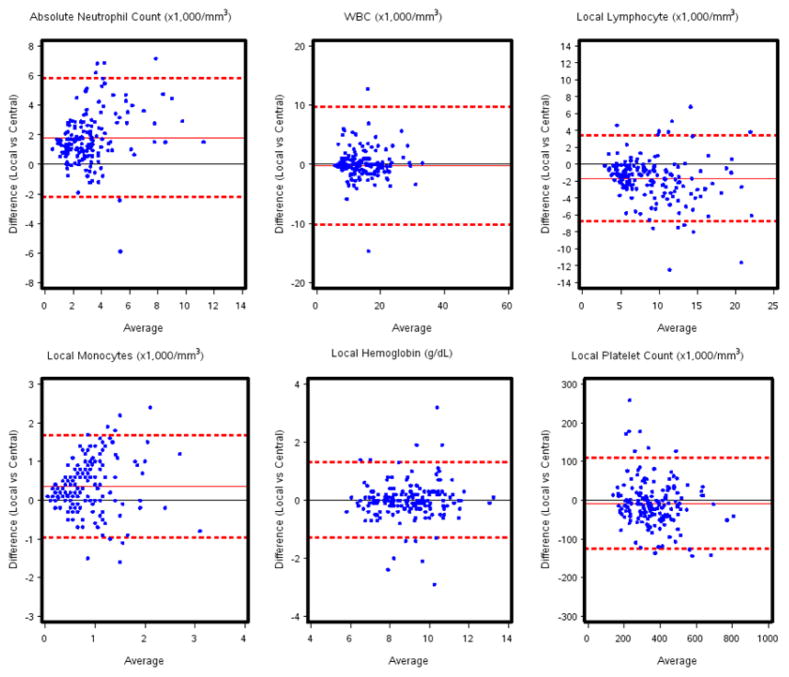
Quantitative differences in CBC measurements obtained at local and central laboratories were compared using a paired t-test. The table presents the measurement mean obtained from the central laboratory and the local laboratories, the difference of the two means, the 95% confidence interval of the difference, and the p-value. Comparisons between local and central laboratory data were displayed for each subject using a Bland-Altman plot, with the x axis representing the average of the local and central measurements and the y axis representing the difference between the local and central measurements of each CBC value. The red solid line in the middle of each figure is the mean of the local vs. central differences and the red dashed lines represent the 95% upper and lower confidence limits. Method comparison analyses were also performed using the methods of Deming. The results of these analyses are presented in the text of this report. All analyses were performed using SAS V9.2 (SAS Inc, Cary, NC).
Results
The results of 167 paired blood samples were compared. The paired t test for each CBC measurement showed statistically significant differences for ANC, lymphocyte count, AMC and MCV (Table 1), with local laboratory mean values higher than central lab mean values for ANC and AMC and lower for ALC and MCV. Deming regression analysis reflected very poor correlation between the local and central measures for ANC and AMC, and better correlation and thus method validation for the ALC and MCV. There was no significant difference between the local and central means for the measures of WBC, RBC and platelets. This was confirmed by the Deming Regression analysis which showed good correlation between the central measures and the local measures for these indices and acceptable method validation for the local versus central measurements.
Table 1.
Hematologic differences between local and central laboratories
| Hematology Indices | Mean Local | Mean Central | Mean Difference* | 95% C.I. | P-Value (paired t Test) |
|---|---|---|---|---|---|
| WBC (X1,000 cells/mm3) | 14.00 | 14.29 | +0.29 | −0.48, 1.07 | 0.4564 |
| ANC (x1,000 cells/mm3) | 4.11 | 2.33 | −1.78 | −2.09, −1.47 | <.0001 |
| AMC (x1,000 cells/mm3) | 1.01 | 0.66 | −0.35 | −0.45, −0.24 | <.0001 |
| Lymphocytes (x1,000 cells/mm3) | 8.14 | 9.85 | +1.71 | 1.31, 2.11 | <.0001 |
| RBC (x 1,000,000 cells/mm3) | 3.45 | 3.41 | −0.04 | −0.08, −0.01 | 0.0276 |
| Hemoglobin (g/dL) | 8.96 | 8.97 | +0.01 | −0.09, 0.11 | 0.8245 |
| MCV (fL) | 77.8 | 80.3 | +2.5 | 1.81, 3.21 | <.0001 |
| Reticulocytes (x1,000 cells/mm3) | 285.8 | 282.7 | −3.1 | −15.2, 9.0 | 0.6109 |
| Platelets (x1,000 cells/mm3) | 358.0 | 367.3 | +9.3 | 0.1, 18.5 | 0.0481 |
WBC, white blood cells; ANC, absolute neutrophil count; AMC, absolute monocyte count; RBC, red blood cell count; MCV, mean corpuscular volume.
Positive value denotes increased counts during transit whereas negative value denotes decreased counts during transit.
Figure 1 shows the Bland-Altman plots of various hematology indices. For WBC count, hemoglobin level and platelet count, the differences between local and central measurements were very small, as indicated by the solid red line (mean differences) almost coinciding with the solid black line (zero difference reference line). However, for the ANC, AMC, and ALC, there was significant separation between the two lines.
Figure 1.
Bland-Altman plots of hematological measurements. Y-axis is the difference between local and central measurements; x-axis is the average of the local and central measurements.
Discussion
Published data are limited as to the effects of shipping and delayed central processing on various blood indices. Overnight shipping reduced lymphocyte proliferative response using various assays, particularly if mononuclear cells were not separated from whole blood prior to shipping5,6. Lymphocyte viability has also been reported to be diminished, especially if a 72-hour delay in processing occurred7. Bayle et al addressed the potential impact of transportation and/or delay on neutrophils. In blood samples obtained from 40 neutropenic subjects, no change in ANC occurred over periods up to 48 hours1. There was a not statistically significant trend toward low ANC and high ALC if a delay (more than 8 hours) occurred in sample measurement using an automated analyzer. Interestingly, the authors reported morphological changes in neutrophils (which resembled lymphocytes) in the stored, transported specimens, which could explain low ANC and high lymphocyte counts using the automated method 1. Tatsumi et al reported that there were no significant differences in RBC, WBC and red cell indices if counts were measured within 24 hours after samples were collected and stored at room temperature, or 48 hours after storage at 4°C 8. The reticulocyte count was reliable up to 48 hours at either 4°C or 23°C. However, the effects of transportation and delayed processing on ANC and other CBC indices and the exact values were not reported 8. In a more recent study, Cornett et al compared the effects of storage conditions on various CBC indices including the differential count2. The authors reported that changes (increase in MCV, hematocrit and ANC) mainly occurred in samples stored at room temperature. There were no major differences in CBC and differential parameters in samples stored at 4°C. The authors did not provide information about transportation of the samples in that study. Peripheral blood samples also were not used in that study; however the authors noted changes in neutrophil morphology (by NEUT-X) as reported by Cornett et al. The authors recommended storing blood samples at 4°C in case of remote CBC analyses.
Our data (Table 1) show that several common blood counts (WBC, Hb, platelets, reticulocytes) were not affected by shipping and/or delay in processing. These results may encourage investigators to use a central laboratory for uniformity of these measurements in clinical trials since central laboratories minimize instrumentation and technical variations in results and may decrease costs for multi-institutional trials. However, in our study, the monocyte and neutrophil counts were lower when measured at a central laboratory compared to local laboratories, and the spuriously low ANC values led to unwarranted toxicity alerts and inappropriate dosage reductions. Central laboratory measurements had been planned for the trial to minimize lab to lab variation, which might have impacted HU dosing. However, after our observations, the protocol was amended so that local laboratory ANC values were utilized to determine the need for HU dosage adjustments.
The discrepancy in hematological indices between local and central laboratories is most likely due to morphological changes and apoptosis of blood cells resulting from transportation variables and/or time delay in shipping and processing samples. Similar to the study by Bayle et al, we observed significantly higher lymphocyte counts in the central laboratory measurements compared to local sites. In fact, the increase in lymphocyte count [1400/mm3] was similar to the combined reduction in neutrophil plus monocyte counts [1780+370= 2150/mm3] [p = 0.2], and consistent with the similar total WBC counts in the central and local laboratories; we hypothesize that the failure of the central laboratory to correctly measure ANC and AMC reflected misidentification of neutrophils and monocytes as lymphocytes. We could not confirm this hypothesis since peripheral smear analysis was not required for BABY HUG. Significantly higher MCV levels in central laboratory measurements may have been due to storage-related osmotic changes. Because MCV is important in monitoring compliance and the therapeutic impact of hydroxyurea therapy, use of central values could have led to further confusion in data analysis. In contrast to the report by Cornett et al, we noticed a decrease in ANC despite samples being shipped in frozen ice pack containers; however we did not use temperature monitoring. Our study has some potential limitations. Different automated analyzers were used to measure CBCs at the various clinical sites, with uncertain impact on inter-site variability. However, our statistical model did include an adjustment for these potential effects. Second, although shipped in an insulted container on frozen gel packs the temperatures during shipping and transportation were not monitored. This may be important, as maintaining specimen temperature above 22°C or preferably near 30°C during transportation yields a higher peripheral blood mononuclear cell counts the report by Olson et al9. However, the effects of temperature and storage on ANC and AMC were not reported in that study. Third, we did not repeat CBC measurements on the same blood samples locally after 20 hours, which could have identified or distinguished the effects of transportation vs. storage of specimens. Fourth, we do not know the contribution of underlying SCA to these findings, particularly ANC, AMC and ALC. Since there are no previously reported data available, a prospective study of samples obtained from healthy and sickle cell subjects would need to be compared. Finally, microscopic examination of blood cells may have confirmed our hypothesis that the apparent increase in lymphocytes was due to degeneration of neutrophils and monocytes.
Information as to the impact of shipping and storage of specimens on laboratory values should be made widely available to investigators and, in studies that require overnight shipment of specimens for which such data are unavailable, validity of these measurements should be confirmed. Recently, Vaught et al encouraged investigators to publish methodology papers related to bio-repositories and bioscience10. Overnight shipping with processing delay causes spurious changes in ANC, AMC and MCV that may result in incorrect monitoring decisions and conclusions. Larger controlled studies are needed to assess the impact of transportation, storage conditions and delayed processing on blood cell parameters when remote CBC analyses are required as part of clinical trials.
Highlights.
Shipment and storage of blood samples to central laboratories for uniform analysis is commonly used as an integral part of clinical trials. However, limited information is available to investigators regarding quality and preservation of these samples. In a multi-center clinical trial of hydroxyurea therapy in infants with sickle cell anemia (BABY HUG), the concordance between blood count indices measured locally and at a central laboratory was investigated. Local laboratory measurements of neutrophil and monocyte counts were significantly higher (44% and 37%, respectively) compared to the central measurements (p<0.0001), and mean corpuscular volume (MCV) was higher centrally. Overnight shipping with processing delay causes spurious reductions in ANC and AMC that may result in incorrect monitoring decisions in multicenter clinical trials. Since total WBC counts were similar, the decrease in centrally measured ANC and AMC likely reflected mis-identification of neutrophils and monocytes as “lymphocytes” as a result of apoptosis causing morphologic changes during transport. Increased RBC volume may have been due to storage-related osmotic changes.
Acknowledgments
We acknowledge the efforts of the BABY HUG subjects and their families, the contributions of all who participated in BABY HUG (http://www.c-tasc.com/sites/default/files/StudySites/babyhug.html).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayle C, Bensmaine M, Henry-Amar M. effect of time factors and transportation on circulating neutrophil counts in patients with chemotherapy-induced neutropenia. Pathol Biol (Paris) 1992;40:25–30. [PubMed] [Google Scholar]
- 2.Cornet E, Behier C, Troussard X. Guidance for storing blood samples in laboratories performing complete blood count with differential. Int J Lab Hematol. 2012 doi: 10.1111/j.1751-553X.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (baby hug) Lancet. 377:1663–72. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson BW, Miller ST, Rogers ZR, Rees RC, Ware RE, Waclawiw MA, et al. The pediatric hydroxyurea phase iii clinical trial (baby hug): Challenges of study design. Pediatr Blood Cancer. 2010;54:250–5. doi: 10.1002/pbc.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betensky RA, Connick E, Devers J, Landay AL, Nokta M, Plaeger S, et al. Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin Diagn Lab Immunol. 2000;7:759–63. doi: 10.1128/cdli.7.5.759-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg A, Betensky RA, Zhang L, Ray G. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol. 1998;5:804–7. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristal AR, King IB, Albanes D, Pollak MN, Stanzyk FZ, Santella RM, et al. Centralized blood processing for the selenium and vitamin e cancer prevention trial: Effects of delayed processing on carotenoids, tocopherols, insulin-like growth factor-i, insulin-like growth factor binding protein 3, steroid hormones, and lymphocyte viability. Cancer Epidemiol Biomarkers Prev. 2005;14:727–30. doi: 10.1158/1055-9965.EPI-04-0596. [DOI] [PubMed] [Google Scholar]
- 8.Tatsumi N, Miwa S, Lewis SM. Specimen collection, storage, and transmission to the laboratory for hematological tests. Int J Hematol. 2002;75:261–8. doi: 10.1007/BF02982039. [DOI] [PubMed] [Google Scholar]
- 9.Olson WC, Smolkin ME, Farris EM, Fink RJ, Czarkowski AR, Fink JH, et al. Shipping blood to a central laboratory in multicenter clinical trials: Effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26. doi: 10.1186/1479-5876-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaught JB. Biorepository and biospecimen science: A new focus for cebp. Cancer Epidemiol Biomarkers Prev. 2006;15:1572–3. doi: 10.1158/1055-9965.EPI-06-0632. [DOI] [PubMed] [Google Scholar]