Abstract
Previous studies have shown that bone morphogenetic proteins (BMPs) promote dendritic growth in sympathetic neurons; however, the downstream signaling molecules that mediate the dendrite promoting activity of BMPs are not well characterized. Here we test the hypothesis that reactive oxygen species (ROS)-mediated signaling links BMP receptor activation to dendritic growth. In cultured rat sympathetic neurons, exposure to any of three mechanistically distinct antioxidants, diphenylene iodinium (DPI), nordihydroguiaretic acid (NGA) or desferroxamine (DFO), blocked de novo BMP-induced dendritic growth. Addition of DPI to cultures previously induced with BMP to extend dendrites caused dendritic retraction while DFO and NGA prevented further growth of dendrites. The inhibition of the dendrite promoting activity of BMPs by antioxidants was concentration-dependent and occurred without altering axonal growth or neuronal cell survival. Antioxidant treatment did not block BMP activation of SMAD 1,5 as determined by nuclear localization of these SMADs. While BMP treatment did not cause a detectable increase in intracellular ROS in cultured sympathetic neurons as assessed using fluorescent indicator dyes, BMP treatment increased the oxygen consumption rate in cultured sympathetic neurons as determined using the Seahorse XF24 Analyzer, suggesting increased mitochondrial activity. In addition, BMPs upregulated expression of NADPH oxidase 2 (NOX2) and either pharmacological inhibition or siRNA knockdown of NOX2 significantly decreased BMP-7 induced dendritic growth. Collectively, these data support the hypothesis that ROS are involved in the downstream signaling events that mediate BMP7-induced dendritic growth in sympathetic neurons, and suggest that ROS-mediated signaling positively modulates dendritic complexity in peripheral neurons.
Keywords: Antioxidants, bone morphogenetic proteins, dendrites, free radicals, reactive oxygen species, sympathetic neurons
Introduction
Dendrites are the primary sites of synapse formation in the nervous system (Purves and Hume, 1981; Rubin, 1985), and the length and branching pattern of dendrites is tightly correlated to neuronal function (Elston, 2000; Jan and Jan, 2001; McAllister, 2000). Therefore, understanding the signaling molecules that influence the size and complexity of the dendritic arbor is crucial for gaining insight into the functioning of the nervous system. Bone morphogenetic protein (BMP) family members have been shown to promote dendritic growth in hippocampal (Withers et al., 2000), cortical (Le Roux et al., 1999) and retinal ganglion neurons (Hocking et al., 2008). In sympathetic neurons, BMPs are known to not only enhance the growth of existing dendrites (Lein et al., 1996; Majdazari et al., 2013) but also to induce de novo dendritic formation (Lein et al., 1995).
The signaling pathways that mediate the dendrite promoting activity of BMPs are not well characterized. BMPR1A is required for BMP-induced dendritic growth in cultured sympathetic neurons, and genetic deletion of this receptor subunit results in significant reduction of dendritic arborization of sympathetic neurons in the adult animal (Majdazari et al., 2013). BMPRII is required for BMP-induced dendritic growth in cultured cortical neurons (Lee-Hoeflich et al., 2004). There is at least one report suggesting that the dendrite promoting activity of BMPs requires SMAD 1 activation (Guo et al., 2001). However, there are also reports that the dendrite promoting activity of BMPs may be mediated by SMAD-independent signaling pathways involving c-jun kinase or p21 kinase (Lee-Hoeflich et al., 2004; Podkowa et al., 2013, 2010). But how SMAD-dependent or independent signaling pathways ultimately enhance dendritic arborization remains unknown.
Reactive oxygen species (ROS) are byproducts of normal cellular metabolism and include superoxide ion (O2·), hydroxyl radical (OH·) and hydrogen peroxide (H2O2). High levels of ROS have been shown to have deleterious effects on cells including lipid peroxidation, DNA damage and cell death (Valko et al., 2007), and have been implicated in neurodegenerative diseases and cellular senescence (Furukawa et al., 2007; Jenner, 2003; Jomova et al., 2010). However, there is growing evidence that ROS can also act as signaling molecules under normal physiologic conditions. ROS have been shown to be involved in Ca2+-dependent signaling downstream of many growth factors, and are known to activate transcription factors such as NF-κB (Rhee, 2006; Valko et al., 2007). ROS are required for neurogenesis in the central nervous system and have been shown to modulate synaptic plasticity in the hippocampus (Hongpaisan et al., 2004; Kennedy et al., 2012).
In this study, we test the hypothesis that ROS are involved in BMP-induced dendritic growth in sympathetic neurons. This hypothesis derives from the following observations: (1) ROS are important for neurite outgrowth in PC12 cells downstream of NGF stimulation or under hyperoxic conditions (Katoh et al., 1997; Suzukawa, 2000); (2) c-jun kinase and p21 kinase, which have been implicated in SMAD-independent mechanisms of BMP-induced dendritic growth (Podkowa et al., 2013, 2010), are also known to function upstream of ROS signaling in various cell types (Valko et al., 2007); (3) in non-neuronal cells, BMP-2 has been shown to activate NADPH oxidase, one of the enzymes that is important for production of ROS (Liberman et al., 2011; Simone et al., 2012); and (4) various isoforms of NADPH oxidase, the enzyme responsible for ROS production, are present in neonatal sympathetic neurons, in sympathetic ganglia and in sensory ganglia (Cao et al., 2009; Hilburger et al., 2005). Collectively, these data suggest a potential role for ROS signaling during BMP-induced dendritic growth in sympathetic neurons, and the data from this study support this hypothesis.
Experimental Methods
Materials
Recombinant human bone morphogenetic proteins (BMPs) were generously provided by Curis (Cambridge, MA, USA). Nordihydroguaiaretic acid (NGA), desferroxamine (DFO), diphenyleneiodonium (DPI), cytosine-β-D arabinoside (Ara-C), 2,4-dinitrophenol (DNP) xanthine, xanthine oxidase, buthionine sulfoximine (BSO) and tertiary butyl H2O2 were obtained from Sigma Aldrich Corporation (St. Louis, MO). β-nerve growth factor was obtained from Harlan Laboratories (Indianapolis, IN). Thr101 and Ebselen, which are specific NOX2 inhibitors, were obtained from Millipore (Billerica, MA). Other tissue culture media components, gel electrophoresis supplies, DCF-DA and Mitosox™ were purchased from Life Technologies (Grand Island, NY).
Animals
All procedures involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committees at the University of Buffalo (Buffalo, NY) or the University of California, Davis (Davis, CA). Timed-pregnant Holtzman rats were purchased from Harlan Laboratories (Indianapolis, IN) and timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratories (Hollister, CA). All rats were housed individually in standard plastic cages in a temperature (22 ± 2°C) controlled room on a 12 h reverse light-dark cycle. Food and water were provided ad libitum. Dams and pups were humanely euthanized prior to harvesting of superior cervical ganglia (SCG) from the pups for culture; no experimental manipulations were performed prior to euthanasia.
Tissue Culture and Transfection
Sympathetic neurons were dissociated from the SCG of perinatal (embryonic day 20 – postnatal day 1) Holtzman or Sprague Dawley rats according to previously described methods (Ghogha et al., 2012). Comparable results were obtained using cultures derived from the two different rat strains (data not shown). Cells were plated onto glass coverslips (Bellco Glass, Vineland, NJ) pretreated with poly-D-lysine (100 μg/ml, BD Biosciences, San Jose, CA) and maintained in serum-free medium containing NGF (100 ng/ml), bovine serum albumin (BSA, 500 μg/ml), bovine insulin (10 μg/ml) and human transferrin (10 μg/ml). To eliminate nonneuronal cells, the cultures were treated with the antimitotic Ara-C (1-2 μM) for 48 h beginning 24 h after plating. . Experimental treatments were begun after the non-neuronal cells were eliminated.
NOX2, NOX4 and control siRNAs (Santa Cruz Biotechnology, Dallas, TX) were labeled with Cy5 using the Label IT siRNA labeling kit (Mirus Bio, Madison, WI). The labeled siRNAs (40 μM) were frozen at −20°C prior to transfection. Sympathetic neurons were transfected using the GenMute™ siRNA transfection kit for primary neurons (SignaGen Laboratories, Rockville, MD) as described below. Following elimination of non-neuronal cells, sympathetic neurons derived from E21 rat pups were incubated with the complex containing GenMute™ transfection reagent and either control (90 pmoles), NOX2 (90 pmoles) or NOX4 (90 pmoles) siRNA in growth medium at 37°C in 5% CO2 incubator. After 4-5 h, the cultures were rinsed and maintained in normal growth medium. At 24 h after transfection, BMP-7 was added to cultures as described below and dendritic morphology was assessed in 50 to 70 Cy5-labelled cells per condition and compared to cells that were exposed to the transfection reagent alone (no siRNA).
Quantification of dendritic morphology
Following 3d of treatment as described in the figure legend, cultures were fixed with 4% paraformaldehyde, immunostained and visualized by indirect fluorescence using previously described methods (Ghogha et al., 2012). Antibody against MAP-2 (1:5000, Millipore, Billerica, MA) or non-phosphorylated forms of M and H neurofilament subunits (SMI-32 at 1:5000, Millipore, Billerica, MA) were used to visualize dendrites. The fluorescent images were acquired using the Nikon Eclipse E400 fluorescent microscope and SPOT camera. Dendritic morphology was quantified in digitized images of neurons immunopositive for nonphosphorylated neurofilaments or MAP-2 using Metamorph software (Universal Imaging, West Chester, PA) and Image J freeware (NIH). Approximately 50 neurons per coverslip were analyzed from 2-3 coverslips per treatment. Experiments were replicated at least twice using cultures derived from independent dissections. Data are expressed as the mean ±SEM and statistically significant treatment-related differences were identified by one way ANOVA using a p value of 0.05 with differences between treatment groups identified using post hoc Tukey's test.
Western Blotting
The effects of the antioxidants on axonal growth were assessed using a monoclonal antibody against the phosphorylated forms of M and H neurofilaments (1: 1000, SMI-31, Millipore, Billerica, MA). NADPH oxidase proteins levels were assessed using polyclonal antibodies against Nox2 or Nox4 (1: 200, Abcam, Cambridge, MA). GAPDH was used as a loading control and protein levels of GAPDH were assessed using a mouse or rabbit monoclonal antibody against GAPDH (Life Technologies, Grand Island, NY, Cell Signaling, Danvers, MA). After 3 d of experimental treatment, SCG cultures were lysed in a buffer containing 20 mM HEPES - pH 7.4, 150 mM sodium chloride, 1% Triton X-100, 10% glycerol, 2mM EGTA and 1X Protease Inhibitor cocktail (Millipore, Billerica, MA). Lysates were heated to 70°C in 4X LDS sample buffer (Life Technologies, Grand Island, NY), centrifuged and the supernatant from each sample separated by electrophoresis on a 4-12% Bis-Tris gel (Life Technologies, Grand Island, NY) and then immunoblotted onto a nitrocellulose membrane (Life Technologies, Grand Island, NY). The blots were incubated in Tris buffered saline (TBS) at pH 7.4 containing 5% dried nonfat powdered milk (Safeway, Phoenix, AZ) for 1 h, followed by overnight incubation at 4°C with primary antibody. The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 in 5% dried milk in TBS) and visualized by chemiluminescence using the ECL Plus Western Blotting substrate (Thermo Scientific, Rockford, IL). The blots were imaged on the ChemiDoc XRS® (Bio-Rad Laboratories, Hercules, CA).
Densitometric analysis of the blots was performed on the Image Lab software (Bio-Rad Laboratories, Hercules, CA). The intensity of the neurofilament M and H bands, NOX4 and NOX2 bands were normalized to the intensity of the GAPDH band from the same sample. The experiment was repeated with lysates derived from two independent dissections. The change in the normalized intensity with respect to control are expressed as mean ±SEM and statistically significant differences between treatment groups identified using one way ANOVA with post hoc Tukey's test.
Measurement of cell survival
Beginning on day in vitro (DIV) 4-5, cultures were treated for 3 days with control medium or BMP-7 (50 ng/ml) in the presence or absence of various antioxidants. Viability was assessed by counting the number of cells in each well and by measuring reducing capability using the MTT assay. For the MTT assay, cultures were incubated with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) at 500 μg/ml for 4 h at 37°C. The blue tetrazolium product was dissolved in 200 μL of DMSO and the absorbance of the solution measured at 562 nm on a Benchmark Plus® Microplate Reader (Bio-Rad Laboratories, Hercules CA). For both analyses, the data are expressed as the mean ±SEM and statistically significant differences between treatment groups identified using one way ANOVA with post hoc Tukey's test.
Immunocytochemical localization of Smad1
On DIV 4-5, neurons were treated with BMP-7 in the presence or absence of antioxidants for 2 h. Cellular distribution of Smad1 was assessed in cultures immunostained for phosphorylated Smad 1, 5 (1:100 dilution, Cell signaling, Danvers, MA) followed by indirect immunofluorescence as previously described (Kim et al., 2009).
Quantification of oxygen consumption rate
The Seahorse XF24 Analyzer was used as per the manufacturer's instructions (Seahorse Biosciences, North Billerica, MA) to measure the oxygen consumption rate (OCR) of cultured sympathetic neurons. Sympathetic neurons dissociated from the SCG of E21 rats were plated at a density of 15,000 cells/well onto 96-well plates specialized for use on the Seahorse XF24 Analyzer (Seahorse Biosciences) precoated with poly-D-lysine (100 μg/ml). Following the elimination of non-neuronal cells, cultures were treated with control media or BMP-7 at 50 ng/ml in the absence or presence of anti-oxidants for 48 h prior to measuring their oxygen consumption. At the end of the treatment period, neurons were incubated in DMEM with 5 mM glucose and 2 mM L-glutamine for 1 h at 37° C prior to loading them into the Seahorse analyzer. Following three measurements for baseline OCR in the first 35 min, the wells were injected with 100 μM 2,4-dinitrophenol (DNP) and three measurements for OCR were recorded in the next 35 min. Data are expressed as % of the first baseline OCR reading and presented as the mean ± SEM (n = 3 replicates per treatment group). Statistically significant differences between treatment groups were identified using one way ANOVA with post hoc Tukey's test.
Results
Antioxidants inhibit BMP-induced dendritic growth in cultured sympathetic neurons
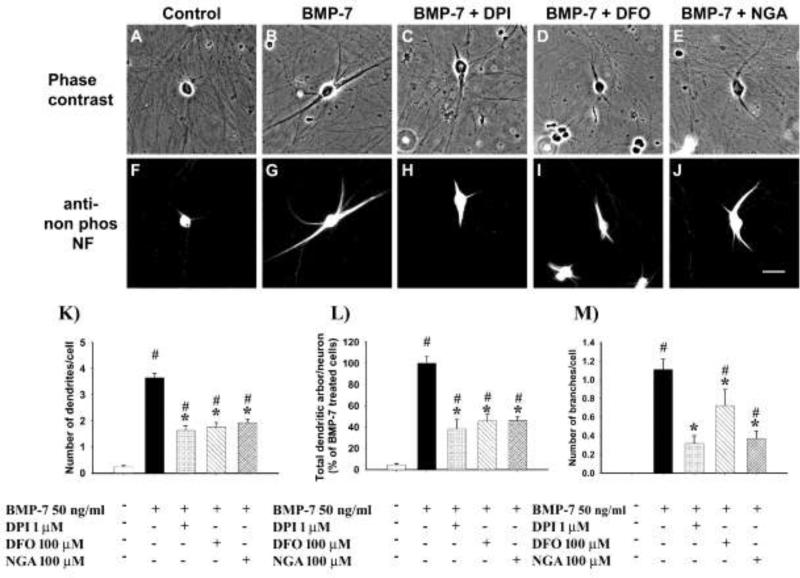
Sympathetic neurons from the SCG of perinatal rats were cultured in the absence of serum or glial cells. In accordance with previous observations (Bruckenstein and Higgins, 1988), sympathetic neurons did not extend dendrites under these conditions (Fig. 1A, 1F, 1K). In contrast, neurons treated with BMP-7 at a maximally effective concentration (Lein et al., 1995) for 3 d extend multiple MAP-2 immunopositive processes that exhibit dendritic morphology (Fig. 1B, 1G, 1K).
Figure 1. Antioxidants inhibited BMP-7-induced dendritic growth in cultured sympathetic neurons.
Representative phase contrast (A-E and fluorescence (F-J) micrographs of sympathetic neurons cultured from perinatal rat SCG immunostained for nonphosphorylated neurofilaments after treatment for 3 d to BMP-7 (50 ng/ml) in the absence or presence of 100 nM diphenylene iodinium (DPI), 100 μM desferroxamine (DFO) or 100 μM nordihydroguaiaretic acid (NGA). Negative controls were maintained in culture medium without any BMP-7; bar = 50 μM. The number of dendrites per cell (K), total length of the dendritic arbor per cell (L) and number of branches per cell (M) were quantified in cultures immunostained for nonphosphorylated neurofilaments or MAP-2 to identify dendritic processes. Data are expressed as the mean ± SEM (N ≥ 60). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *indicates antioxidant treatments that are significantly different from cultures treated with BMP-7 at p ≤ 0.05; # shows conditions that are significantly different from negative control cultures not exposed to BMP-7 at p < 0.05.
To test the hypothesis that ROS is involved in BMP-induced dendritic growth, we first determined whether treatment with antioxidants would block the dendrite promoting activity of BMPs. Treatment of cultured sympathetic neurons with diphenylene iodinium (DPI,1 μM), an antioxidant that irreversibly inhibits NADPH oxidase (Wyatt et al., 1994), in the absence of BMP-7 did not trigger de novo dendritic growth (data not shown). However, when added to cultures simultaneously exposed to BMP-7 at 50 ng/ml, DPI inhibited BMP-induced dendritic growth (Fig. 1). Neurons treated simultaneously with DPI and BMP-7 showed a significant reduction in dendrite number (Fig. 1K), total length of the dendritic arbor (Fig. 1L) and the number of branches (Fig. 1M) compared to BMP-7 treated cells maintained in the absence of DPI. This effect was concentration-dependent with an IC50 of ~ 50 nM (Fig. 2A).
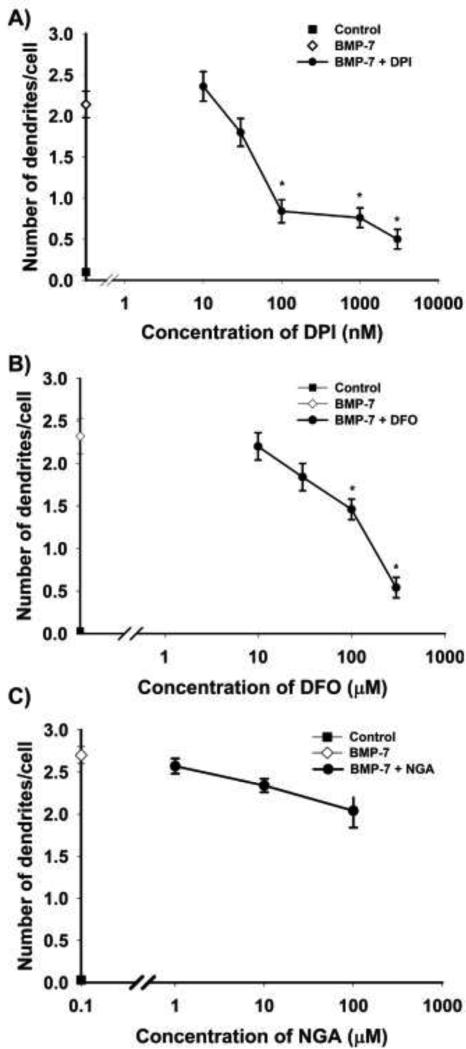
Figure 2. Antioxidant mediated inhibition of BMP-7 induced dendritic growth was concentration-dependent.
Sympathetic neurons cultured from perinatal rat SCG were treated with 50 ng/ml BMP-7 with or without varying concentrations of DPI, DFO and NGA for 3 d. The number of dendrites per cell was quantified in cultures immunostained for nonphosphorylated neurofilaments or MAP-2 to identify dendritic processes. DPI (A), DFO (B) and NGA (C) inhibit BMP-7-induced dendritic growth in a concentration-dependent manner. Data are expressed as the mean + SEM (N = 60). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *Significantly different from positive control cultures treated with BMP-7 at p < 0.05
To corroborate that the inhibition of BMP-induced dendritic growth by DPI was due to its effects as an antioxidant, sympathetic neurons were treated with two mechanistically distinct antioxidants: desferroxamine (DFO), an antioxidant that chelates iron (Farinelli and Greene, 1996), and nordihydroguaiaretic acid (NGA), which prevents lipid peroxidation (Arteaga et al., 2005). Similar to DPI, DFO and NGA had no effect on dendritic growth in cultures not exposed to BMP-7 (data not shown), but both DFO (Fig. 1D, 1I, 1K-M) and NGA (Fig. 1E, 1J, 1K-M) significantly inhibited BMP-7 induced dendritic growth in a concentration-dependent manner.
While the efficacy of DFO was similar to that of DPI, it was less potent with an IC50 value of ~ 100 μM (Fig. 2B). NGA was less efficacious than DPI or DFO at inhibiting dendritic growth as evidenced by the observation that at the highest concentration tested (100 μM), it only inhibited BMP-7-induced dendritic growth by ~ 40 - 50% compared to ~80% inhibition of BMP-7-induced dendritic growth by maximally effective concentrations of either DPI (3 μM) or DFO (300 μM) (Fig. 2)
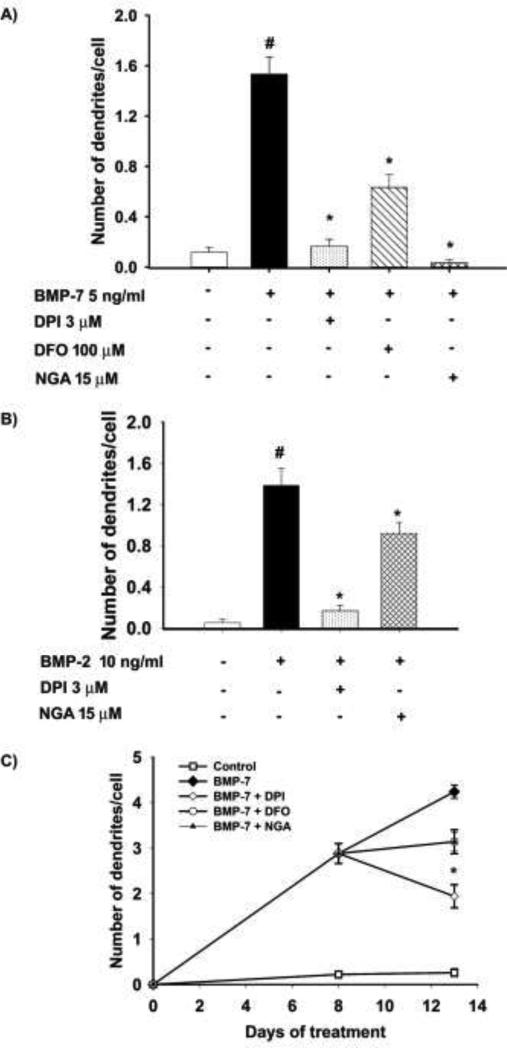
The observation that NGA was not as efficacious as DPI and DFO in inhibiting dendritic growth triggered by maximally effective concentrations of BMP-7 raised the question of whether this discrepancy was of mechanistic relevance. To test this, we next determined whether DPI, DFO and NGA blocked dendritic growth triggered by submaximal concentrations of BMP-7. In cells exposed to BMP-7 at 5 ng/ml, DPI at 3 μM blocked BMP-induced dendritic growth by 85 %, DFO at 100 μM, by 60 % and NGA at 15 μM, by > 90% (Fig. 3A). Dendritic growth in sympathetic neurons is triggered by not only BMP-7, a member of the 60A BMP subfamily, but also by BMPs of the dpp subfamily (Guo et al., 1998). Dendritic growth induced by submaximal concentrations of BMP-2, a dpp BMP, was inhibited by 88 % by DPI at 3 μM and by 34 % by NGA at 15 μM (Fig. 3B).
Figure 3. Antioxidants inhibited dendritic growth induced by multiple members of the BMP subfamily and caused inhibition or retraction of existing dendrites.
Sympathetic neurons cultured from perinatal rat SCG were treated for 3 d with (A) BMP-7 at a submaximal concentration (5 ng/ml) or (B) BMP-2 at a submaximal concentration (5 ng/ml) in the absence or presence of DPI (3 μM), DFO (100 μM) or NGA (15 μM). The number of dendrites per cell was quantified in cultures immunostained for nonphosphorylated neurofilaments or MAP-2 to identify dendritic processes. Data are expressed as the mean + SEM (N = 60). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *Significantly different from cultures treated with BMP-7 only at p ≤ 0.05; #significantly different from negative control cultures not exposed to BMP-7 at p < 0.05. (C) The effects on existing dendrites was measured by treating cultured sympathetic neurons with BMP-7 (50 ng/ml) for 8 days to induce dendritic growth, then treated for an additional 5 days with BMP-7 alone or BMP-7 plus DPI (100 nM), DFO (100 μM) or NGA (100 μM). Cultures were immunostained for MAP-2 and the number of dendrites/cell was quantified. Data are expressed as the mean ± SEM (N = 60). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *Significantly different from cultures treated with BMP-7 only at p ≤ 0.05.
Some agents that inhibit de novo dendritic growth can also cause dendritic retraction (Chandrasekaran et al., 2000; Guo et al., 1997; Kim et al., 2002) To determine whether antioxidants cause the elimination of preexisting processes, cultures were pretreated with BMP-7 (50 ng/ml) for 8 d to induce dendritic growth. During the subsequent 5 d, a subset of cultures continued to receive only BMP-7 while other cultures were treated with both BMP-7 and an antioxidant (DPI at 100 nM, DFO at 100 μM or NGA at 100 μM). Neurons that continued to receive just BMP-7 showed increased dendritic growth; in contrast, neurons treated with DPI showed retraction of dendrites as evidenced by a decreased number of dendrites at DIV 13 relative to DIV 8 (Fig. 3C). Cultures treated with either DFO and NGA did not exhibit dendritic retraction but rather showed no further extension of dendrites beyond DIV 8 (Fig. 3C).
Antioxidants selectively interfere with dendritic growth
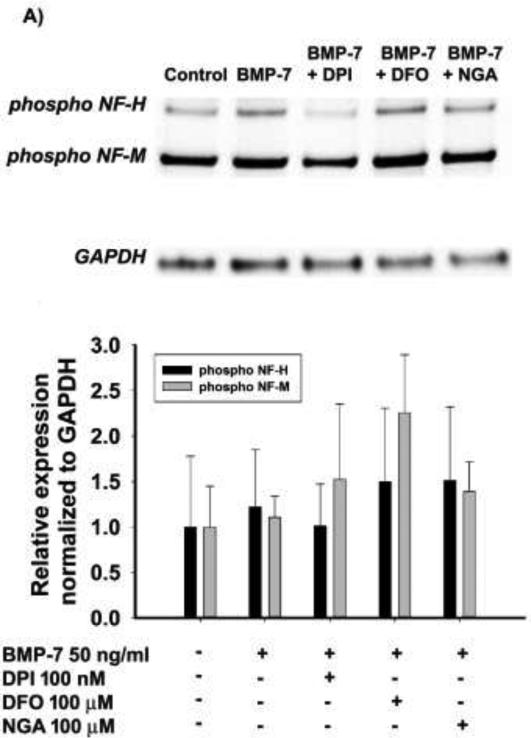
To determine whether antioxidants specifically and selectively target dendrites, we evaluated the effects of antioxidants on axonal growth and cell viability. The phosphorylated forms of neurofilaments are enriched in axons and their expression can be used as an indicator of axonal growth (Guo et al., 1998; Sternberger and Sternberger, 1983). There was no significant decrease in the levels of phosphorylated neurofilaments between cultures treated with BMP-7 alone versus BMP-7 plus antioxidant (Fig. 4).
Figure 4. Antioxidants did not affect axonal growth.
Cell lysates from sympathetic cultures treated with control medium or BMP-7 (50 ng/ml) in the absence or presence of DPI (100 nM), DFO (100 μM) or NGA (100 μM) for 3d were immunoblotted for phosphorylated neurofilaments as marker of axonal growth and GAPDH as a loading control. (A) Representative western blots probed for phosphorylated forms of NF-H (200 kD), NF-M (160 kD) and GAPDH (37 kD). (B) Densitometric values of the phospho-NF-H and phospho-NF-M bands from samples obtained from 3 independent dissections were normalized to GAPDH within the same sample. The data are expressed as the mean ± SEM (N = 3-5). As determined by one way ANOVA (p ≤ 0.05), there were no statistically significant difference between treatment groups.
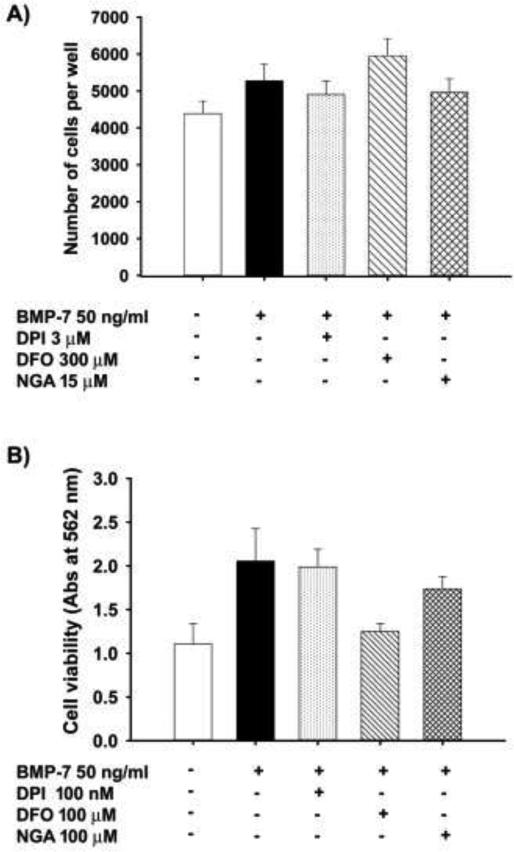
The observation that axonal growth was not generally inhibited by antioxidants suggests that the effects of antioxidants were specific to dendrites and not a result of deleterious effects of antioxidants on the health of sympathetic neurons. In confirmation of this conclusion, the number of neurons/well was not affected by simultaneous treatment of cultures with BMP-7 and DPI, DFO or NGA (Fig. 5A).
Figure 5. Antioxidants did not affect cell numbers or cell viability.
(A) The number of surviving cells was quantified in sympathetic neuronal cell cultures treated for 5d with BMP-7 at 50 ng/ml in the absence or presence of DPI (3 μM), DFO (300 μM), NGA (15 μM) (N= 3). (B) The effects of antioxidants on cell viability were measured in cultured sympathetic neurons treated with BMP-7 (50 ng/ml) in the absence or presence of DPI (100 nM), DFO (100 μM) or NGA (100 μM) using the MTT assay. Data are expressed as the mean ± SEM (N = 3 cultures per treatment). As determined by one way ANOVA (p < 0.05), there were no statistically significant differences between treatment groups for either cell survival (A) or cell viability (B).
In addition, cell viability was assessed using the MTT assay, which measures metabolic activity and is used as an indicator of general cellular health (Kim et al., 2009; van Meerloo et al., 2011). While the absorbance of MTT appeared to be higher in BMP-7 treated cells compared to control cells, this difference was not found to be statistically significant. Co-exposure to BMP-7 and antioxidants appeared to decrease MTT values compared to cultures exposed to BMP-7 alone, but the levels were not lower than in cultures not exposed to BMP-7 and they were not significantly different from cultures treated with BMP-7 only (Fig. 5B).
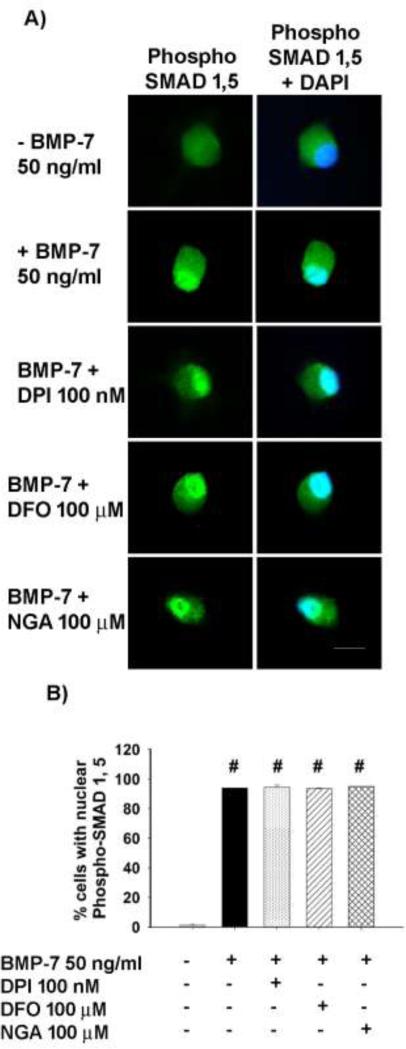
Antioxidants do not affect the nuclear translocation of SMADs
The canonical pathway of BMP signaling involves transcription factors known as SMADs. Binding of BMPs to BMP receptors causes phosphorylation of SMAD 1 and Smad 5, which then complex with Smad 4 and translocate to the nucleus to alter gene expression (Massagué and Chen, 2000). In cultures not exposed to BMP-7, there was slight immunoreactivity for phosphorylated SMAD 1, 5 and this was primarily localized to the cytoplasm (Fig. 6A). In contrast, in cultures treated with BMP-7 (50 ng/ml) for 2 h, phospho-SMAD 1, 5 immunoreactivity was localized predominantly to the nucleus, as indicated by colocalization with DAPI (Fig. 6A). Treatment with DPI (100 nM), DFO (100 μM) or NGA (100 μM) did not alter BMP-induced nuclear localization of phosphorylated SMAD 1, 5 (Fig. 6A). Quantification of the percentage of cells exhibiting nuclear staining for phospho-SMAD 1,5 confirmed that treatment with antioxidants did not interfere with BMP-7 induced nuclear translocation of SMAD 1,5 (Fig. 6B)
Figure 6. Antioxidants did not inhibit BMP-mediated nuclear translocation of SMADs.
Cultured sympathetic neurons were treated with BMP-7 (50 ng/ml) in the absence or presence of DPI (100 nM), DFO (100 μM) or NGA (100 μM) for 2 h and then immunostained for phosphorylated (phospho) SMAD 1 and SMAD 5. Negative control cultures were maintained in the absence of BMP-7. (A) Representative images of cells from each treatment group showing phospho-SMAD 1,5 immunoreactivity alone or with co-labelling with DAPI to define the nucleus. Bar = 20 μM (B) The percentage of neurons with nuclear staining for SMAD 1,5 was determined from 100 neurons in each condition. Data are expressed as mean ± SEM. Statistical significance was assessed using one way ANOVA, followed by post hoc Tukey's test. #significantly different from negative control cultures not exposed to BMP-7 at p < 0.05.
BMP-7 induction of ROS in sympathetic neurons
The observation that three antioxidants with different modes of action inhibited BMP-induced dendritic growth suggests that reactive oxygen species (ROS) may play a role in BMP-induced dendritic growth. In initial tests of this hypothesis, two fluorescent dyes - DCF-DA (2',7'-dichlorodihydrofluorescein diacetate) and Mitosox™, which are routinely used for detection of ROS, were used to label control and BMP-7 treated neurons (Gomes et al., 2005; Kuznetsov et al., 2011). However, these dyes were unable to detect increased ROS in sympathetic neurons treated with BMP-7 at 50 ng/ml for 30 min, 2h, 24h and 5d (data not shown). Also, treatment of sympathetic neurons with agents to induce ROS production such as xanthine/xanthine oxidase (100 mM/0.05 U/mL), buthionine sulfoximine (BSO, 100 μM) and tertiary butyl H2O2 (0.1 μM) for 72 hours alone or in the presence of submaximal concentrations of BMP-7 were not effective in promoting dendritic growth (data not shown). Therefore, two indirect methods were used to determine the potential involvement of ROS in BMP-induced dendritic growth.
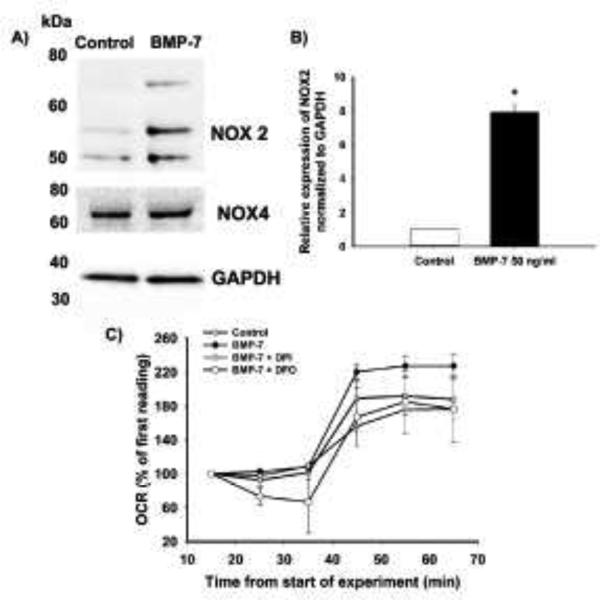
First, it is known that NADPH oxidase is important for ROS production and is inhibited by DPI (Hilburger et al., 2005; Sorce and Krause, 2009). Since DPI inhibited dendritic growth, the levels of NOX2 and NOX4 were compared in sympathetic neurons grown in the absence or presence of BMP-7 (50 ng/ml) for 3 d. Western blot analyses using antibody specific for NOX4 detected a band of ~60 kD, which was unchanged in control versus BMP-7 treated cells (Fig. 7A). Previous studies using an antibody against NOX2 identified multiple bands at 50 – 80 kD and a band of ~30 kD (Ambasta et al., 2004). Similar size bands were observed in lysates from control and BMP-7 treated sympathetic neurons probed with NOX2-specific Ab. However, unlike NOX4 expression, the three bands around 60 kD showed significantly increased expression in BMP-7 treated cells compared to control cells (Fig. 7A, B).
Figure 7. BMP-7 increased NOX2 expression and oxygen consumption in cultured sympathetic neurons.
Cell lysates from sympathetic cultures treated with control medium or BMP-7 (50 ng/ml) for 3 d were immunoblotted for NOX2, NOX4 or GAPDH. (A) Representative western blots probed for NOX2 (50 – 70kD), NOX4 (65 kD) and GAPDH (37 kD). (B) Densitometric values of the NOX2 bands from samples obtained from 3 independent dissections were normalized to GAPDH within the same sample. The data are expressed as the mean ± SEM. Statistical significance was assessed using Student t-test at a P < 0.05. (C) To measure OCR, neuronal cell cultures dissociated from SCG were treated with control media or BMP-7 (50 ng/ml) in the absence or presence of DPI, DFO or NGA for 48 h. OCR was measured using the XF24 extracellular flux analyzer. The first three measurements were done at baseline. The cultures were then injected with 2,4-dinitrophenol and the three measurements of OCR were collected. The OCR results are expressed as a % of first baseline reading. Data are expressed as the mean ± SEM (N = 3)
Increased metabolic activity is known to be associated with increased production of free radicals (Kuznetsov et al., 2011). Therefore, an increase in oxygen consumption upon treatment with BMP-7 was measured as another indirect indication of BMP-induced ROS production. The oxygen consumption rate (OCR) was measured in cultured sympathetic neurons after 48 h (Fig. 7C) of exposure to BMP-7. Increased oxygen consumption was evident in the cultures treated with BMP-7 compared to control cells (Fig. 7C). BMP-treated cultures exposed to antioxidants for 48 h exhibited a lower OCR than cultures treated with BMP-7 only and in fact the OCR in the antioxidant-exposed cultures was similar to that observed in the control cells not treated with BMP-7 (Fig. 7C).
Inhibition of NOX2 leads to decrease in BMP-7 induced dendritic growth
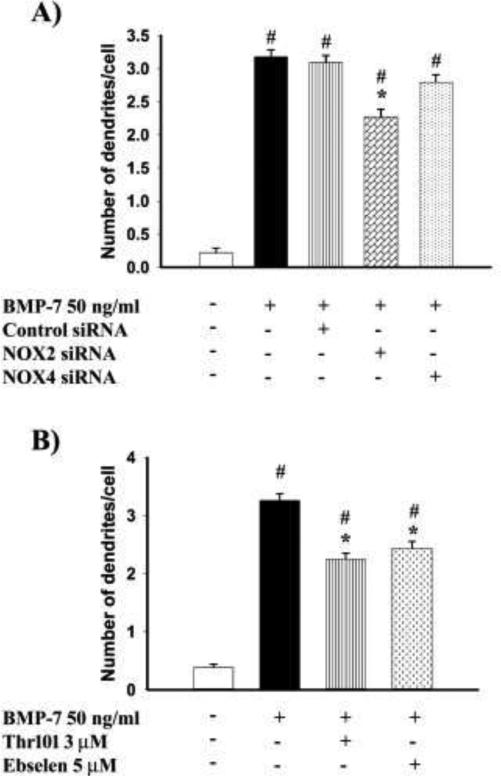
The increased expression of NOX2 but not NOX4 in BMP-7 treated neurons suggests that NOX2 may play a role in BMP induced dendritic growth. To determine the importance of NOX2 for dendritic growth, sympathetic neurons were transfected with siRNAs previously shown to inhibit either NOX2 or NOX4 in PC12 cells (Khan et al., 2011). Cultured sympathetic neurons transfected with NOX2 siRNA, followed by treatment with BMP-7 (50 ng/ml) showed reduced dendritic growth compared to BMP-7 treated neurons transfected with either control siRNA or no siRNA (Fig. 8A). In contrast, neurons transfected with NOX4 siRNA, followed by BMP-7 treatment did not show a statistically significant difference in dendritic number compared to neurons treated with BMP-7 following transfection with no siRNA or control siRNA (Fig. 8A).
Figure 8. Inhibition of NOX2 decreases BMP-7-induced dendritic in cultured sympathetic neurons.
(A) Sympathetic neurons cultured from perinatal rat SCG were transfected with either control siRNA (90 pmoles), NOX2 siRNA (90 pmoles) or NOX4 siRNA (90 pmoles), followed after 24 hours by treatment with BMP-7(50 ng/ml) for 3 d. The number of dendrites per cell were quantified in cultures immunostained for MAP-2 to identify dendritic processes. Data are expressed as the mean ± SEM (N ≥ 85). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *indicates antioxidant treatments that are significantly different from cultures treated with BMP-7 at p ≤ 0.05; # shows conditions that are significantly different from negative control cultures not exposed to BMP-7 at p < 0.05.
(B) The effect of NOX2 inhibitors on dendritic growth in sympathetic neurons was measured in cultured sympathetic neurons that were treated with BMP-7 (50 ng/ml) in the presence or absence of either Thr101 (3 μM) or Ebselen (5 μM). The number of dendrites per cell were quantified in cultures immunostained for MAP-2 to identify dendritic processes. Data are expressed as the mean ± SEM (N ≥ 100). Statistical significance was assessed using one way ANOVA, followed by Tukey's post hoc test. *indicates antioxidant treatments that are significantly different from cultures treated with BMP-7 at p ≤ 0.05; # shows conditions that are significantly different from negative control cultures not exposed to BMP-7 at p < 0.05.
To further test the relevance of NOX2 in BMP-induced dendritic growth, sympathetic neurons were exposed to two NOX2 inhibitors, Thr 101 and Ebselen, both of which have low IC50 values for NOX2 (0.3 and 0.5 μM respectively) compared to other NADPH oxidases (Smith et al., 2012). Co-treatment of neurons with BMP-7 (50 ng/ml and either Thr 101 (3 μM) and Ebselen (5 μM) inhibited BMP-7 induced dendritic growth as determined by number of dendrites (Fig. 8B) and total length of the dendritic arbor (data not shown).
Discussion
Our results show that three antioxidants – DPI, DFO and NGA – inhibit BMP-7 induced dendritic growth in sympathetic neurons. In addition, these antioxidants inhibit dendritic growth induced by BMP-2, another member of the BMP family suggesting that their inhibitory effects target a part of the signaling pathway that is common among various BMP family members. Two of the inhibitors, DPI and DFO caused 80-90% inhibition of dendritic growth induced by maximally effective concentrations of BMP-7, with an IC50 for their inhibition of BMP-induced dendritic growth of approximately 50 nM and 100 μM, respectively. In contrast, even at 100 μM, NGA inhibition of dendritic growth induced by a maximally effective concentration of BMP-7 was around 40 - 50%. This may be due to either the efficacy of NGA as an antioxidant in sympathetic neurons or mechanistic differences between NGA and the other antioxidants studied. NGA is a known lipoxygenase inhibitor and has been shown to inhibit lipoxygenase with an IC50 of around 3-5 μM. However, it is also known to inhibit other enzymes such as cyclooxygenase, with a higher IC50, and to also promote the activity of glutathione peroxidase and catalase, which contribute to its antioxidant effects (Hernández-Damián et al., 2014). It is possible, therefore, that NGA mediated inhibition of dendritic growth is due to its effects on some of these other pathways, but not due to its role as a lipoxygenase inhibitor. Interestingly, NGA was able to effectively block dendritic growth triggered by a submaximal concentration of BMP-7. This may reflect the inability of NGA to neutralize the free radicals generated by high levels of BMP-7 or there may be additional signaling pathways induced by higher concentrations of BMP-7 that are important for dendritic growth but not inhibited by NGA.
Antioxidants did not affect cell survival or lower the metabolic activity below control levels in the presence of BMP-7. In addition, antioxidant-mediated inhibition of dendritic growth was not associated with changes in the levels of phosphorylated neurofilaments, suggesting that there was no inhibition of axonal growth. These observations indicate that the decrease in dendritic arborization in the presence of antioxidants was not due to deleterious effects on the health of the cells and was not a global repression of process outgrowth but a specific effect on dendrites.
The observation that antioxidants blocked dendritic growth induced by two BMP family members suggests the possibility that antioxidant effects are due to inhibition of the canonical BMP signaling pathway, which involves the nuclear translocation of SMADs (Guo et al., 2001; Massagué and Chen, 2000). However, antioxidants did not affect the nuclear translocation of SMAD 1,5, suggesting that the antioxidants are altering proteins downstream from SMAD activation or are modulating dendritic growth via a SMAD-independent signaling pathway.
Though antioxidants inhibited dendritic growth, our studies were unable to detect increased ROS following exposure to BMPs. Fluorescent staining using DCF-DA and MitoSox™ did not show significant differences between cells treated with control media and BMP-7. One possible reason for the inability to detect a difference in ROS production is that there was significant fluorescent staining with the dyes in SCG neurons under control conditions, which makes it difficult to detect increases in ROS upon treatment with BMP-7. In agreement with our observations, a previous study reported the presence of significant fluorescent staining in non-apoptotic SCG neurons (Kirkland et al., 2010). It is also possible that the increase in ROS production upon exposure to BMP-7 is a local increase within certain cellular compartments but not an overall increase in ROS throughout the cell. Localized ROS production has been observed in paraventricular hypothalamic neurons and in rat sympathetic neurons (Coleman et al., 2013; Natera-naranjo et al., 2012). A localized increase in ROS would be consistent with our observation that there was no toxicity associated with BMP-7 treatment. This would provide one explanation for the inability of ROS generating agents such as xanthine-xanthine oxidase, hydrogen peroxide and BSO to promote dendritic growth in these neurons.
Though we were unable to detect ROS in SCG neurons upon BMP treatment, our data provide evidence for upregulation of pathways that usually lead to ROS production. Consistent with a ROS-dependent mechanism of BMP-7-induced dendritic growth, we observed a small but significant increase in oxygen consumption in sympathetic neurons exposed to BMP-7 for 48 h. The BMP-7-induced increase in oxygen consumption was decreased to control levels in the presence of antioxidants, suggesting that there was an increase in metabolic rate in the presence of BMP-7. Previous studies have shown that increased OCR is correlated to cell size and cell volume in different cell types (Wagner et al., 2011). The increase in the number of dendrites following BMP-7 exposure would likely result in BMP treated sympathetic neurons having greater surface area compared to control neurons. It is therefore possible that the increased surface area in BMP-7 treated cells may be causing the increased OCR in these cells. Interestingly, we also observed a slight increase in MTT reduction in the presence of BMP-7 compared to control cultures. Since increased OCR and increased mitochondrial activity are associated with increased ROS production (Ambrosio et al., 1993; Hongpaisan et al., 2004; Kowaltowski et al., 2009; Kuznetsov et al., 2011), the increased oxygen consumption observed in BMP-7 treated cells likely reflects an increase in ROS.
It is known that NADPH oxidase is an important enzyme in the production of ROS (Valko et al., 2007). NOX2 protein expression, but not NOX4 protein was upregulated in sympathetic neurons treated with BMP-7. Furthermore, siRNA knockdown of NOX2 but not NOX4 decreased BMP-induced dendritic growth. Consistent with these data, pharmacological inhibitors specific for NOX2 also decreased BMP-7-induced dendritic growth. Our study provides the first evidence for the role for these oxidases in dendritic growth. Previous studies have shown that NADPH oxidases, including NOX2 and NOX4, are detected in many areas of the brain as well as in the cell bodies and axons of sensory and sympathetic neurons, and that increased NADPH oxidase levels and are associated with increased ROS production in nervous system (Cao et al., 2009; Infanger et al., 2006; Sorce and Krause, 2009). Therefore, it is likely that the increased NADPH oxidase activity in sympathetic neurons is associated with ROS production following BMP-7 treatment. Previous studies of podocytes demonstrated that BMP-2 increased NAPDH oxidase-dependent ROS production via upregulation of Id-1, a negative regulator of bHLH transcription factors (Pache et al., 2006). Interestingly, it has been shown that Id-1 is upregulated in cultured sympathetic neurons following BMP-7 treatment (Garred et al., 2011). However, the effects of Id-1 on BMP-7 induced dendritic growth and on NOX2 expression in sympathetic neurons have not yet been explored. These results, together with the increase in OCR upon BMP-7 treatment, provide indirect evidence for the upregulation of ROS by BMP-7 in sympathetic neurons.
It is well documented that high levels of ROS cause harmful effects including DNA damage and apoptosis (Jomova et al., 2010; Valko et al., 2007); however, there is increasing evidence that ROS function as critical signaling molecules (Massaad and Klann, 2011; Valko et al., 2007). Free radicals have been found downstream of signaling mediated by various growth factors including PDGF, EGF, β-FGF and VEGF (Bae et al., 1997; Colavitti et al., 2002; Sundaresan et al., 1996). In addition, other studies have implicated ROS in the activities of TGF–β and BMPs (Liberman et al., 2011; Pache et al., 2006; Podkowa et al., 2013; Simone et al., 2012; Valko et al., 2007). This study provides further evidence to support the role of ROS in low concentrations and/or in localized subcellular compartments as signaling molecules involved in neuronal morphogenesis.
In summary, our findings show that BMP-7 induced dendritic growth can be inhibited by antioxidants, suggesting a role for ROS mediated signaling during primary dendritogenesis. In addition, our results show that BMP-7 mediated regulation of NOX2, an enzyme required for ROS production in many cell types, is important for dendritic growth in sympathetic neurons.
Highlights.
Antioxidants concentration-dependently inhibit BMP-induced dendritic growth.
Antioxidants do not alter cell viability or axonal growth.
Antioxidants do not inhibit BMP-7 induced nuclear translocation of SMAD 1,5.
BMP-7 upregulates oxygen consumption rate and NADPH oxidase 2 (NOX2).
Pharmacological or siRNA inhibition of NOX2 decreases BMP-induced dendritic growth.
Acknowledgements
We thank Dr. Paula Goines, Mr. Donald Bruun and Mr. Hao Chen at UC Davis for assistance in setting up and maintaining sympathetic cultures. We also acknowledge Donald Bruun and Hao Chen at UC Davis and Barbara Liepe at Seahorse Biosciences for training and providing technical advice regarding experiments on the Seahorse XF24 Analyzer. This work was supported by the Saint Mary's College Summer Research Program, Saint Mary's College Faculty Development Fund and by the National Institute of Environmental Health Sciences (grants ES014901 and ES017592 to PJL). The funding agencies were not involved in the study design, in the collection, analysis, or interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Abbreviations
- BMP
bone morphogenetic protein
- DPI
diphenylene iodinium
- NGA
nordihydroguiaretic acid
- DFO
desferroxamine
- MAP-2
microtubule-associated protein-2
- NOX
NADPH oxidase
- OCR
oxygen consumption rate
- ROS
reactive oxygen species
- SCG
superior cervical ganglia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambasta RK, Kumar P, Griendling KK, Schmidt HHHW, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004;279:45935–41. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993;268:18532–41. [PubMed] [Google Scholar]
- Arteaga S, Andrade-Cetto A, Cárdenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005;98:231–9. doi: 10.1016/j.jep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–21. [PubMed] [Google Scholar]
- Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton. Neurosci. 2009;151:90–7. doi: 10.1016/j.autneu.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran V, Zhai Y, Wagner M, Kaplan PL, Napoli JL, Higgins D. Retinoic acid regulates the morphological development of sympathetic neurons. J. Neurobiol. 2000;42:383–93. [PubMed] [Google Scholar]
- Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002;277:3101–8. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, Milner T. a, Iadecola C, Pickel VM. Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J. Neurosci. 2013;33:4308–16. doi: 10.1523/JNEUROSCI.3061-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J. Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J. Neurosci. 1996;16:1150–62. doi: 10.1523/JNEUROSCI.16-03-01150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Tada-oikawa S, Kawanishi S, Oikawa S. H2O2 Accelerates Cellular Senescence by Accumulation of Acetylated p53 via Decrease in the Function of SIRT1 by NAD + depletion. Cell Physiol. Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- Garred MM, Wang MM, Guo X, Harrington CA, Lein PJ. Transcriptional Responses of Cultured Rat Sympathetic Neurons during BMP-7-Induced Dendritic Growth. PLoS One. 2011;6:e21754. doi: 10.1371/journal.pone.0021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghogha A, Bruun D. a, Lein PJ. Inducing dendritic growth in cultured sympathetic neurons. J. Vis. Exp. 2012;61:e3546. doi: 10.3791/3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JLFC. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Guo X, Lin Y, Horbinski C, Drahushuk KM, Kim IJ, Kaplan PL, Lein P, Wang T, Higgins D. Dendritic growth induced by BMP-7 requires Smad1 and proteasome activity. J. Neurobiol. 2001;48:120–130. [PubMed] [Google Scholar]
- Guo X, Metzler-Northrup J, Lein P, Rueger D, Higgins D. Leukemia inhibitory factor and ciliary neurotrophic factor regulate dendritic growth in cultures of rat sympathetic neurons. Brain Res. Dev. Brain Res. 1997;104:101–10. doi: 10.1016/s0165-3806(97)00142-9. [DOI] [PubMed] [Google Scholar]
- Guo X, Rueger D, Higgins D. Osteogenic protein-1 and related bone morphogenetic proteins regulate dendritic growth and the expression of microtubule-associated protein-2 in rat sympathetic neurons. Neurosci. Lett. 1998;245:131–4. doi: 10.1016/s0304-3940(98)00192-x. [DOI] [PubMed] [Google Scholar]
- Hernández-Damián J, Andérica-Romero AC, Pedraza-Chaverri J. Paradoxical cellular effects and biological role of the multifaceted compound nordihydroguaiaretic Acid. Arch. Pharm. (Weinheim) 2014;347:685–97. doi: 10.1002/ardp.201400159. [DOI] [PubMed] [Google Scholar]
- Hilburger EW, Conte EJ, McGee DW, Tammariello SP. Localization of NADPH oxidase subunits in neonatal sympathetic neurons. Neurosci. Lett. 2005;377:16–19. doi: 10.1016/j.neulet.2004.11.066. [DOI] [PubMed] [Google Scholar]
- Hocking JC, Hehr CL, Chang R-Y, Johnston J, McFarlane S. TGFbeta ligands promote the initiation of retinal ganglion cell dendrites in vitro and in vivo. Mol. Cell. Neurosci. 2008;37:247–60. doi: 10.1016/j.mcn.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Winters C. a, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J. Neurosci. 2004;24:10878–87. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Dendrites. Genes Dev. 2001;15:2627–41. doi: 10.1101/gad.916501. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative Stress in Parkinson's Disease. 2003:26–38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- Katoh S, Mitsui Y, Kitani K, Suzuki T. Hyperoxia induces the differentiated neuronal phenotype of PC12 cells by producing reactive oxygen species. Biochem. Biophys. Res. Commun. 1997;241:347–51. doi: 10.1006/bbrc.1997.7514. [DOI] [PubMed] [Google Scholar]
- Kennedy K. a M., Sandiford SDE, Skerjanc IS, Li SS-C. Reactive oxygen species and the neuronal fate. Cell. Mol. Life Sci. 2012;69:215–21. doi: 10.1007/s00018-011-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH Oxidase 2 Mediates Intermittent Hypoxia-Induced Mitochondrial Complex I Inhibition: Relevance to Blood Pressure Changes in Rats. Antioxid. Redox Signal. 2011;14:533–542. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. Forum Review. Antioxid. Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kim I-J, Beck HN, Lein PJ, Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J. Neurosci. 2002;22:4530–9. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-Y, Gonsiorek EA, Barnhart C, Davare MA, Engebose AJ, Lauridsen H, Bruun D, Lesiak A, Wayman G, Bucelli R, Higgins D, Lein PJ. Statins decrease dendritic arborization in rat sympathetic neurons by blocking RhoA activation. J. Neurochem. 2009;108:1057–1071. doi: 10.1111/j.1471-4159.2008.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Saavedra GM, Cummings BS, Franklin JL. Bax Regulates Production of Superoxide in Both Apoptotic and Nonapoptotic Neurons: Role of Caspases. J. Neurosci. 2010;30:16114–16127. doi: 10.1523/JNEUROSCI.2862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–43. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, Grimm M, Troppmair J. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal. Bioanal. Chem. 2011;400:2383–90. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- Le Roux P, Behar S, Higgins D, Charette M. OP-1 enhances dendritic growth from cerebral cortical neurons in vitro. Exp. Neurol. 1999;160:151–63. doi: 10.1006/exnr.1999.7194. [DOI] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004;23:4792–801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P, Guo X, Hedges AM, Rueger D, Johnson M, Higgins D. The effects of extracellular matrix and Osteogenic Protein-1 on the morphological differentiation of rat sympathetic neurons. Int. J. Dev. Neurosci. 1996;14:203–215. doi: 10.1016/0736-5748(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Liberman M, Johnson RC, Handy DE, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem. Biophys. Res. Commun. 2011;413:436–41. doi: 10.1016/j.bbrc.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdazari A, Stubbusch J, Müller CM, Hennchen M, Weber M, Deng C-X, Mishina Y, Schütz G, Deller T, Rohrer H. Dendrite complexity of sympathetic neurons is controlled during postnatal development by BMP signaling. J. Neurosci. 2013;33:15132–44. doi: 10.1523/JNEUROSCI.4748-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011;14:2013–54. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- McAllister a. K. Cellular and Molecular Mechanisms of Dendrite Growth. Cereb. Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- Natera-Naranjo O, Kar AN, Aschra A, Gervasi NM, Macgibeny MA, Gioio AE, Kaplan BB. Molecular and Cellular Neuroscience Local translation of ATP synthase subunit 9 mRNA alters ATP levels and the production of ROS in the axon. 2012;49:263–270. doi: 10.1016/j.mcn.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Pache G, Schäfer C, Wiesemann S, Springer E, Liebau M, Reinhardt HC, August C, Pavenstädt H, Bek MJ. Upregulation of Id-1 via BMP-2 receptors induces reactive oxygen species in podocytes. Am. J. Physiol. Renal Physiol. 2006;291:F654–62. doi: 10.1152/ajprenal.00214.2004. [DOI] [PubMed] [Google Scholar]
- Podkowa M, Christova T, Zhao X, Jian Y, Attisano L. p21-Activated kinase (PAK) is required for Bone Morphogenetic Protein (BMP)-induced dendritogenesis in cortical neurons. Mol. Cell. Neurosci. 2013;57:83–92. doi: 10.1016/j.mcn.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Podkowa M, Zhao X, Chow C-W, Coffey ET, Davis RJ, Attisano L. Microtubule stabilization by bone morphogenetic protein receptor-mediated scaffolding of c-Jun N-terminal kinase promotes dendrite formation. Mol. Cell. Biol. 2010;30:2241–50. doi: 10.1128/MCB.01166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Hume RI. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion. J. Neurosci. 1981;1:441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. doi:10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rubin E. Development of the Rat Superior Cervical Ganglion : Initial Stages of Synapse Formation. J. Neurosci. 1985;5:697–704. doi: 10.1523/JNEUROSCI.05-03-00697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone S, Cosola C, Loverre A, Cariello M, Sallustio F, Rascio F, Gesualdo L, Schena FP, Grandaliano G, Pertosa G. BMP-2 induces a profibrotic phenotype in adult renal progenitor cells through Nox4 activation. Am. J. Physiol. Renal Physiol. 2012;303:F23–34. doi: 10.1152/ajprenal.00328.2011. [DOI] [PubMed] [Google Scholar]
- Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol. 2012;19:752–763. doi: 10.1016/j.chembiol.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Krause K. NOX Enzymes in the Central Nervous System : From Signaling to Disease. Antioxid. Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad.Sci. USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 1996;318:379–82. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K. Nerve Growth Factor-induced Neuronal Differentiation Requires Generation of Rac1-regulated Reactive Oxygen Species. J. Biol. Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van Meerloo J, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Venkataraman S, Buettner GR. The Rate of Oxygen Utilization by Cells. Free Radic. Biol. Med. 2011;51:700–712. doi: 10.1016/j.freeradbiomed.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BA, Venkataraman S, Buettner GR. The Rate of Oxygen Utilization by Cells. Free Radic. Biol. Med. 2011;51:700–712. doi: 10.1016/j.freeradbiomed.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers GS, Higgins D, Charette M, Banker G. Bone morphogenetic protein-7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. Eur. J. Neurosci. 2000;12:106–116. doi: 10.1046/j.1460-9568.2000.00889.x. [DOI] [PubMed] [Google Scholar]