Highlights
-
•
Animal coronaviruses can cause severe respiratory illness and death in humans.
-
•
Coronavirus vaccines and antiviral drugs are not yet available.
-
•
Animal models are needed to study pathogenesis and evaluate vaccines and antivirals.
-
•
No single animal model can be used to study both SARS and MERS coronaviruses.
-
•
The most appropriate animal model should be selected to meet experimental goals.
Abstract
The emergence of Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome coronavirus (MERS-CoV), two strains of animal coronaviruses that crossed the species barrier to infect and cause severe respiratory infections in humans within the last 12 years, have taught us that coronaviruses represent a global threat that does not recognize international borders. We can expect to see other novel coronaviruses emerge in the future. An ideal animal model should reflect the clinical signs, viral replication and pathology seen in humans. In this review, we present factors to consider in establishing an animal model for the study of novel coronaviruses and compare the different animal models that have been employed to study SARS-CoV and MERS-CoV.
Current Opinion in Virology 2015, 13:123–129
This review comes from a themed issue on Animal models for viral diseases
Edited by Alexander Ploss and Christopher Walker
For a complete overview see the Issue and the Editorial
Available online 14th July 2015
http://dx.doi.org/10.1016/j.coviro.2015.06.009
1879-6257/Published by Elsevier B.V.
Introduction
Members of the Coronaviridae family infect a wide range of animal species in nature and most are limited in their host range [1]. Human coronaviruses including OC43, 229E, NL63 and HKU1 are generally associated with self-limiting respiratory tract infections (Table 1 ) [1, 2•]. However, in the past 12 years, two outbreaks of severe respiratory tract infection, SARS and MERS, have been caused by animal coronaviruses that have crossed the species barrier. Despite the severe disease and high case fatality rate associated with SARS and MERS, coronavirus vaccines and antiviral drugs are not yet available. Animal models are needed for pathogenesis studies as well as for evaluation of vaccines and antiviral drugs. We will focus on animal models for these two coronaviruses in this review.
Table 1.
Coronaviruses associated with disease in humans.
| Primary site of disease | Virus | Receptor | Other systems involved |
|---|---|---|---|
| Upper respiratory tract | OC43 | Unknown | Gastrointestinal |
| 229E | Aminopeptidase N | Gastrointestinal | |
| NL63 | ACE2 | – | |
| HKU1 | Unknown | Gastrointestinal | |
| Lower respiratory tract | SARS-CoV | ACE2 | – |
| MERS-CoV | DPP4 | Renal failure |
Coronaviruses contain a 30 KB long positive-sense RNA genome. Receptor binding domains of the viral spike protein on SARS-CoV and MERS-CoV attach to angiotensin-converting enzyme 2 (ACE2) [3, 4] and dipeptidyl-peptidase 4 (DPP4) proteins [5••, 6••], respectively. SARS was first reported in Hong Kong in 2003, and went on to cause over 8000 infections with an approximately 10% case-fatality rate [7, 8]. The newly emerged MERS-CoV, identified in 2012, has caused over 800 infections associated with a case fatality rate of approximately 40% [9•, 10, 11••]. In 2014, the Centers for Disease Control and Prevention confirmed the first MERS case imported into the United States. The development and evaluation of antiviral drugs and vaccines for SARS and MERS has been challenging, in part because of difficulties in developing animal models that provide consistent and reproducible results.
The ideal animal model is one that mimics human disease in sharing the route of infection, increased severity of disease in the corresponding demographic groups and comparable levels of mortality/morbidity. The presence and distribution of viral receptors should be similar to that in humans. The virus should replicate in the selected animal species and a correlation should exist between virus titer and disease severity. Finally, animal models should be carefully assessed and selected to meet experimental goals (Figure 1 ). For example, if the primary focus is to elucidate pathogenesis, the animal model should fully replicate key aspects of the disease and immunological reagents must be available. By contrast, the primary outcome in a vaccine efficacy study is a meaningful difference between vaccinated and the unvaccinated control groups; the ability of a vaccine to prevent clinical disease and/or pathology associated with viral replication following challenge provides compelling evidence of vaccine efficacy [12] though at a minimum, differences in challenge virus replication can be assessed as a measure of vaccine efficacy.
Figure 1.
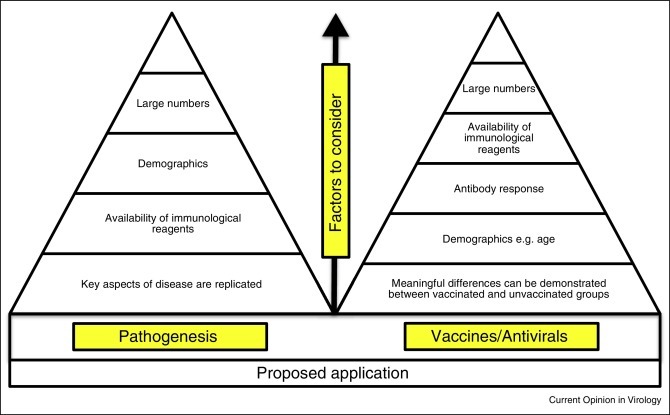
Factors to consider when selecting an animal model. Animal models should be tailored to the goals of the study. If the primary goal is to elucidate pathogenesis, the model should replicate key aspects of the disease and immunological reagents should be available. The demographic background (e.g. age for SARS) of the animal should be taken into consideration. By contrast, animal models used in vaccine/antiviral efficacy studies must demonstrate meaningful differences between vaccinated and unvaccinated control groups. Special consideration should be given to how animals from different demographic backgrounds respond to the vaccine/antiviral under investigation. To determine the correlate of protection, it is necessary to study the immune response to the vaccine as well as the response to challenge with the homologous coronavirus. It may be of interest to evaluate the response to challenge with other coronaviruses that the vaccinated host may encounter.
Coronavirus disease in humans
People infected with SARS-CoV and MERS-CoV present with initial symptoms that include fever, myalgia and respiratory signs including a nonproductive cough and dyspnea [9•, 11••, 13, 14, 15, 16, 17, 18•]. Chest radiograph abnormalities are evident in almost all cases. Etiologic diagnosis is made by virus isolation in culture, polymerase chain reaction assays or serological testing for antibodies to the virus. SARS associated lung pathology was described from examination of post-mortem tissue samples [7, 19, 20, 21]; however, pathologic changes associated with MERS have not been reported, perhaps because autopsies are rarely performed. The findings in SARS were consistent with prolonged inflammation with destruction and desquamation of alveolar pneumocytes. Hyaline-membrane formation, interstitial inflammatory infiltration and intraalveolar hemorrhage were observed [7] and multinucleated giant cells were also seen. The presence of viral antigen was demonstrated by immunohistochemistry (IHC) in the lungs.
The median age of patients infected with SARS-CoV and MERS-CoV is different. MERS-CoV tends to affect middle-aged males, while SARS-CoV had a predilection for older people. The overall case-fatality rate for MERS (40%) is greater than was seen with SARS (10%). Finally, preexisting chronic illnesses such as diabetes, renal disease and heart disease were less common in SARS-CoV patients [18•].
Animal models for SARS and MERS
Non-human primates
SARS-CoV was shown to infect rhesus macaques [22, 23], cynomolgus macaques [22, 23, 24, 25, 26] and African green monkeys (AGMs) [22]. Clinical signs, viral replication and pathology depended upon the species. There is at least one report of pneumonitis in each species but the findings in non-human primates (NHPs) were variable, likely because of genetic variability in subspecies and differences in experimental methods including inoculum dose and route [22, 23, 25]. Greenough et al. reported multi-organ involvement with fever, diarrhea and hepatitis in common marmosets [27].
Infection of rhesus macaques and common marmosets with MERS-CoV has resulted in different outcomes. Rhesus macaques showed a transient pulmonary infection [28•, 29•]. Radiographs of the chest revealed localized infiltration and interstitial markings. Clinical illness was associated with viral replication in the pneumocytes around the terminal bronchioles [28•, 29•]. These findings were consistent with viral load detected by reverse transcription polymerase chain reaction (RT-PCR) and viral antigen in alveolar pneumocytes detected by IHC. By contrast, the clinical symptoms in the marmoset model were much more severe [30••]. In addition to bronchointerstitial pneumonia and viral antigen detected in the lungs, the marmosets supported viral titers a thousand-fold higher than rhesus macaques [30••].
The anatomical, physiological and immunological similarities of NHPs to humans make them ideal models to recapitulate the pathogenesis of coronavirus infection in humans. However, costs, limited availability and individual variation among NHPs make it difficult to conduct studies in large enough sample sizes for statistical evaluation and to draw robust conclusions. Despite these limitations, it is desirable to evaluate coronavirus vaccine candidates in NHPs before proceeding to clinical trials because we have no clinical experience with human coronavirus vaccines. Special consideration should be given to the demographic background (age, sex and source) and the presence of co-pathogens and studies should be carried out in large sample sizes in order to assess statistical significance.
Mice
SARS-CoV replication was observed in several inbred strains of mice (BALB/c, C57BL6 and 129S) following intranasal infection, and 129S mice were more susceptible than BALB/c mice [31, 32, 33]. Young inbred mice supported viral replication but failed to show clinical signs of disease [31, 33]. As in humans infected with SARS-CoV, age seemed to play an important role in disease susceptibility in mice. Twelve-month-old BALB/c mice developed more severe disease than young mice [34, 35]. On intranasal infection, the older mice developed weight loss, ruffled fur and dehydration [34]. Histopathology showed interstitial pneumonitis along with diffuse alveolar damage and viral antigens were detected by IHC in the lungs. The older BALB/c mouse provided an opportunity to study the age-dependent susceptibility of humans to SARS-CoV [35, 36]. Several knockout mice (Rag1−/−, CD1−/−, Beige) were also infected with SARS-CoV in order to determine the role of immune effectors in the disease [31]. STAT 1−/− mice in the 129S background supported prolonged viral replication and histopathology similar to humans [32, 37]. However, mice with targeted immune defects are of limited value in vaccine studies.
Because infection in young mice was cleared rapidly without clinical disease, in addition to infecting older mice, two approaches were employed to enhance clinical signs of disease in young mice: the development of transgenic mice expressing the human ACE2 (hACE2) receptor and the adaptation of SARS-CoV to mice by serial passage. McCray et al. demonstrated that expression of hACE2 under the control of an epithelial cell-specific promoter K18 resulted in lethal SARS-CoV infection [38]. However, SARS-CoV infection in K18-hACE2 mice was associated with central nervous system disease, which was not a feature of SARS in humans. Tseng et al. developed two lineages of transgenic mice expressing hACE2 under the CAG promoter, a strong composite promoter consisting of the cytomegalovirus immediate early enhancer, the chicken β-actin promoter, rabbit globulin splicing and polyadenylation sites to drive high levels of gene expression in mammalian expression vectors [39]. The transgene-positive mice (AC70 and AC63) showed robust viral growth, generalized illness and tissue pathology after infection with SARS-CoV [39]. The lethal lineage of mice (AC70) showed a wider spectrum of clinical manifestations, including death, than the nonlethal lineage mice (AC63). Transgenic mice were used for pathogenesis studies and evaluation of vaccines and other therapeutics [40, 41].
Three mouse-adapted (MA) strains of SARS-CoV were developed independently by serial passage of SARS-CoV (Urbani strain) in the respiratory tract of mice [40, 42, 43]. The MA15, MA20 and v163 mouse-adapted SARS-CoV strains replicated to high titer in the lungs of mice, associated with pathological changes, dissemination of the virus to extrapulmonary sites and mortality. The disease in mice resembled the disease seen in severe human cases of SARS [40, 42, 43]. These three MA viruses shared mutations in specific viral proteins such as the replicase nonstructural protein nsp9 and the spike glycoprotein, which attests to the importance of these proteins in viral pathogenesis [40, 43]. Infection of older mice with the MA15 virus produces clinical disease particularly reminiscent of acute respiratory distress syndrome (ARDS) in humans [43].
By contrast to SARS-CoV, mice are not naturally susceptible to infection by MERS-CoV because the mouse DPP4 receptor differs from human DPP4 (hDPP4) in crucial areas of interaction with the MERS-CoV spike protein [44]. BALB/c and B6 mice were transduced with an adenoviral vector expressing hDPP4 (Ad5-hDPP4); these mice supported replication of MERS-CoV associated with interstitial pneumonia and viral antigen in the lungs [45••]. Older Ad5-hDPP4 transduced mice lost weight but mortality was not observed. Agrawal et al. recently developed a transgenic mouse model globally expressing hDPP4 under the control of the CAG promoter used to generate the SARS transgenic mice [46••]. The hDPP4 mice were fully permissive to MERS-CoV infection, supporting a robust infection with severe respiratory and generalized illness that led to death within days after infection. High viral titers were recovered from multiple organs and pathological changes were consistent with extensive inflammation.
When mouse models are available, they are useful in evaluating the pathogenesis of viruses and testing vaccines and antiviral drugs. Mice are advantageous due to their low cost, small size and availability. They can also be manipulated at the genetic level and immunological reagents are available to study viral pathogenesis.
Hamsters
The golden Syrian hamster was an excellent model for SARS-CoV because the virus replicates to high titers in the lung with associated pathology. Following intranasal inoculation of SARS-CoV, viral replication was observed in the upper and lower respiratory tract with peak replication three days after infection. The virus was cleared seven to ten days after infection [47]. Viral replication was accompanied by pronounced histopathological changes in the lungs including interstitial inflammation, pneumonitis and consolidation. Since hamsters showed no outward signs of clinical illness, exercise wheels (Nalgene activity wheel) were employed to measure their activity (revolutions/night); these activity wheels showed that SARS-CoV infected hamsters were less active from days two to seven post-infection [47, 48]. Primary infection elicited a neutralizing antibody response that provided protection from subsequent infections [47]. Hamsters were suitable for immunoprophylaxis and treatment studies because objective clinical signs were accompanied by high viral titers and pulmonary histopathology [49].
Attempts to experimentally infect hamsters with MERS-CoV were not successful [50].
Ferrets
Ferrets are frequently used as a model for the study of respiratory viruses that infect humans. However, conflicting data were reported when ferrets were infected with SARS-CoV [51, 52]; one group observed clinical illness [51], but another group did not [52]. The ferret model was further characterized to resolve these inconsistent results; fever and sneezing were associated with high viral titers in the upper respiratory tract and histologic changes in the lungs characterized by lymphohistiocytic bronchointerstitial pneumonia [53].
Ferrets do not support replication of MERS-CoV [54].
The application of animal models for vaccine development
SARS-CoV and MERS-CoV research have demonstrated that a single animal species will not serve as a model for all coronaviruses (Table 2 ). The ability to elicit clinical disease, viral replication and pathology depends on the expression of the viral receptor, the species and the demographic characteristics of the animal. Infection of young mice with SARS-CoV was not ideal because there was limited histopathology and no clinical disease. However the combination of two approaches, using mouse-adapted SARS-CoV in older mice, resulted in a model of ARDS that represents a more stringent challenge for the evaluation of vaccine efficacy than either alone. Unfortunately, immune defects associated with aging are complex and can influence results of vaccine evaluations [55, 56].
Table 2.
Clinical signs, viral replication and pathology of SARS-CoV and MERS-CoV in humans and various animal models.
| Species | Virus |
|
|---|---|---|
| SARS-CoV | MERS-CoV | |
| Humans | • Clinical signs include fever and respiratory illness. • Lung pathology is consistent with pneumonia and acute lung injury. |
• Clinical signs include fever and respiratory illness. Some patients develop renal failure. • Lung pathology samples are not available for investigation. |
| NHP | • Rhesus macaques, cynomolgus macaques, African green monkeys and common marmosets are susceptible to infection. Clinical signs, viral replication and pathology depend on the species. | • Rhesus macaques develop a transient infection with moderate viral replication and pathology in the lung. • Common marmosets have a more severe response to the virus with higher viral titers and severe pathology in the lungs. Lethality is also observed in this model. |
| Mice | • Young inbred mice (BALB/c, C57BL6, 129S) support viral replication but fail to show clinical signs of disease. • Older inbred mice (BALB/c), knockout mice (STAT 1−/−, Rag 1−/−, CD1−/−, Beige) and transgenic mice (K18-hACE2, A70-hACE2) develop generalized illness, robust viral growth and pronounced lung pathology consistent with pneumonia and acute lung injury. The K18-hACE2 transgenic mice develop central nervous system disease, which is not a feature in humans. |
• Inbred mice are not naturally susceptible to infection. • Transduced mice (Ad5-hDPP4) develop clinical signs and support replication of virus with interstitial pneumonia and viral antigen found in the lungs. • Transgenic mice (hCD26/DPP4) develop robust respiratory and generalized illness with high viral titers and extensive inflammation in the lungs. Lethality was also observed in this model. |
| Hamsters | • Clinical illness (measured by a decrease in activity on the exercise wheel) is accompanied by viral replication and pronounced histopathological changes such as inflammation, pneumonitis and consolidation in the lungs. | • Hamsters do not support replication. |
| Ferrets | • Clinical illness (fever and sneezing), is accompanied by viral replication and histologic changes in the lungs. | • Ferrets do not support replication. |
| Rabbits | • The rabbit model has not been investigated. | • The rabbit model is currently under investigation. |
Several animal models were developed for SARS — largely because the crucial domains of the ACE2 receptor that binds the SARS-CoV spike protein are conserved across several species. This has not been the case for MERS-CoV. There are several point mutations in the DPP4 protein of different animal species that limit the ability of the MERS-CoV spike protein to attach to the host receptor. Therefore, without modification of either the receptor or the viral spike protein, animal models for MERS are limited to non-human primates and camels. Recent studies have shown that there is sequence homology between rabbit and human DPP4, raising the possibility that the rabbit may be a promising model for MERS-CoV infection [56a].
Several SARS vaccine candidates elicited neutralizing antibodies and were effective in protecting young mice or hamsters from challenge [48, 57, 58, 59, 60, 61, 62, 63]. However, reports of immunopathologic reactions in older mice and in non-human primates vaccinated with SARS-CoV vaccines that were subsequently challenged with SARS-CoV [57, 59, 62, 64] have revealed two concerns about proceeding to clinical trials with SARS-CoV vaccines. First, there is a precedent for coronavirus-vaccine associated disease enhancement; kittens immunized with a vaccinia virus vectored feline infectious peritonitis virus vaccines developed severe disease when they were subsequently infected with FIPV [65]. In these kittens, non-neutralizing or sub-neutralizing antibodies facilitated viral entry into macrophages. The concern that is extrapolated from the FIPV vaccine experience to human SARS-CoV vaccines is whether vaccine recipients will develop more severe disease if they are exposed to or infected with SARS-CoV after neutralizing antibody titers decline. The second concern is whether recipients of a SARS-CoV vaccine would be at risk of developing pulmonary immunopathology following infection with an unrelated human coronavirus, for example, 229E, OC43, HKU1 or NL63 that usually causes mild, self limited disease. Although findings from preclinical evaluation have revealed these concerns, studies in animal models may not be able to provide data to confirm or allay these concerns.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors’ research is supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). LMGs research is made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
References
- 1.Masters P.S., Perlman S. Coronaviridae. In: Knipe DM, Howley PM, editors. Field's Virology. edn 6th. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. pp. 825–858. [Google Scholar]
- 2•.Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review of the pathogenesis of SARS and MERS coronavirus infections focuses on the interplay between a dysregulated host immune response and the development of ARDS.
- 3.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]; The molecular basis of the interaction between the MERS-CoV spike protein and DPP4 is delineated from crystal structures of the receptor binding domain of the MERS-CoV spike protein free and complexed with DPP4.
- 6••.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study identifies dipeptidyl peptidase 4 (also known as CD26) as the functional receptor for MERS.
- 7.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 8.WHO: Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available from http://www.who.int/csr/sars/country/table2004_04_21/en/.
- 9•.Bermingham A., Chand M.A., Brown C.S., Aarons E., Tong C., Langrish C., Hoschler K., Brown K., Galiano M., Myers R. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]; This paper documents the diagnostic approach and the clinical and virological features of the second reported case of MERS, in a patient transferred to London, United Kingdom, from Qatar.
- 10.WHO: Middle East respiratory syndrome coronavirus (MERS-CoV) – update May 9, 2014. Available at http://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_Update_09_May_2014.pdf?ua=1 [DOI] [PMC free article] [PubMed]
- 11••.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]; The first case report of MERS-CoV infection, in a 60-year-old man who presented with acute pneumonia and subsequent renal failure with a fatal outcome in Saudi Arabia. The clinical data, virus isolation, and molecular identification are presented.
- 12.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 14.Lee N., Wu H.D., Chan A., Cameron P., Joynt P., Ahuja G.M., Yung A., Leung M.Y., To C.B., Lui K.F. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 15.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 17.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 18•.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the epidemiological, demographic, clinical, and laboratory characteristics of 47 cases of MERS-CoV infection from Saudi Arabia. The authors report that MERS can present with a wide range of clinical signs and is associated with high mortality in patients with medical comorbidities.
- 19.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., Selbs E., McEvoy C.P., Hayden C.D., Fukuoka J. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAuliffe J., Vogel L., Roberts A., Fahle G., Fischer S., Shieh W.J., Butler E., Zaki S., St Claire M., Murphy B. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology. 2004;330:8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe T., Gao G., Hogan R.J., Crystal R.G., Voss T.G., Grant R.L., Bell P., Kobinger G.P., Wivel N.A., Wilson J.M. Macaque model for severe acute respiratory syndrome. J Virol. 2004;78:11401–11404. doi: 10.1128/JVI.78.20.11401-11404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stohr K., Osterhaus A.D. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawler J.V., Endy T.P., Hensley L.E., Garrison A., Fritz E.A., Lesar M., Baric R.S., Kulesh D.A., Norwood D.A., Wasieloski L.P. Cynomolgus macaque as an animal model for severe acute respiratory syndrome. PLoS Med. 2006;3:e149. doi: 10.1371/journal.pmed.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenough T.C., Carville A., Coderre J., Somasundaran M., Sullivan J.L., Luzuriaga K., Mansfield K. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol. 2005;167:455–463. doi: 10.1016/S0002-9440(10)62989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.de Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L., Fischer E.R., Martellaro C., Okumura A., Chang J. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that experimentally infected rhesus macaques develop a transient lower respiratory tract infection with a multifocal, mild to marked interstitial pneumonia, and virus replication occurring mainly in alveolar pneumocytes. Clinical signs, virus shedding, virus replication in respiratory tissues, gene expression, and cytokine and chemokine profiles peaked early in infection and decreased over time.
- 29•.Yao Y., Bao L., Deng W., Xu L., Li F., Lv Q., Yu P., Chen T., Xu Y., Zhu H. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis. 2014;209:236–242. doi: 10.1093/infdis/jit590. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another paper presenting evidence that rhesus macaques can be experimentally infected with MERS-CoV. The infected monkeys showed mild clinical signs of disease, virus replication, histological lesions, and neutralizing antibody production.
- 30••.Falzarano D., de Wit E., Feldmann F., Rasmussen A.L., Okumura A., Peng X., Thomas M.J., van Doremalen N., Haddock E., Nagy L. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10:e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first description of a severe, partially lethal, disease model of MERS-CoV in common marmosets. Most of the marmosets developed a progressive severe pneumonia and some animals were also viremic; high viral loads were detected in the lungs of all infected animals, and total RNAseq demonstrated the induction of immune and inflammatory pathways.
- 31.Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- 32.Hogan R.J., Gao G., Rowe T., Bell P., Flieder D., Paragas J., Kobinger G.P., Wivel N.A., Crystal R.G., Boyer J. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J Virol. 2004;78:11416–11421. doi: 10.1128/JVI.78.20.11416-11421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts A., Paddock C., Vogel L., Butler E., Zaki S., Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baas T., Roberts A., Teal T.H., Vogel L., Chen J., Tumpey T.M., Katze M.G., Subbarao K. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J Virol. 2008;82:9465–9476. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M., Lamirande E.W., Roberts A., Heise M., Subbarao K. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng C.T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M., Makino S., Packard M.M., Zaki S.R., Chan T.S. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J Virol. 2007;81:1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day C.W., Baric R., Cai S.X., Frieman M., Kumaki Y., Morrey J.D., Smee D.F., Barnard D.L. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netland J., Zhao D.M., Fett J., Alvarez C., Nieto-Torres E., Enjuanes J.L., Perlman L.S. Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology. 2010;399:120–128. doi: 10.1016/j.virol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frieman M., Yount B., Agnihothram S., Page C., Donaldson E., Roberts A., Vogel L., Woodruff B., Scorpio D., Subbarao K. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol. 2012;86:884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cockrell A.S., Peck K.M., Yount B.L., Agnihothram S.S., Scobey T., Curnes N.R., Baric R.S., Heise M.T. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88:5195–5199. doi: 10.1128/JVI.03764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J., Gale M.J., Jr., Baric R.S., Enjuanes L., Gallagher T. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a novel approach to developing a mouse model for MERS by transducing mice with a recombinant, nonreplicating adenovirus expressing the hDPP4 receptor. Transduced mice could be subsequently infected with MERS-CoV and could also be used to evaluate anti-MERS-CoV vaccines and therapies.
- 46••.Agrawal A.S., Garron T., Tao X., Peng B.H., Wakamiya M., Chan T.S., Couch R.B., Tseng C.T. Generation of transgenic mouse model of Middle East respiratory syndrome-coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the generation of transgenic mice expressing human DPP4. The transgenic mice are fully permissive to MERS-CoV infection, resulting in weight loss and death within days postinection. High titers of infectious virus were detected in the lungs and brains and viral RNA was also detected in the heart, spleen, and intestine, indicating a disseminating viral infection. Infected transgenic mice developed a progressive pneumonia, characterized by extensive inflammatory infiltration.
- 47.Roberts A., Vogel L., Guarner J., Hayes N., Murphy B., Zaki S., Subbarao K. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol. 2005;79:503–511. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamirande E.W., DeDiego M.L., Roberts A., Jackson J.P., Alvarez E., Sheahan T., Shieh W.J., Zaki S.R., Baric R., Enjuanes L. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden Syrian hamsters. J Virol. 2008;82:7721–7724. doi: 10.1128/JVI.00304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts A., Thomas W.D., Guarner J., Lamirande E.W., Babcock G.J., Greenough T.C., Vogel L., Hayes N., Sullivan J.L., Zaki S. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Wit E., Prescott J., Baseler L., Bushmaker T., Thomas T., Lackemeyer M.G., Martellaro C., Milne-Price S., Haddock E., Haagmans B.L. The Middle East respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters. PLoS One. 2013;8:e69127. doi: 10.1371/journal.pone.0069127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G., Peiris J.S., Lim W., Osterhaus A.D. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu Y.K., Ali G.D., Jia F., Li Q., Kelvin D., Couch R.C., Harrod K.S., Hutt J.A., Cameron C., Weiss S.R. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374:151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raj V.S., Smits S.L., Provacia L.B., van den Brand J.M., Wiersma L., Ouwendijk W.J., Bestebroer T.M., Spronken M.I., van Amerongen G., Rottier P.J. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruver A.L., Hudson L.L., Sempowski G.D. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.(a) Haagmans B.L., van den Brand J.M.A., Provacia L.B., Raj V.S., Stittellar K.J., Getu S., de Waal L., Bestebroer T.M., van Amerongen G., Verjans G.M.G.M. Asymptomatic Middle East Respiratory Syndrome Coronavirus infection in rabbits. J Virol. 2015;89:6131–6135. doi: 10.1128/jvi.00661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Haynes L., Swain S.L. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Enjuanes L., Dediego M.L., Alvarez E., Deming D., Sheahan T., Baric R. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133:45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lokugamage K.G., Yoshikawa-Iwata N., Ito N., Watts D.M., Wyde P.R., Wang N., Newman P., Kent Tseng C.T., Peters C.J., Makino S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26:797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.See R.H., Zakhartchouk A.N., Petric M., Lawrence D.J., Mok C.P., Hogan R.J., Rowe T., Zitzow L.A., Karunakaran K.P., Hitt M.M. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87:641–650. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 61.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., Gerencer M., Bruhl P., Grillberger L., Reiter M., Tauer C. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24:652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S., Kase R., Sekiguchi S., Morita K., Hishima T. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M., Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C., Spaan W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64:1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


