Abstract
Background and Purpose
In primary intracerebral hemorrhage (ICH), the presence of contrast extravasation following CT angiography (CTA), termed the ‘spot sign’, predicts hematoma expansion and mortality. Since the biological underpinnings of the spot sign are not fully understood, we investigated whether the rate of contrast extravasation - which may reflect the rate of bleeding - predicts expansion and mortality beyond the simple presence of the spot sign.
Methods
Consecutive ICH patients with first-pass CTA followed by a 90-second delayed post-contrast CT (delayed CTA) were included. CTAs were reviewed for spot sign presence by two blinded readers. Spot sign volumes on first-pass and delayed CTA and ICH volumes were measured using semi-automated software. Extravasation rates were calculated and tested for association with hematoma expansion and mortality using uni- and multivariable logistic regression.
Results
162 patients were included, 48 (30%) of whom had ≥1 spot sign. Median spot sign volume was 0.04mL on first-pass CTA and 0.4mL on delayed CTA. Median extravasation rate was 0.23mL/min overall, and 0.30mL/min among expanders versus 0.07mL/min in non-expanders. Extravasation rates were also significantly higher in patients who died in hospital: 0.27mL/min versus 0.04mL/min. In multivariable analysis, the extravasation rate was independently associated with in-hospital mortality (OR1.09 [95%CI 1.04–1.18], p=0.004), 90-day mortality (OR1.15 [95%CI 1.08–1.27], p=0.0004), and hematoma expansion (OR1.03 [95%CI 1.01–1.08], p=0.047).
Conclusions
Contrast extravasation rate, or spot sign growth, further refines the ability to predict hematoma expansion and mortality. Our results support the hypothesis that the spot sign directly measures active bleeding in acute ICH.
Keywords: Intracerebral Hemorrhage, Hematoma Expansion, CTA Spot Sign, Mortality, CT angiography
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) is the deadliest form of stroke, with a one-month mortality rate of about 40%.1 With no medical or surgical therapy proven to benefit clinical outcomes, ICH is a pressing public health concern in need of effective therapies and better understanding of patients at highest risk of poor outcomes.
Although initial hematoma volume upon hospital arrival remains among the strongest predictors of mortality, it is a non-modifiable determinant of outcome.2 On the other hand, hematoma expansion is a potentially modifiable predictor tightly correlated with poor functional outcome and death, occurring in up to 40% of ICH patients.3–5 Expansion is therefore an attractive and common treatment target in previous and current clinical trials.6–9
Nevertheless, the specific targeting of hematoma expansion has yet to yield functional improvement in large randomized controlled trials, including the recently published INTERACT2 trial.9 This may be attributed to the challenge of accurately identifying those patients most likely to benefit from an intervention; that is, those who will experience expansion severe enough to negatively impact outcome.10,11
Adequate selection tools are therefore warranted and contrast extravasation following CT angiography (CTA), commonly termed the ‘spot sign’, has been a widely studied phenomenon.12–15 The spot sign is thought to represent active bleeding, but the biological underpinnings of the spot sign are not fully understood.14,16 We therefore investigated whether the size of the spot sign changes on delayed imaging, and if so, whether the rate of change predicts hematoma expansion and mortality beyond the simple presence of the spot sign.
METHODS
Study Design
Primary ICH patients presenting to Massachusetts General Hospital (MGH), an urban academic tertiary care center, were approached for enrollment in an ongoing prospective cohort study of ICH. Written informed consent was obtained from all patients or their legally authorized health care proxies, or their consent was waived by a protocol specific allowance. The Institutional Review Board at MGH approved all portions of the study.
Study Subjects
During the study period April 2012 to May 2013, consecutive ICH patients enrolled in the aforementioned prospective cohort study were considered eligible for the current analysis based on the following criteria: 1.) Primary ICH confirmed on CT of sufficient quality for volumetric analyses; 2.) Baseline (first-pass) CTA for spot sign reading and spot sign volume measurements; 3.) 90-second delayed CTA for repeated spot sign reading and volumetric measurements.
Patients with secondary ICH were excluded, including vascular malformations, neoplasms, trauma, or hemorrhagic transformation of an ischemic stroke. Patients with primary intraventricular hemorrhage and those who underwent surgical evacuation were also excluded.
Clinical Data
Clinical data including age, sex, past medical history, and prior medication use (e.g. oral anticoagulants) were all collected through patient interviews (or their surrogates). Prospectively recorded admission variables comprised Glasgow Coma Scale (GCS), systolic and diastolic blood pressure, and time from symptom onset to CTA. At discharge and 90 days, trained study staff ascertained mortality. The Social Security Death Index, a database of deaths reported to the United States Social Security Administration, was used to supplement mortality data.17
Imaging Analyses
Experienced neurologists or neuroradiologists assigned hemorrhage locations for all patients, blinded to clinical data, functional outcome, and CTA readings. ICH location was categorized into lobar, deep, brainstem, and cerebellar. Volumetric measurements for baseline and follow-up ICH volumes were carried out by experienced readers, blinded to other data points, according to previously published protocols.13,18 Analyze 10.0 (AnalyzeDirect, Overland Park, KS) was used for semi-automated volume segmentation. Significant hematoma expansion, assessed as secondary endpoint, was defined as either an absolute volume increase of 6mL or a relative increase of 33% between the first and second CT.15,19
Of note, delayed CTA imaging became standard of care at our institution in April 2012 and encompasses a 90-second delayed post-contrast CT (without a new bolus of contrast). This acquisition will be termed ‘delayed CTA’ throughout this manuscript for consistency with definitions used in the recent literature.
Spot sign status (absent or present) was assigned by two blinded readers in accordance with standard methods with excellent inter-rater reliability.18 Spot sign volumes on first-pass and delayed CTA were measured using the previously described Analyze software (Figure 1). Contrast extravasation rates were calculated using the following formula: follow-up spot sign volume minus baseline spot sign volume divided by time elapsed between first-pass and delayed CTA (formula at end of the paragraph). Following this formula, spot sign negative patients were assigned a contrast extravasation rate of zero.
CER = Contrast Extravasation Rate; SSVFU = Spot Sign Volume (follow-up); SSVBL = Spot Sign Volume (baseline); tDEL-FP = Time delayed minus first-pass CTA
Figure 1. CT Angiography Spot Sign Volume Measurements.
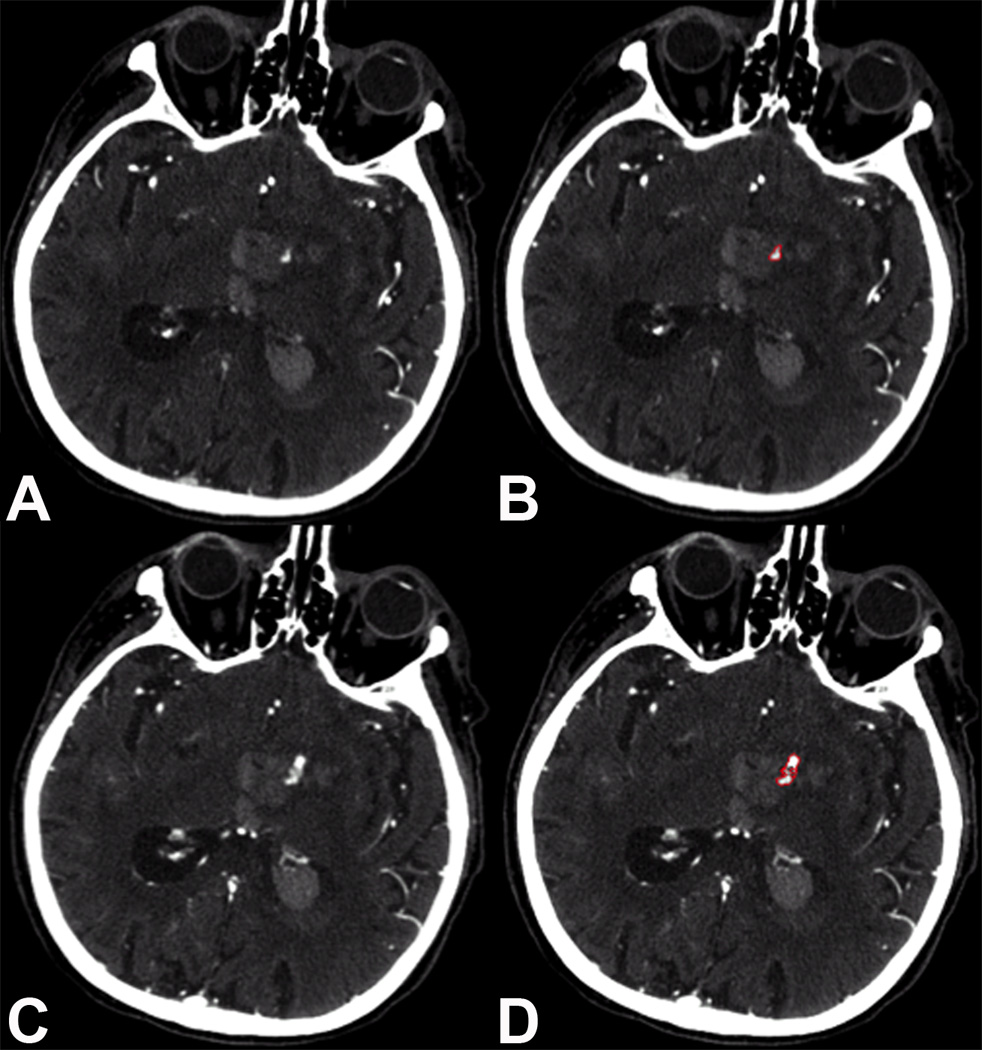
First-pass and delayed CT angiography visualizing active contrast extravasation (i.e. spot sign growth) in a patient with left deep intracerebral hemorrhage (panels A and C). Semi-automated measurement techniques were used to quantify the spot sign volumes and calculate the contrast extravasation rate (panels B and D).
Statistical Analyses
Discrete variables are presented as count and percentage (%), while continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR) where appropriate. CTA contrast extravasation rates are presented as milliliter per minute (mL/min) or milliliter per hour (mL/h). The primary analysis comprised uni- and multivariable logistic regression to test the relationship between contrast extravasation rate and in-hospital mortality. Hematoma expansion was tested as a secondary endpoint in the subgroup of patients with an available follow-up CT. Multivariable models included age, sex, and variables with p < 0.20 in the univariable analyses. Collinear variables, measured using the variance inflation factor, were removed from the multivariable model as appropriate. Of note, the spot sign was not included in the multivariable models due to strong collinearity with contrast extravasation rate. All analyses were also repeated using interaction terms, returning identical results (data not shown). In addition, median contrast extravasation rates were compared for spot sign positive patients. All statistical analyses were performed using JMP Pro version 11.0 (SAS Institute Inc., Cary, NC). The threshold for significance was set at p < 0.05.
RESULTS
Study Population
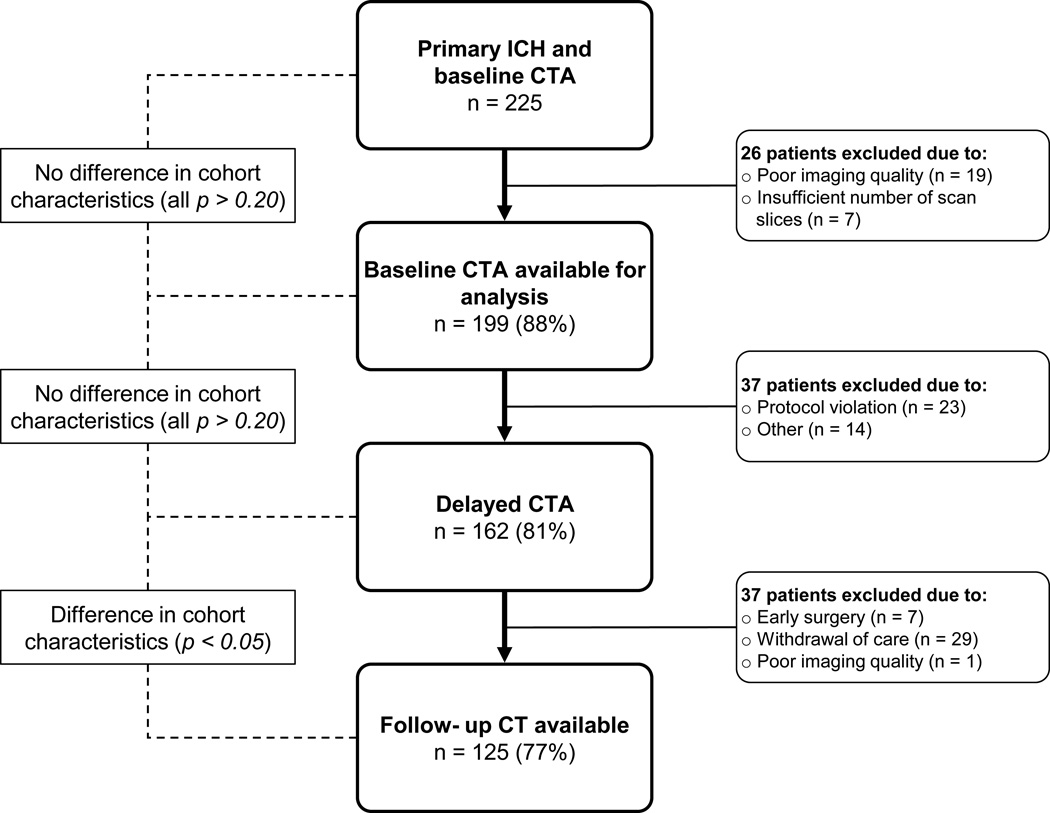
During the study period, 225 patients presented to our institution with primary ICH and had an available CTA. After application of the eligibility criteria, 162 patients had a first-pass and delayed CTA of sufficient quality for analysis (Figure 2). Baseline cohort characteristics are provided in table 1. Briefly, the mean age was 73 (SD 13) years, 65 (40%) were females, and 31 (19%) patients were taking warfarin prior to admission. 125 (77%) patients had a follow-up CT available for analysis. Patients without an available follow-up CT were more likely to be on pre-admission oral anticoagulation, had lower GCS scores at hospital admission, larger baseline ICH volumes, and higher in-hospital mortality rates (all p < 0.05).
Figure 2. Cohort flowchart.
ICH = Intracerebral Hemorrhage; CTA = CT Angiography
Table 1.
Cohort Characteristics
| Variable | All n (%) |
|---|---|
| Number of patients | 162 |
| Age (mean, SD) | 73 (13) |
| Sex (female) | 65 (40) |
| Hypertension | 131 (82) |
| Diabetes | 37 (24) |
| Antiplatelets | 82 (51) |
| Warfarin | 31 (19) |
| GCS (median, IQR) | 12 (6 – 15) |
| SBP (median, IQR) | 176 (155 – 204) |
| Time to CTA, in hours (median, IQR) | 4 (2 – 8) |
| ICH location Lobar Deep Brainstem Cerebellar |
81 (50) 60 (37) 7 (4) 14 (9) |
| Baseline ICH volume (median, IQR) | 23 (7 – 66) |
| Intraventricular extension | 94 (58) |
| Baseline IVH volume* (median, IQR) | 7 (2 – 16) |
| Spot sign presence First-pass CTA Delayed CTA |
41 (25%) 48 (30%) |
| Spot sign volume (median, IQR) First-pass CTA Delayed CTA |
0.04 (0.01 – 0.09) 0.4 (0.06 – 0.91) |
| Extravasation rate (mL / min) | 0.23 (0.06 – 0.64) |
| Extravasation rate (mL / hour) | 13.8 (3.58 – 38.42) |
| Hematoma expansion** | 26 (21%) |
| In-hospital mortality | 65 (40%) |
| 90-day mortality | 70 (43%) |
Data refer to ICH patients with intraventricular extension only (n = 94, 58%).
Data refer only to ICH patients with a follow-up CT (n = 125, 77%)
SD = Standard Deviation; GCS = Glasgow Coma Scale; IQR = Interquartile Range; SBP = Systolic Blood Pressure; CTA = CT Angiography; ICH = Intracerebral Hemorrhage; IVH = Intraventricular Hemorrhage; mL = Milliliter
CT and CTA Imaging
Of the 162 patients included, 81 (50%) had lobar, 60 (37%) deep, 14 (9%) cerebellar, and 7 (4%) brainstem ICH. Median baseline ICH volume was 23 mL (IQR 7 – 66). At least one spot sign was identified in 41 (25%) patients on first-pass CTA and in 48 (30%) patients on delayed CTA (median delay 84 seconds [IQR 65 – 141]). Median spot sign volumes on first-pass and delayed CTA were 0.04 mL (IQR 0.01 – 0.09) and 0.4 mL (IQR 0.06 – 0.91), respectively. Hematoma expansion was present in 26 (21%) of the 125 patients with an available follow-up CT.
Contrast Extravasation Rates
Median contrast extravasation rate was 0.23 mL/min (IQR 0.06 – 0.64) among spot sign positive patients (spot sign negative patients were assigned a rate of zero). Median extravasation rates were significantly higher in spot sign positive patients who died in hospital versus those who survived: 0.27 mL/min (IQR 0.13 – 0.77) versus 0.04 mL/min (IQR 0.009 – 0.08), p < 0.0001. Among spot sign positive patients, those who experienced significant expansion had higher median extravasation rates: 0.30 mL/min (IQR 0.06 – 0.68) versus 0.07 mL/min (IQR 0.03 – 0.29), p = 0.16. Patients with a lobar ICH had higher median extravasation rates compared to those with deep ICH: 0.33 mL/min (IQR 0.11 – 0.77) versus 0.11 mL/min (IQR 0.05 – 0.26), p = 0.046.
Predictors of In-hospital Mortality and Hematoma Expansion
In univariable analysis, baseline ICH volume, CTA spot sign, and contrast extravasation rate were all associated with in-hospital mortality (Table 2). In multivariable analysis (adjusted for age, sex, and warfarin use), time to CTA, baseline ICH volume, and contrast extravasation rate were all independently associated with in-hospital mortality (Table 3). Contrast extravasation rate was also associated with 90-day mortality (available for all patients; OR 1.15 [95% CI 1.08 – 1.27], p = 0.0004).
Table 2.
Univariable Analysis of In-Hospital Mortality and Hematoma Expansion
| Variable | In-hospital death | Hematoma Expansion* | ||
|---|---|---|---|---|
| OR (95% CI) | P–value | OR (95% CI) | P–value | |
| Age | 1.01 (0.98 – 1.03) | 0.55 | 1.01 (0.98 – 1.05) | 0.45 |
| Sex (male) | 1.95 (1.01 – 3.77) | 0.05 | 1.34 (0.54 – 3.29) | 0.65 |
| Hypertension | 1.87 (0.77 – 4.55) | 0.20 | 1.11 (0.37 – 3.30) | 1.00 |
| Antiplatelet | 1.33 (0.70 – 2.51) | 0.42 | 0.86 (0.35 – 2.11) | 0.82 |
| Warfarin | 1.79 (0.81 – 3.93) | 0.16 | 1.15 (0.38 – 3.47) | 0.78 |
| Time to CTA** | 1.05 (1.00 – 1.11) | 0.09 | 1.17 (1.02 – 1.42) | 0.01 |
| Baseline ICH volume | 1.05 (1.03 – 1.06) | <0.0001 | 1.02 (1.01 – 1.03) | 0.0003 |
| CTA spot sign | 7.93 (3.51 – 17.93) | <0.0001 | 3.84 (1.40 – 10.52) | 0.01 |
| Extravasation rate (mL / hour) | 1.17 (1.09 – 1.29) | 0.0002 | 1.03 (1.01 – 1.06) | 0.02 |
Data refer only to ICH patients with a follow-up CT (n = 125, 77%)
Odds ratio for shorter time to CTA
OR = Odds Ratio; 95% CI = 95% Confidence Interval; CTA = CT Angiography; ICH = Intracerebral Hemorrhage; mL = milliliter
Table 3.
Multivariable Analysis of In-Hospital Mortality and Hematoma Expansion
| Variable | In-hospital death | Hematoma Expansion* | ||
|---|---|---|---|---|
| OR (95% CI) | P–value | OR (95% CI) | P–value | |
| Age | 1.00 (0.96 – 1.05) | 0.90 | --- | -- |
| Sex (male) | 1.70 (0.54 – 5.62) | 0.37 | --- | -- |
| Warfarin | 1.70 (0.28 – 14.00) | 0.59 | --- | -- |
| Time to CTA** | 1.11 (1.02 – 1.23) | 0.02 | 1.10 (1.02 – 1.32) | 0.01 |
| Baseline ICH volume | 1.04 (1.03 – 1.07) | <0.0001 | 1.01 (1.00 – 1.02) | 0.10 |
| Extravasation rate (mL / hour) | 1.09 (1.04 – 1.18) | 0.004 | 1.03 (1.01 – 1.08) | 0.047 |
Data refer only to ICH patients with a follow-up CT (n = 125, 77%). Analysis restricted to three covariables based on the rule of one covariate per ten outcome events (n = 26).
Odds ratio for shorter time to CTA
OR = Odds Ratio; 95% CI = 95% Confidence Interval; ICH = Intracerebral Hemorrhage; CTA = CT Angiography; mL = milliliter
Baseline ICH volume, CTA spot sign, and contrast extravasation rate were also associated with hematoma expansion, as well as shorter time to CTA, in univariable analysis (Table 2). A limited multivariable analysis (based on only 26 outcome events) showed an association with hematoma expansion for shorter time to CTA (odds ratio [OR] 1.10 [95% confidence interval {95% CI} 1.02 – 1.32], p = 0.01) and contrast extravasation rate (OR 1.03 [95% CI 1.01 – 1.08], p = 0.047) (Table 3).
DISCUSSION
This study demonstrates that CTA contrast extravasation rate predicts hematoma expansion, in-hospital death, and 90-day mortality in patients with primary ICH. Our results add to the body of literature suggesting that spot sign represents active bleeding, and may additionally help to better select patients for clinical trials aimed at the attenuation of hematoma expansion.
The CTA spot sign is strongly correlated with both hematoma expansion and poor functional outcome.12–15 It has been widely hypothesized that the spot sign represents active contrast extravasation, and therefore serves as a visual manifestation of continued bleeding.14,16 Alternative hypotheses of what the spot sign represents include Charcot-Bouchard aneurysms, micro-dissections, and pseudo-aneurysms.12,20 Our findings lend credence to the active bleeding theory, as spot signs were shown to increase in size between the first-pass and delayed CTAs (Table 1). The solely positive extravasation rates likely mark an increase in blood (mixed with contrast agent) leaving the injured vessel and entering the brain parenchyma over time. The association of higher extravasation rates with more hematoma expansion (i.e. larger final ICH volumes) further supports this active bleeding theory. This demonstration of active bleeding, as well as the increase in the number of spot signs visible on delayed CTA, provide additional evidence for an ‘avalanche’ expansion model of cascading small vessel injury as originally proposed by Dr. C. Miller Fisher.21 Based on the observation of multiple recently ruptured vessels at the periphery of serially sectioned hematomas, this model describes the process of hematoma expansion as secondary mechanical shearing of neighboring vessels caused by expansion of the initial hemorrhage.21 When these neighboring vessels rupture, contrast leaks out and is seen as an additional spot sign on the delayed CTA.
Our results show that the contrast extravasation rate better distinguishes between expanders and non-expanders than the CTA spot sign alone. Further refinement of the CTA spot sign as a prognostic tool is needed, as the sensitivity for predicting significant hematoma expansion in two recent prospective validation studies only reached 0.51 and 0.64, with positive predictive values of 0.61 and 0.52, respectively.15,22 By the superior selection of those patients most likely to expand, clinical trials will also be more likely to show a benefit for treatments aimed at arresting expansion, as the potential benefit of any treatment must be balanced against its potential harms.11 The enhanced selection of those patients who are actively bleeding while in the CT scanner is an important first step in this process.
Additional provocative data come from the raw extravasation rates in spot sign positive patients: 0.23 mL/min (IQR 0.06 – 0.64), which translates into an hourly bleeding rate of nearly 14 mL per hour (Table 1). In the phase III recombinant factor VIIa trial, the mean increase in ICH volume between the baseline and 24-hour CT was only 7.5 mL (95% CI 5.4 – 9.6) in the non-treated (placebo) arm.7 Therefore, the bleeding rate of 14 mL per hour described here seems very high, although this only represents spot sign positive patients. Such a bleeding rate seems unsustainable given the limited intracranial volume and would probably lead to rapid deterioration and early death in these patients. These amounts of hematoma expansion were typically not seen on follow-up CTs in our cohort. It is therefore more plausible that hematoma expansion occurs at a variable pace as opposed to a linear fashion. The inherent downside of these findings is that interventions aimed at the restriction of expansion need to be implemented early in the course of disease, as extravasation rates are high in the acute phase (when patients undergo their CTA) and may plateau later on, although the latter needs to be established in future studies. This brings to mind similarities with the time-dependent effectiveness of intravenous tissue plasminogen activator for the treatment of acute ischemic stroke.23
Our study has several strengths, including its prospective design, the standard acquisition of delayed CTAs at our institution since 2012, and the large sample size compared to previous studies assessing the role of delayed CTA acquisitions. Nevertheless, 162 patients is still a limited number to establish definite associations. Furthermore, our study is limited by its single-center design and the lack of follow-up CTs in almost a quarter of the patients. The latter is a recurring problem in all non-randomized (observational) studies without standardized follow-up imaging. Lastly, our study is limited by its outcome measures of expansion and mortality, more refined measures like quality of life were not collected as part of the present study.
In conclusion, our results demonstrate that contrast extravasation rate (i.e. spot sign growth) further refines the ability to predict hematoma expansion and in-hospital mortality. These data support the hypothesis that the spot sign directly measures active bleeding in the acute phase of ICH.
Acknowledgements
None.
Sources of Funding
All funding entities had no involvement in study design, data collection, analysis, and interpretation, writing of the manuscript, and in the decision to submit for publication. The project described was supported by National Institutes of Health – National Institute of Neurological Disorders and Stroke (NIH-NINDS) grants R01NS073344, R01NS059727, and 5K23NS059774. Drs. Brouwers and Falcone were supported by the NIH-NINDS SPOTRIAS fellowship grant P50NS051343.
J.N. Goldstein, Research Grant NIH-NINDS, Consultant / advisory board CSL Behring; R.G. Gonzalez, None; J. Rosand, Research Grant NIH, Consultant Boehringer Ingelheim; J.M. Romero, Imaging Committee DIAS trial / advisory board Lundbeck pharmaceuticals.
Footnotes
Disclosures
H.B. Brouwers, None; T.W.K. Battey, None; H.H. Musial, None; V.A. Ciura, None; G.J. Falcone, None; A.M. Ayres, None; A. Vashkevich, None; K. Schwab, None; A. Viswanathan, None; C.D. Anderson, Research Fellowship American Brain Foundation, Research Fellowship Biogen Idec; S.M. Greenberg, Research Grant NIH; S.R. Pomerantz, Research Fellow Salary Support GE Healthcare; C.J. Ortiz, None;
REFERENCES
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 3.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 4.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology. 2012;79:314–319. doi: 10.1212/WNL.0b013e318260cbba. [DOI] [PubMed] [Google Scholar]
- 6.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J. Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 7.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J. Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II design, methods, and rationale. Neurocrit Care. 2011;15:559–576. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J. Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 10.Mayer SA, Davis SM, Skolnick BE, Brun NC, Begtrup K, Broderick JP, et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke. 2009;40:833–840. doi: 10.1161/STROKEAHA.108.524470. [DOI] [PubMed] [Google Scholar]
- 11.Brouwers HB, Greenberg SM. Hematoma Expansion following Acute Intracerebral Hemorrhage. Cerebrovasc. Dis. 2013;35:195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 14.Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 16.Dowlatshahi D, Hogan MJ, Sharma M, Stotts G, Blacquiere D, Chakraborty S. Ongoing bleeding in acute intracerebral haemorrhage. Lancet. 2013;381:152. doi: 10.1016/S0140-6736(12)60829-0. [DOI] [PubMed] [Google Scholar]
- 17.Wojcik NC, Huebner WW, Jorgensen G. Strategies for using the National Death Index and the Social Security Administration for death ascertainment in large occupational cohort mortality studies. Am J Epidemiol. 2010;172:469–477. doi: 10.1093/aje/kwq130. [DOI] [PubMed] [Google Scholar]
- 18.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Huynh TJ, Keith J, Aviv RI. Histopathological characteristics of the "spot sign" in spontaneous intracerebral hemorrhage. Arch Neurol. 2012;69:1654–1655. doi: 10.1001/archneurol.2012.672. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Romero JM, Brouwers HB, Lu J, Delgado-Almondoz J, Kelly H, Heit J, et al. Prospective Validation of the Computed Tomographic Angiography Spot Sign Score for Intracerebral Hemorrhage. Stroke. 2013;44:3097–3102. doi: 10.1161/STROKEAHA.113.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]