Abstract
Objective
Little is known regarding the etiological factors which lead to the occurrence of intraluminal thrombus (ILT) during abdominal aortic aneurysm (AAA) development. Recent work has suggested that macrophages may play an important role in progression of a number of other vascular diseases, including atherosclerosis; however, it is unknown whether these cells are present within the ILT of a progressing AAA. The purpose of this work was to define the presence, phenotype and spatial distribution of macrophages within ILT excised from six human patients. We hypothesized that the ILT contains a population of activated macrophages with a distinct, non-classical phenotypic profile.
Methods
ILT samples were examined using histologic staining and immunofluorescent labeling for multiple markers of activated macrophages (CD45, CD68, HLA-DR, MMP9), as well as additional markers α-SMA, CD34, CD105, FLK-1, and collagen I and III.
Results
Histologic staining revealed a distinct laminar organization of collagen within the shoulder region of the ILT lumen and a spatially heterogeneous cell composition within the ILT. The majority of the cellular constituents of the ILT were in the luminal region and predominantly expressed markers of activated macrophages but also concurrently expressed α-SMA, CD105 and synthesized collagen I and III
Conclusions
Our results demonstrate that the luminal layer of human ILT is rich in a distinct population of activated macrophages. Additional work is needed to assess the contribution of these cells to ILT formation and the pathobiology of AAA as a whole.
Summary
The present manuscript presents evidence for the presence of a distinct macrophage population within the luminal region of AAA ILT. These cells express a set of markers indicative of a unique population of activated macrophages. The exact contributions of these previously unrecognized cells to ILT formation and AAA pathobiology remains unknown.
1. Introduction
Abdominal aortic aneurysm (AAA) is the 13th leading cause of death in the United States 1. Although the pathogenesis of AAA has not been fully elucidated, it is increasingly recognized to involve a chronic inflammatory process 2-5. There are multiple types of inflammatory cells (neutrophils, T cells, B cells, macrophages, mast cells, and NK cells) within the aortic wall during AAA progression, and these cells have been associated with increased levels of pro-inflammatory cytokines and diverse proteases. Taken together, this suggests an active inflammatory remodeling environment in the AAA wall that promotes progressive tissue degradation, focal dilatations, and potential rupture6-9. However, much less is known about the process which leads to the formation of an intraluminal thrombus (ILT), which occurs in the majority (>75%) of AAA cases 8.
Altered hemodynamics resulting from AAA is likely among the drivers of initial ILT formation and progression 10, 11. It is presumed that these hemodynamic disturbances activate platelets which leads to subsequent accumulation of fibrin and entrapment of erythrocytes within a layered structure 12. A number of reports describe a three-layered structure consisting of luminal (in contact with flowing blood), medial, and abluminal (in contact with AAA wall) layers, with the majority of cells residing within the luminal layer 13-16. Evidence also suggests that these layers possess a heterogeneous structure that matures with time and disease progression. Although the cells within the ILT are predominantly platelets, lesser numbers of other cells, including macrophages, have also been observed 9, 13, 17. However, due to their relatively small numbers in comparison to platelets, the exact phenotypes and roles of these cells in the formation or maturation of the ILT and overall pathology of AAA have gone largely unstudied. Given that recent evidence suggests that activated macrophages play an important and determinant role in the pathology of a number vascular diseases 17-20, the purpose of this work was to define the presence, phenotype, and distribution of macrophages within ILT of patients with AAA. We hypothesized that the ILT contains a population of activated macrophages with a distinct, non-classical phenotypic profile.
2. Methods
2.1. Subjects
This study met the necessary requirements of HHS regulation 45 CFR 46.101(b)(4) to be designated as “research with no human subjects involved”; i.e., research involving the collection or study of pathological specimens collected in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. Therefore, this study was found to be exempt for the need for patient consent and was approved by the University of Pittsburgh Institutional Review Board.
For this study, AAA wall and ILT samples were obtained from 9 males (8 were smokers) between the ages of 66-75 who were undergoing elective AAA repair surgery. All patients were taking statins and antiplatelet drugs. The aortic dilatation for these patients was between 43-76 mm. All of the AAA studied were degenerative, fusiform atherosclerotic aneurysms and not mycotic or associated with genetic abnormalities.
2.1. Sample procurement
Tissue was obtained from the operating room immediately following excision, de-identified, provided to the research team, and transported to the laboratory in ice cold phosphate buffered saline (PBS, pH 7.4). ILT samples were then processed for either histologic examination (6 patients) or isolation of cells for culture experiments (3 patients) as described below.
2.2. Sample preparation
Samples for histologic examination and immunofluorescent labeling were prepared as follows. Briefly, the ILT was washed thoroughly in PBS, placed immediately in 4% paraformaldehyde and kept at 4°C for 24 h. Samples were then transferred to a 30% sucrose solution for at least 24 h before being cut luminally to abluminally through the thickness of the thrombus in two locations: the shoulder region and the midsection (Fig. 1). The resulting pieces were loaded into OCT mounting media (Tissue-Tex, Sakura, 4583) in cryomolds and frozen at -80°C for 24 h before being sectioned at 6 -7 microns thickness.
Fig. 1. Gross morphological appearance of ILT.
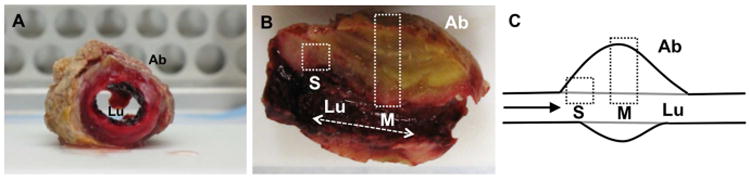
Image of cross-sections at time of surgery (A, B). Note layered structure. Samples were cut in two and then further dissected for microscopic investigation (B, C). A schematic view of the ILT aspect depicted in (B) is provided in (C). Boxes represent areas sampled: shoulder (S) and midsection (M). “Ab” and “Lu” represent the abluminal and luminal aspects of the sample, respectively.
Samples for cell isolation were left unfixed and processed as described below.
2.3. Histologic staining
Samples were subjected to histologic examination following staining with hematoxylin and eosin (H&E), Picrosirius red (PSR) and a modified Herovici's polychrome stain 21. The PSR and Herovici were used to evaluate the presence, type, and distribution of collagen within the ILT. Each stain was performed on consecutive sections taken from each sample. Images were taken using either a Nikon Eclipse E600 with a 10× objective (H&E, Herovici) or a Provis III with a 10× objective (PSR) across the width (luminal to abluminal) of the sample and stitched using ImageJ software (NIH).
2.4. Immunofluorescent labeling
Further characterization of the spatial distribution of the cellular population within the ILT was performed by immunofluorescent labeling. Following a standard staining protocol, samples were permeabilized in 0.1% Triton X-100 (Fisher Scientific, BP151-100) in PBS for 10 min, followed by 3 washes in PBS and incubation in a blocking agent (5% goat serum; Life Technologies, 16210) for one hour. Next the sample was exposed to an immunospecific primary antibody diluted in 5% goat serum overnight at 4°C, then to a fluorescent-conjugated secondary antibody for one hour before examination using a Nikon Eclipse E800 microscope with fluorescent optical cubes and a 20× lens. Samples were also stained with Hoechst (Sigma, B2883) to identify the presence of nuclear material. Antibodies included those specific for common leukocyte antigen (CD45, eBioscience, clone HI30, 1:1000), hematopoietic progenitor cell antigen (CD34, abcam, clone EP373Y, 1:100), endothelial cell marker, (FLK1, Santa Cruz, clone A-3, 1: 200), pan-macrophage marker (CD68, Santa Cruz, clone KP1, 1:200), matrix metalloproteinase 9 (MMP9, Millipore, clone 56-2A4, 1:500), major histocompatibility complex II (HLA-DR, eBioscience clone LN3, 1:250), smooth muscle alpha actin (αSMA, Sigma, clone 1A4, 1:1000), endoglin (CD105, Dako, clone SN6h, 1:250), collagen I, (Sigma, clone COL-1, 1:2000), collagen III (Sigma, clone FH-7A, 1:2000). Coverslips were mounted using Dako Fluorescent mounting media (Dako North America, S3023). Images were saved and processed utilizing NIS Elements software. The number of CD45, CD68, CD105, HLA-DR, and αSMA positive cells were counted within 3 separate fields, every 100 microns apart across the width of the ILT from luminal to abluminal region. Counts were averaged and normalized to ILT thickness/depth.
2.5. Cell isolation
The luminal, medial, and abluminal layers of the ILT specimens designated for cell isolation were dissected individually and then digested with PBS containing 10 mg/ml of collagenase Type IV (Worthington, 40N12270) and 5 mg/ml of elastase (Worthington, 31P13140) for 30 min at 37°C. After digestion, the pellet was washed once in PBS. The pieces of the ILT were placed onto wells of six-well tissue culture plates and allowed to adhere for 15 min. Media containing fungizone and gentamycin was then carefully added to the plates and the plates were incubated at 37°C. This media consisted of one of three formulations corresponding to basal media (DMEM/F12 [Life Technologies, 11330-032], 10% fetal bovine serum [Atlanta Biologicals, S11550], 1% penicillin/streptomycin [Life Technologies, 15140-163]), fibroblast growth media (Lonza, Walkersville Inc. CC3131+CC4525), and smooth muscle cell growth media (Cell Applications, Inc. 311K-500). All medias were supplemented with 1× Fungizone (Life Technologies, 15290-018) and 1% gentamycin (Life Technologies, 15710-064). The plates were monitored and media was changed every 4 d. After two weeks, when the outgrowth of cells was seen, the tissue was removed and fresh media was added.
Cells were also harvested from the AAA wall from the same patients. The wall tissue was scraped to remove the luminal layer and the aortic medial and adventitial layers were teased apart. The two layers were cut into small pieces and digested with the digestion buffer and processed as described above. The medial layer was placed in smooth muscle cell growth media and the adventitial layer was placed in fibroblast growth media.
All cultured cells were subjected to immunofluorescent labeling for CD45, CD68, HLA-DR, αSMA, collagen I, and collagen III following the protocol described above.
3. Results
3.1. Histologic appearance and collagen organization of ILT
The histologic appearance of the ILT (Fig. 2A-C) was characterized by a layered structure consisting of three distinct regions: luminal, medial, and abluminal. The medial and abluminal regions consisted primarily of fibrin with little to no collagen present. In contrast, both Herovici's polychrome and Picrosirius red (PSR) staining revealed the presence of collagen within the luminal region of the samples. Limited islands of less intense staining were also observed within the portion of the medial layer closest to the luminal layer, with little to no staining observed within the abluminal layer. Few if any nucleated cells were observed outside the luminal region. The number of platelets also decreased from the luminal to abluminal regions of the ILT.
Fig. 2. Asymmetric composition of the ILT.

Representative histologic appearance of the ILT (A-C). Samples were stained with hematoxylin and eosin (A), Herovici's polychrome (B), and picrosirius red (C). Note the distinct appearances of the luminal (Lu), medial, and abluminal (Ab) regions of the full thickness ILT sample. Herovici's polychrome and picrosirius red staining suggest the presence of collagen within the luminal region of the ILT (B, C). “Ab” and “Lu” represent the abluminal and luminal aspects of the sample, respectively. Scale bar = 250 μm (A-C).
Further examination of the collagenous tissue within the luminal region of the ILT revealed differences in collagen content and organization depending on the location along the luminal surface (Fig. 3). A laminar arrangement of collagen was observed parallel to the luminal surface in the shoulder region of the ILT (Fig. 3A-B), whereas the collagen within the midsection of the luminal region consisted of disorganized and less mature collagen. Despite the difference in extracellular collagen between the shoulder region and midsection, immunofluorescent staining showed that luminal cells within both the shoulder region and the midsection expressed collagen types I and III (Fig. 3E-F). No cell associated labeling of collagen I or III was observed outside the luminal region.
Fig. 3. Representative images of histologic and immunohistochemical evaluation of the luminal aspect of the ILT.
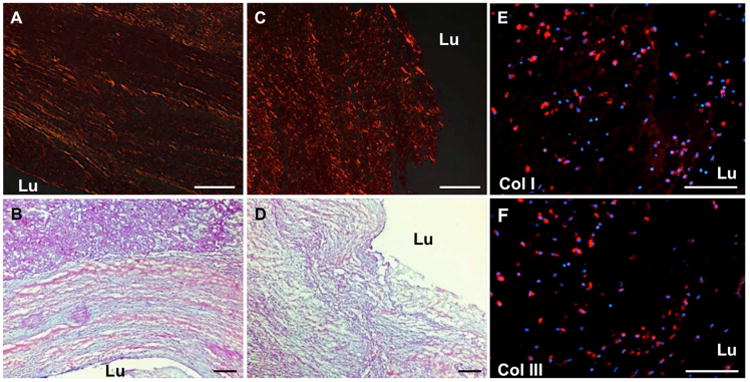
Picrosirius red (A, C) and Herovici's polychrome (B, D) suggest the presence of collagen with alignment parallel to the luminal surface within the shoulder region (A, B) of the ILT. Collagen is present but less organized within the midsection of the luminal surface (C, D). Immunolabeling (red) demonstrated collagen I (E) and III (F) within the extracellular matrix and within cytoplasm of cells of the luminal aspect, and there was no distinct difference between midsection or shoulder regions. “Lu” represents the luminal aspect of the sample. Scale bar = 100 μm.
3.2. Phenotypic analysis of cells within the luminal region
The distribution and phenotypic profile of cells within the luminal region of the ILT was examined using immunofluorescent microscopy (Fig. 4). Nuclear labeling demonstrated a highly nucleated region – or “nuclear stratus” - within the luminal region of the ILT (Fig. 4A). The density of nucleated cells was shown to decrease rapidly with increasing distance from the luminal surface, consistent with earlier reports 13. This nuclear stratus varied in thickness, depending on sample, from a few microns to over 500 microns; however, this stratus thickness was not proportional to ILT sample thickness, which varied patient to patient (Fig. 5). Few cells were found outside of the nuclear stratus.
Fig. 4. Representative images of immunolabeling performed to examine the phenotype and location of cells within the ILT.
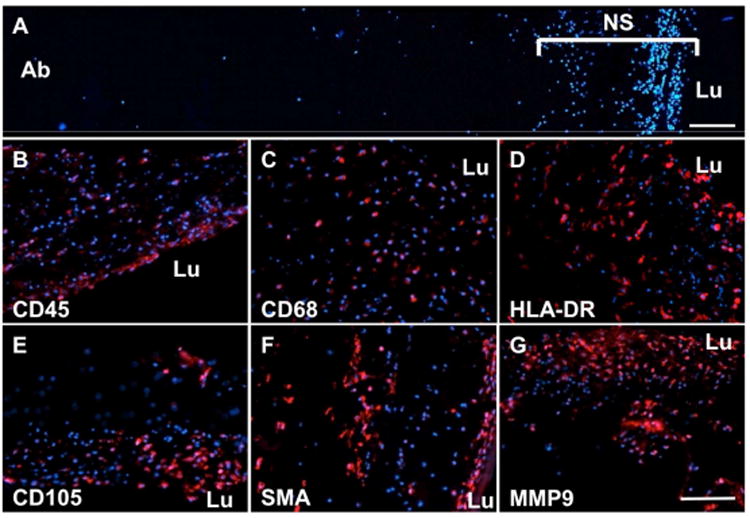
A significant population of nucleated cells was observed within a “nuclear stratus” (NS) of DAPI stained sections of the lumen of the sample, with few cells found within the medial or abluminal regions (A). Nucleated cells within the lumen express a heterogeneous phenotype including CD45 (B), CD68 (C), HLA-DR (D), CD105 (E), SMA (F), and MMP9 (G). “Lu” represents the luminal aspect of the sample. Scale bar = 100 μm.
Fig. 5. Ratio of Nuclear stratus to Total ILT width.
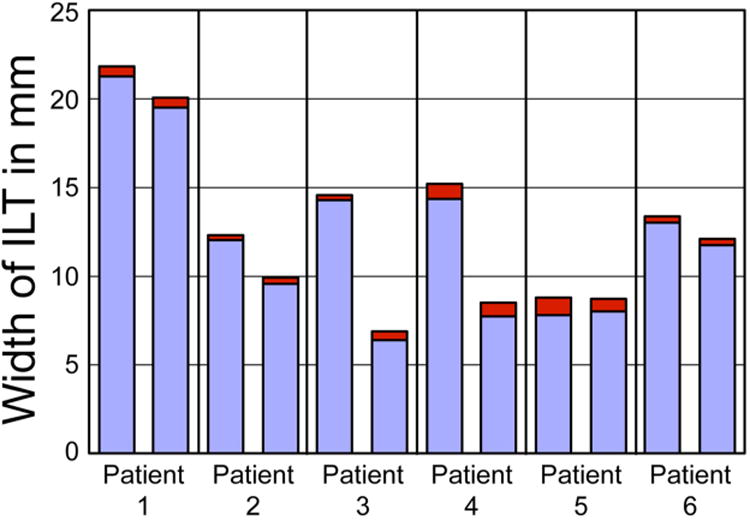
Data from 6 patients is shown. Two samples from each patient were used. The nuclear stratus (red) is not proportional to the total ILT thickness (red+blue).
Further characterization of the spatial distribution of the cellular population within the ILT was performed by immunofluorescent labeling (Fig. 4B-G). Results demonstrate presence of cells expressing common markers of activated macrophages (CD45, CD68, HLA-DR, MMP9), as well as aSMA and CD105. These cells are largely located within the inner luminal region of the ILT. However, labeling for CD34 (hematopoietic progenitor) and FLK1 (endothelial cell) were not observed. When the spatial distribution of a subset of these markers (CD45, CD68, HLA-DR, CD105, and aSMA), was quantified we found that expression of these markers decreased rapidly with distance from the luminal surface (Fig. 6). Few, if any, cells were observed beyond the first 5 to 8% of the sample thickness from the luminal surface. This characteristic pattern was similar in all 6 patient samples examined (2 areas per patient, 12 specimens total). MMP9 was observed to exhibit a similar labeling pattern to other markers of activated macrophages with decreasing MMP9 labeling with increasing distance from the luminal surface. However, staining was observed both in the intracellular and extracellular space. Therefore, it was not amenable to the type of particle analysis performed for the other markers.
Fig. 6. Spatial distribution of CD45 (A), CD68 (B), CD105 (C), HLA-DR (D), and SMA (E) positive cells within the ILT.
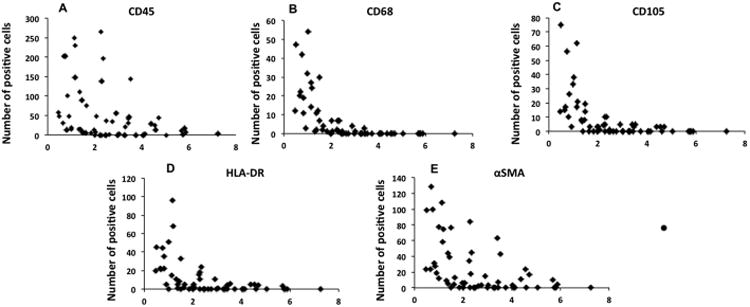
Data from 6 patients is displayed. Two samples per patient were examined. Results demonstrate presence of cells expressing myeloid, mesenchymal and progenitor cell markers, which are largely located within the luminal region. Few cells of any type were observed within the medial or abluminal portions of the ILT.
Co-localization of a number of the markers was investigated to determine if cells expressed markers of both activated macrophages and other markers concurrently (Fig. 7). Cells expressing markers of activated macrophages (CD68) also expressed αSMA and collagens I and III.
Fig. 7. Coexpression of fibrocyte markers in cells from the ILT.
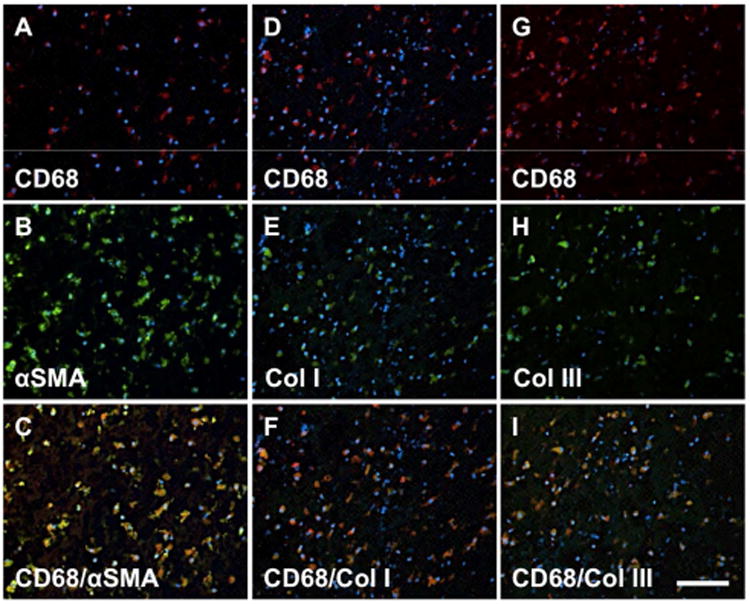
Cells within the ILT express a unique “fibrocyte-like” phenotype. Cells colocalizing expression of CD68+/SMA+ (A-C), CD68+/Collagen I+ (D-F), and CD68+/Collagen III+ cells (G-I) were all observed. Red: CD68, Blue: DAPI, Green: SMA (B), Collagen I (E), Collagen III (H). Scale bar = 100 μm.
3.3. Phenotypic analysis of cells from ILT and AAA wall in multiple culture conditions
Immunofluorescent labeling of cells cultured from the ILT and AAA wall stained for individual markers (CD45, CD68, HLA-DR, αSMA, collagen I, collagen III) confirmed that cells from the ILT expressed markers of both activated macrophages as well as other markers, whereas in comparison, cells from the AAA wall expressed only those of indicative of a smooth muscle cell phenotype (Table 1). These markers co-localized to individual cells within the culture. The phenotype of cells from the ILT was not affected by media type (basal, smooth muscle, fibroblast), and marker profile was consistent across samples obtained from three individual patients (Table 1). Of note, cells derived from the ILT were not observed to proliferate, suggestive of a differentiated macrophage phenotype, while cells from the AAA wall were observed to proliferate, suggestive of smooth muscle cell phenotype. All culture times and conditions were identical between ILT and AAA wall derived cells.
Table 1. Phenotypic evaluation of cells isolated from the ILT and AAA wall.
Cells from the ILT co-expressed both innate immune cell markers (CD45, CD68, HLA-DR) and markers of smooth muscle cell phenotype (αSMA, collagen I, collagen III), whereas cells from the AAA wall only expressed smooth muscle markers.
| Marker | ILT- Basal | ILT - FBR | ILT - SMC | Wall – FBR | Wall – SMC |
|---|---|---|---|---|---|
| CD45 | + | + | + | - | - |
| CD68 | + | + | + | -/+(few cells) | -/+(few cells) |
| HLA-DR | + | + | + | - | - |
| α-SMA | + | + | + | + | + |
| Collagen I | + | + | + | + | + |
| Collagen III | + | + | + | + | + |
4. Discussion
This study examined the phenotype and distribution of the cellular population present within ILT obtained from AAA at surgery. The histologic results of this study are consistent with previous observations that the ILT is heterogeneous consisting of three distinct regions corresponding to luminal, medial, and abluminal layers 12, 13, 15, 16. Further characterization of the luminal layer by multiple histologic methods did, however, reveal the presence of fibrillar collagen and a distinct laminar organization within the shoulder region of the luminal layer, a tissue organization which has not been previously observed in the literature.
The three distinct layers in the ILT have been shown to exhibit differences in both biochemical and biomechanical properties 15, 16, 22, 23. It has been suggested that the structure of the layered ILT evolves through four phases identified by their histologic appearance as fresh, young, intermediate, and old 22. The ILT is commonly considered to be composed primarily of fibrin with few additional constituents. In the present study however, histologic staining with Herovici's polychrome and PSR revealed the presence of collagen within the luminal layer of the ILT. This collagen was found to have a laminar organization within the shoulder regions with a less organized appearance in the mid-section. The exact mechanism which drives this organization cannot be discerned from the present study. However, it has been suggested that the distinct layers of the ILT develop over time with the age of the aneurysm and are accompanied by changes in local flow regimes, which logically may also affect local tissue organization. Small amounts of disorganized collagen I and III were observed within the surrounding medial layer closest to the luminal layer of the ILT as evidenced by histologic staining, although the intensity of this staining was less intense than observed in the luminal region. This finding that there is organized collagen only in the luminal layer correlates well with studies that have demonstrated increased stiffness and strength of the luminal layer as compared to the abluminal and medial layers 15, 22, 23. Further examination of the luminal surface using immunofluorescent labeling demonstrated the presence of cells expressing collagen I and III within the cytoplasm, suggesting a potential role for the observed cell population in the organization and development of the thrombus
It has been reported previously that the nucleated cell population within the ILT consists predominantly of neutrophils, with few macrophages or lymphocytes present 24. The present study suggests the presence of a unique macrophage population within the luminal aspect of the ILT. These cells, upon further examination, were shown to be producing collagen with an organization which varied with position within the ILT.
While the exact role of the observed cells is not known, activated macrophages have received increasing attention for both their protective and pathogenic roles in diverse diseases, including vascular diseases such as atherosclerosis 25, 26. Macrophages are highly plastic and express phenotypes ranging from M1 to M2 extremes 27-29. Monocytes/macrophages may also be plastic beyond the standard myeloid lineage30-32. A number of studies have shown that macrophages can exhibit mesenchymal cell properties and adopt a myofibroblast-like phenotype under certain inflammatory conditions 33. These cells have been implicated in a number of chronic inflammatory and fibro-proliferative states 33, 34, although the exact origin and differentiation pathways for such cells remain unclear.
Fibrocytes are a population of cells suggested to represent a circulating, bone marrow-derived cell population that expresses mixed morphological and molecular characteristics of leukocytes, fibroblasts, and hematopoietic progenitor cells 33. Much of the literature describing fibrocytes has focused upon their role in wound healing where they contribute to the population of fibroblasts and myofibroblasts that participate in wound healing. Yet, the apparent myeloid origin of these cells has led to speculation that they may also play an important role in the development of fibro-proliferative vascular diseases such as atherosclerosis. For example, macrophages have been suggested contribute to the stability of the atherosclerotic cap by transdifferentiating into fibrocytes and producing collagen 35. A reduction in this population of cells is a predictor of instability of the atherosclerotic plaque, often with a concurrent increase in the M1 pro-inflammatory macrophage phenotype 35.
In the present study, the ILT luminal surface was found to be rich in CD45+, CD68+, and HLA-DR+ cells which also expressed αSMA, collagen I, collagen III, and CD105, a combination highly suggestive of a fibrocyte-like phenotype 33. However, this population did not express the characteristic fibrocyte marker CD34, suggesting that these cells may represent a unique population of macrophages with fibroblastoid characteristics, similar to the population recently described by Mooney, et al 36. It has been reported that a reduction of CD34 and upregulation αSMA and collagen I has been observed in endothelial to mesenchymal transition, a process implicated in fibrogenic processes in a number of cardiovascular diseases 37, 38. However, the presence of the myeloid marker CD45 and the absence of endothelial cell marker FLK1 suggests that these cells are not derived from endothelial cells through endothelial to mesenchymal transition. The observed phenotype persisted when the cells were isolated and cultured in basal, fibroblast, and smooth muscle cell growth medias and was in direct contrast to that observed for cells isolated from the AAA wall, which expressed only those markers indicative of a smooth muscle cell phenotype. While these culture conditions may not reflect the in vivo reality, the results demonstrate that the phenotype of these cells is not mediated by changes in media composition. Taken together, these results suggest a potential role for mechanics in driving the observed phenotype, a suggestion which is supported by recent reports of strain dependent modulation of macrophage phenotype 39.
Due to their varied phenotypic traits, we propose that the cells observed in the present study may contribute both to the formation of the ILT through the production of a collagen matrix that stabilizes the luminal surface and to the eventual degradation of the aneurysmal wall or the destabilization of the ILT via production of inflammatory mediators and proteases. Of note, expression of CD105 has also been observed on smooth muscle cells, macrophages and endothelial cell populations in non-aneurysmal atherosclerotic aorta 40, suggesting that a cellular population similar to that observed in the present study may be present during or prior to the development of AAA in atherosclerotic vessels.However, further studies must be performed to identify the specific functions and activities of these cells in multiple inflammatory and mechanical environments to determine their role in the formation of the ILT and the progression of AAA as a whole.
There were several limitations of the present study. First, the patient samples were from a limited number of patients. However, the patients were characteristic of the general population which experiences AAA and the results observed in the present study were consistent across all samples examined despite differences in ILT thickness. Second, all of the tissues harvested were considered “end-stage”. Further studies describing the temporal and spatial distribution of cells appearing during the course of ILT formation would have the potential to greatly enhance the understanding of the role of these cells, if any, in early AAA progression and ILT formation. Third, the present study was observational in nature, and based upon histologic and immunofluorescence based outcomes. Future studies examining a larger cohort of patients could investigate additional quantitative metrics related to biochemical composition, ratio of collagen I to III, and relationships between patient characteristics (e.g., age, race, obesity, smoking, underlying vascular disease, pharmacologic interventions) and ILT composition.
5. Conclusion
The present study demonstrates the presence of cells expressing a previously unrecognized phenotype largely confined to the luminal region of the ILT in AAA. While the exact origin and function of this macrophage population within the formation of the ILT and the progression of AAA was not investigated, both the origin and specific functions of these cells within the pathogenesis of AAA are an important avenue for future research and the phenotypic makeup of these cells represent a potential predictor and/or suggest potential biomarkers of AAA progression.
Clinical Relevance.
ILT formation in AAA is well recognized. However, little is known regarding the factors that lead to ILT formation and influence upon AAA pathobiology. Given recent evidence of the important role of macrophages in vascular diseases including atherosclerosis, we examined the phenotype of cells within ILT to determine the potential for involvement of macrophages in ILT pathobiology. The lumen of the ILT was found to include cells expressing markers of a unique activated macrophage phenotype as well as synthesizing collagen I and III. Investigation of the relevance of these cells in in AAA progression is ongoing.
Acknowledgments
We would like to thank Deborah A. Cleary and Joseph E. Pichamuthu for their help with tissue procurement. This research was supported in part by grants from the National Institute of Health (HL086418).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research Conducted: Department of Bioengineering, University of Pittsburgh, 300 Technology Drive, Pittsburgh, PA 15219
Contributor Information
Jayashree Rao, Department of Bioengineering, Center for Vascular Remodeling and Regeneration, University of Pittsburgh.
Bryan N. Brown, Department of Bioengineering, McGowan Institute for Regenerative Medicine, University of Pittsburgh.
Justin S. Weinbaum, Department of Bioengineering, Center for Vascular Remodeling and Regeneration, and McGowan Institute for Regenerative Medicine, University of Pittsburgh.
Emily Ofstun, Department of Bioengineering, Center for Vascular Remodeling and Regeneration, University of Pittsburgh.
Michel S. Makaroun, Department of Surgery, Division of Vascular Surgery, Center for Vascular Remodeling & Regeneration, University of Pittsburgh.
Jay D. Humphrey, Department of Biomedical Engineering, Yale University.
David A. Vorp, Department of Bioengineering, Department of Cardiothoracic Surgery, Department of Surgery, Center for Vascular Remodeling and Regeneration, McGowan Institute for Regenerative Medicine, University of Pittsburgh.
References
- 1.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361(11):1114–6. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–74. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 3.Eberlova L, Tonar Z, Witter K, Krizkova V, Nedorost L, Korabecna M, et al. Asymptomatic abdominal aortic aneurysms show histological signs of progression: a quantitative histochemical analysis. Pathobiology. 2013;80(1):11–23. doi: 10.1159/000339304. [DOI] [PubMed] [Google Scholar]
- 4.Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90(1):18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiernicki I, Millo B, Safranow K, Gorecka-Szyld B, Gutowski P. MMP-9, homocysteine and CRP circulating levels are associated with intraluminal thrombus thickness of abdominal aortic aneurysms: new implication of the old biomarkers. Dis Markers. 2011;31(2):67–74. doi: 10.3233/DMA-2011-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrell TW, Burnand KG, Booth NA, Humphries J, Smith A. Intraluminal thrombus enhances proteolysis in abdominal aortic aneurysms. Vascular. 2006;14(1):9–16. doi: 10.2310/6670.2006.00008. [DOI] [PubMed] [Google Scholar]
- 7.Coutard M, Touat Z, Houard X, Leclercq A, Michel JB. Thrombus versus wall biological activities in experimental aortic aneurysms. J Vasc Res. 2010;47(4):355–66. doi: 10.1159/000265569. [DOI] [PubMed] [Google Scholar]
- 8.Khan JA, Abdul Rahman MN, Mazari FA, Shahin Y, Smith G, Madden L, et al. Intraluminal thrombus has a selective influence on matrix metalloproteinases and their inhibitors (tissue inhibitors of matrix metalloproteinases) in the wall of abdominal aortic aneurysms. Ann Vasc Surg. 2012;26(3):322–9. doi: 10.1016/j.avsg.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Sagan A, Mrowiecki W, Mikolajczyk TP, Urbanski K, Siedlinski M, Nosalski R, et al. Local inflammation is associated with aortic thrombus formation in abdominal aortic aneurysms. Relationship to clinical risk factors. Thromb Haemost. 2012;108(5):812–23. doi: 10.1160/TH12-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34(2):291–9. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 11.Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol. 2005;25(8):1558–66. doi: 10.1161/01.ATV.0000174129.77391.55. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JS, Virag L, Di Achille P, Karsaj I, Humphrey JD. Biochemomechanics of intraluminal thrombus in abdominal aortic aneurysms. J Biomech Eng. 2013;135(2):021011. doi: 10.1115/1.4023437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25(5):916–26. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey JD, Holzapfel GA. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech. 2012;45(5):805–14. doi: 10.1016/j.jbiomech.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DH, Makaroun M, Webster MW, Vorp DA. Mechanical properties and microstructure of intraluminal thrombus from abdominal aortic aneurysm. J Biomech Eng. 2001;123(6):536–9. doi: 10.1115/1.1411971. [DOI] [PubMed] [Google Scholar]
- 16.Wang DH, Makaroun MS, Webster MW, Vorp DA. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg. 2002;36(3):598–604. doi: 10.1067/mva.2002.126087. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29(10):1419–23. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 18.Boytard L, Spear R, Chinetti-Gbaguidi G, Acosta-Martin AE, Vanhoutte J, Lamblin N, et al. Role of proinflammatory CD68(+) mannose receptor(-) macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler Thromb Vasc Biol. 2013;33(2):431–8. doi: 10.1161/ATVBAHA.112.300663. [DOI] [PubMed] [Google Scholar]
- 19.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalhoub J, Falck-Hansen MA, Davies AH, Monaco C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm (Lond) 2011;8:9. doi: 10.1186/1476-9255-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner NJ, Pezzone MA, Brown BN, Badylak SF. Quantitative multispectral imaging of Herovici's polychrome for the assessment of collagen content and tissue remodelling. J Tissue Eng Regen Med. 2013;7(2):139–48. doi: 10.1002/term.508. [DOI] [PubMed] [Google Scholar]
- 22.Tong J, Cohnert T, Regitnig P, Holzapfel GA. Effects of age on the elastic properties of the intraluminal thrombus and the thrombus-covered wall in abdominal aortic aneurysms: biaxial extension behaviour and material modelling. Eur J Vasc Endovasc Surg. 2011;42(2):207–19. doi: 10.1016/j.ejvs.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Vande Geest JP, Sacks MS, Vorp DA. A planar biaxial constitutive relation for the luminal layer of intra-luminal thrombus in abdominal aortic aneurysms. J Biomech. 2006;39(13):2347–54. doi: 10.1016/j.jbiomech.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Folkesson M, Silveira A, Eriksson P, Swedenborg J. Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis. 2011;218(2):294–9. doi: 10.1016/j.atherosclerosis.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(6):1120–6. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 28.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86(5):1105–9. doi: 10.1189/jlb.0209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charriere GM, Cousin B, Arnaud E, Saillan-Barreau C, Andre M, Massoudi A, et al. Macrophage characteristics of stem cells revealed by transcriptome profiling. Exp Cell Res. 2006;312(17):3205–14. doi: 10.1016/j.yexcr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Mooney JE, Rolfe BE, Osborne GW, Sester DP, van Rooijen N, Campbell GR, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol. 2010;176(1):369–80. doi: 10.2353/ajpath.2010.090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seta N, Kuwana M. Derivation of multipotent progenitors from human circulating CD14+ monocytes. Exp Hematol. 2010;38(7):557–63. doi: 10.1016/j.exphem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87(9):858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 34.Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol. 2009;86(5):1111–8. doi: 10.1189/jlb.0309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medbury HJ, Tarran SL, Guiffre AK, Williams MM, Lam TH, Vicaretti M, et al. Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int Angiol. 2008;27(2):114–23. [PubMed] [Google Scholar]
- 36.Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, et al. Transcriptional switching in macrophages associated with the peritoneal foreign body response. Immunol Cell Biol. 2014 doi: 10.1038/icb.2014.19. [DOI] [PubMed] [Google Scholar]
- 37.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125(14):1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitao A, Sato Y, Sawada-Kitamura S, Harada K, Sasaki M, Morikawa H, et al. Endothelial to mesenchymal transition via transforming growth factor-beta1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol. 2009;175(2):616–26. doi: 10.2353/ajpath.2009.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballotta V, Driessen-Mol A, Bouten CV, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35(18):4919–28. doi: 10.1016/j.biomaterials.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Piao M, Tokunaga O. Significant expression of endoglin (CD105), TGFbeta-1 and TGFbeta R-2 in the atherosclerotic aorta: an immunohistological study. J Atheroscler Thromb. 2006;13(2):82–9. doi: 10.5551/jat.13.82. [DOI] [PubMed] [Google Scholar]


