Abstract
Oncolytic adenoviruses are modified to exploit the aberrant expression of proteins in cancer cells to obtain cancer-selective replication. Moreover, the natural tropism of oncolytic adenoviruses can be redirected to tumor cells. Clinical trials revealed that oncolytic viruses showed poor replication in the tumor that is due in part to the immune response against the virus. More recent data demonstrated that tumor infection might subvert the tumor immune system and lead to an anti-tumor immune response. In the next few years, combination of adenoviruses with immune checkpoint antibodies and other immune modulators will be tested in clinical trials.
Oncolytic viruses were designed to infect cancer cells preferentially or to replicate and lyse cancer cells selectively [1,2]. Early research focused on two approaches to achieve these goals [4]. One approach involved targeting deregulated cell cycle and cell death in cancer cells. Since adenovirus replication requires subverting host cells to share these characteristics of cancer cells, deletions can be made in viral genes to attenuate their ability to interfere with these cellular processes. Another approach is to target the gain of function of the genes involved in replicative immortality and angiogenesis in cancer. For instance, the expression of the viral proteins that are indispensable for viral replication will be controlled by ectopic promoters inserted in the adenovirus genome, such as human telomerase reverse transcriptase, E2F transcription factor 1, or hypoxia-inducible factor 1-alpha promoters [3].
Oncolytic viruses must not only be cancer-selective but also replicate despite host immunity. Host immunity is probably responsible for the lack of positive results in clinical studies of cancer patients requiring systemic delivery of an adenovirus [4]. However, the immunity of the host may play a more positive role during adenoviral infection of a tumor, and the development of a strong anti-tumor immunity accompanying virotherapy is emerging as a critical factor required to achieve complete cancer suppression. Recently, the field of cancer virotherapy has embraced this shift in the understanding of the mechanism underlying tumor regression after viral infection. Here, we describe our experience working with oncolytic adenoviruses as examples of the evolution of oncolytic virotherapy to viro-immunotherapy.
ONYX-015
Adenoviruses have been explored extensively as viral vectors for gene therapy. However, clinical tests of adenoviral vectors to treat cancer had disappointing results because of the limited delivery [5]. In this context, the ability of oncolytic adenoviruses to replicate in and spread throughout the tumor appeared to be a potential solution to this delivery problem. Pioneer research revived adenovirus-mediated virotherapy by showing that the E1B55KD-deleted oncolytic adenovirus (dl1520 or ONYX-015) might exploit p53 deficiency in tumor cells [6]. The rational behind this strategy was based on the knowledge about the interactions between viral and cellular proteins. E1B55KD viral protein binds and inactivates p53 protein favoring viral replication in the host, probably by preventing cellular apoptosis. The E1B55KD-deleted adenovirus was designed to be able to replicate in p53-deficient cancer cells, but not in normal cells [6]. Eventually, in 2005, oncolytic adenovirus H101 (closely related to ONYX-015) became the first adenovirus to be approved by the China State Food and Drug Administration for treating head and neck cancer in combination with chemotherapy [7,8].
Extensive clinical testing of ONYX-015 has confirmed that this approach is nontoxic [9]. Thus, a maximally tolerated dose of ONYX-015 has never been reached: ONYX-015 is safe at doses up to 2×1012 viral particles with no clinical signs of liver toxicity [9]. Additionally, a phase II trial of ONYX-015 and a phase III trial of H101 in combination with cisplatin and 5-fluorouracil to treat squamous cell cancer of the head and neck showed that the intratumoral injection of the virus was relatively safe and that response rates reached nearly 80% [10,11]. A dose-escalation trial in patients with recurrent malignant glioma showed that injection of ONYX-015 into the tumor cavity after glioma resection was well tolerated at doses up to 1010 plaque-forming units of the virus [12].
Although the antitumor activity of ONYX-015 has been encouraging when combined with chemotherapy, its efficacy as a single agent has been limited (0%-14% local tumor regression rates, depending on tumor histology) [13]. In addition to binding and inactivating p53 protein, E1B55KD is also involved with viral mRNA transport and host cell protein synthesis shut-off [14-16], which explains why ONYX-015 displayed poor replication and subsequent reduced potency in tumors [6,17]. Further studies also showed that p53 status might not be the basis for the tumor selectivity of ONYX-015 [18].
The implication that the attenuated replication of ONYX-015 is responsible, at least in part, for the limited anti-cancer effect prompted the exploration of other deletions in the adenovirus genome that restrict virus replication to tumor cells without sacrificing its potency.
Targeting the cell-cycle checkpoints: constructing a safe and potent oncolytic adenovirus
To induce S-phase entry in the host cell to support their replication, adenoviruses express early region 1A protein (E1A) that binds and inactivates Rb protein, which regulate the G1–S-phase cell-cycle checkpoint [19]. This interaction occurs through the E1A protein conserved region (CR)-2 [20,21] (Figure 1). Thus, the replication of the adenovirus with this type of mutation is restricted in normal cells with an intact G1/S checkpoint [22,23]. Since this checkpoint is deregulated in almost all cancer cells as a result of mutation or deletion in proteins of Rb pathway [24], this type of virus mutant, Delta-24 or dl922-947, replicates efficiently in these cells and showed cancer-selective cytolysis [22,23]. The replication potency of these viruses is close to that of wild-type adenovirus, and it replicates up to 100 times more efficiently than ONYX-015 in the vast majority of cancer cells [22,23].
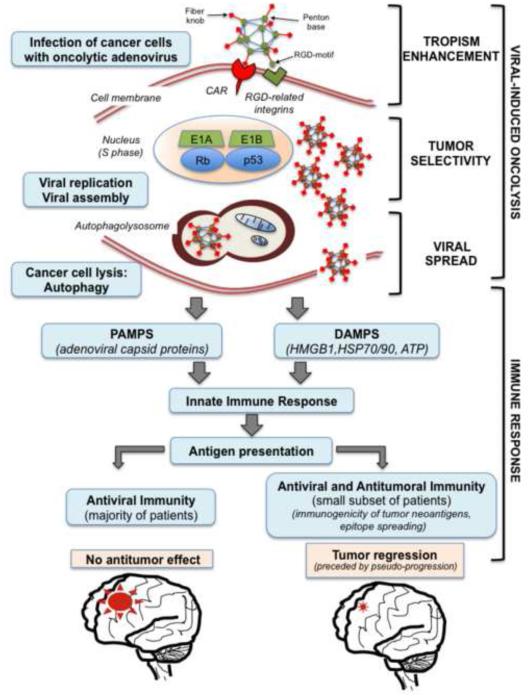
Figure 1. Dual mechanism of cancer virotherapy.
Viral-mediated anticancer effect in vivo would depend on success of the two stages: viral replication with the effect of viral-induced oncolysis and the subsequent immune response. The first stage, viral-induced oncolysis, should probably be 1) enhanced by directing the tropism of the adenovirus to cancer cells, per instance with the insertion of an RGD-motif peptide sequence in the viral fiber knob; 2) restricted to cancer cells, and this can be achieved by modifying the viral proteins that interact with cellular proteins, per instance, via mutations of the early viral proteins E1A or E1B; and 3) modified by accelerating the lysis process, per instance by increasing autophagy with autophagy regulators. The anti-virus and anti-tumor immune responses are elicited by the accumulation of pathogen-associated molecular patterns (PAMPs), which reflect conserved components of microbes and viruses, per instance the adenovirus capsid proteins, together with the release to the tumor milieu of danger-associated molecular patterns (DAMPs), which are responsible for the attraction of innate immune cells and eventually for T cell priming. It is possible that tumor regression after adenoviral infection would exclusively occur if the anti-tumor immune response is elicited and maintained.
To enter the host cell, adenovirus first attaches the coxsackievirus and adenovirus receptor (CAR) on the cell surface through adenovirus fiber protein [25,26]. The process of internalization requires a second interaction between the αvβ3 and αvβ5 integrins on the cell surface and the Arg-Gly-Asp tripeptide motif (RGD) at the adenoviral penton base [27,28]. CAR is expressed by most normal cell types [29], but the level of CAR expression highly varies in cancer cells [30,31]. By contrast, RGD-related integrins are highly expressed by cancer cells or tumor vasculature but are expressed at much lower levels in the normal cells surrounding the tumor [31,32]. Lack of expression of CAR together with the predominant expression of integrins in cancer cells led to the hypothesis that an adenovirus modified to infect by directly binding to integrins (CAR-independent infection) would have a more potent anti-cancer effect than wild-type adenoviruses. To this end, Delta-24 backbone was modified by inserting the RGD motif in the HI loop of the adenoviral fiber protein, generating Delta-24-RGD (Figure 1). This new generated virus demonstrated superior viral propagation and oncolytic effect in vitro and in vivo than did its parental type Delta-24 in cancer cells [31,33].
So far, the first-in-human phase I clinical trial of Delta-24-RGD (DNX-2401) in patients with recurrent glioblastoma has shown encouraging results (Lang et al, manuscript in preparation). Two other trials for DNX-2401 are currently recruiting patients: “Phase 1b, Randomized, Multi-center, Open-label Study of a Conditionally Replicative Adenovirus Delta-24-RGD and Interferon Gamma (IFN-γ) for Recurrent Glioblastoma or Gliosarcoma” (ClinicalTrials.gov Identifier NCT02197169) and “Phase I Trial of Combination of Delta-24-RGD Oncolytic Adenovirus With a Short Course of Temozolomide for Treatment of Glioblastoma at First Recurrence” (Clinical Trial.gov Identifier NCT01956734).
For nearly two decades, research has focused on developing cancer-selective (safe) oncolytic viruses with full cytolysis potential. However, the success of oncolytic adenoviruses in preclinical studies has not fully translated into clinical applications in cancer patients (Figure 1).
From viral oncolysis to viro-immunotherapy: a paradigm shift
The preclinical studies of oncolytic adenoviruses have been conducted mainly in human cancer xenografts in nude mice. Intratumoral injection of Delta-24-RGD in solid tumors demonstrated long-lasting replication and complete wipeout of the glioma in the mouse brain [31]. However, clinical trials have shown no clear evidence that direct cytolysis by the oncolytic virus is the exclusive mechanism of tumor destruction [34]. This disparity between animal models and clinical studies could be due to the lack of an ideal animal model that can be used to test the virus and immune activity in preclinical experiments (Table 1).
Table 1.
The paradigm shift in developing oncolytic adenoviral therapy
| Virus-mediated Oncolysis | Virus- and Immune-mediated Oncolysis | |
|---|---|---|
|
Approaches to improve
safety and therapeutic effect |
Increase cancer selectivity Enhance viral replication potency Improve delivery Suppress host immunity |
Increase cancer selectivity Enhance viral replication potency Control immunity against virus Combination with immune modulators |
|
Limitations and
challenges |
Host antiviral immunity Delivery to distant cells |
Host antiviral immunity Tumor evasion Auto-immunity |
| Preclinical models | Human xenografts in immunodeficient mice |
Syngeneic tumors in immunocompetent animals |
The clinical experience of oncolytic viruses suggests that immunosuppressed patients generally show better response than do those with an intact immune system, but this higher anti-cancer effect is often associated with unacceptable toxicity [35]. These studies support the rationale that better anti-tumor efficacy could be achieved via combining oncolytic viruses with immunosuppressive drugs to delay the immune clearance of the virus [36]. However, the complete regression of the tumor depends on whether the virus can reach every cancer cell. This is a difficult task for the virus, if not impossible, given the physical barriers within the tumor (fibrosis, necrosis, and cellular heterogeneity) and the challenges faced by systemic delivery to metastasized tumors [34]. Further enhancing replication potential is unlikely to increase significantly the efficacy of oncolytic viruses without increasing the risk of virus-mediated toxicity.
Although host immunity has been blamed for blocking the effect of oncolytic viruses, both preclinical and clinical studies have demonstrated that harnessing the immune response could enhance the therapeutic benefits of oncolytic viruses. One of the hallmarks of cancer is evading immune destruction [1], and the anti-tumor immune activity is abrogated by immune inhibitory cytokines, secreted by the tumor, and inhibitory immune cells. This immunosuppressive environment could be disrupted by infecting the tumor with an adenovirus. Targeted viral infection of tumor cells should release to the tumor milieu pathogen-associated molecular pattern molecules (PAMPs) and danger- (damage-) associated molecular pattern molecules (DAMPs) [37,38] (Figure 1). The immune response elicited by PAMPS and DAMPS may overcome immunosuppression within the tumor microenvironment and instigate a localized inflammation within the malignancy, which could facilitate the immune recognition of cancer-specific antigens [34]. For instance, Delta-24-RGD, through multiple intratumoral injections, elicits anti-tumor immunity and prolongs the survival of animals bearing intracranial gliomas in an immunocompetent mouse model [37]. Furthermore, infection of ovalbumin-expressing cancer cells triggered a specific anti-ovalbumin immune response, which strongly indicated that an anti-tumor CD8-mediated immune response can follow adenovirus infection of cancer cells [37].
Success in treating cancer patients with blocking immune checkpoint inhibitors demonstrates that reactivating paralyzed lymphocytes within tumors can subsequently eliminate the tumors [39]. Since patients with increased levels of tumor-infiltrating lymphocytes often have a better prognosis and response to therapy [40] and oncolytic virus infection recruits lymphocytes to the tumor, it is natural to combine the virus with strategies to modulate T lymphocytes activity directly to avoid unwanted systemic effects mediated by cytokines [39] (Table 2 and Figure 2). To this end, combining Newcastle disease virus with blocking immune checkpoint inhibitor cytotoxic T-lymphocyte-associated protein 4 (CTLA4) potentiated the effect of CTLA4 blockade in tumors in preclinical models [41]. Currently, ipilimumab (antagonist anti-CTLA4 antibody) is in early clinical testing with intratumoral injection of talimogene laherparepvec (T-VEC) (ClinicalTrials.gov Identifier NCT01740297), an oncolytic virus derived from herpes simplex virus 1 [42]. Additionally, a Delta-24–based oncolytic adenovirus coding for a full human monoclonal antibody specific for CTLA4 has been tested in preclinical models and in human cancer patients, demonstrating the feasibility of testing the approach in clinical trials [43]. Another approach is to use oncolytic viruses to express immune co-stimulators to enhance anti-tumor immunity [42]. Accordingly, co-expression of 4-1BBL and interleukin 12 by the oncolytic adenovirus increased anti-tumor immune response through enhanced cytolytic activity of cytotoxic T lymphocytes and IFN-γ–releasing immune cells [44]. Both approaches could be highly significant for the development of more effective virotherapy; however, further studies are necessary to confirm these published results.
Table 2.
Examples for combination of oncolytic adenovirus with immune checkpoint modulators
| Advantages | Disadvantages | |
|---|---|---|
|
OA + antagonist antibodies to
block immune inhibitors (CTLA-4, PD-1) |
Abrogate T cell anergy | Affect all T cells Induce global effect, autoimmunity |
|
OA + antagonist antibodies
to block immune inhibitor ligands (PD-L1) |
Target tumor cells | Affect other APCs Induce global effect, autoimmunity |
|
OA expressing single-chain
antibodies against immune inhibitors |
Limit effect at tumor site Achieve high levels of antibodies within the tumor |
Technically possible but complicated; Paucity of publications; require confirmation |
|
OA expressing ligands for
immune co-stimulators |
Confine effect to tumor cells Technically straight forward |
Paucity of publications; require confirmation |
|
OA expressing decoy receptors to
block immune inhibitor ligands |
Limit effect at tumor site Target tumor cells |
Require confirmation |
OA, Oncolytic adenovirus
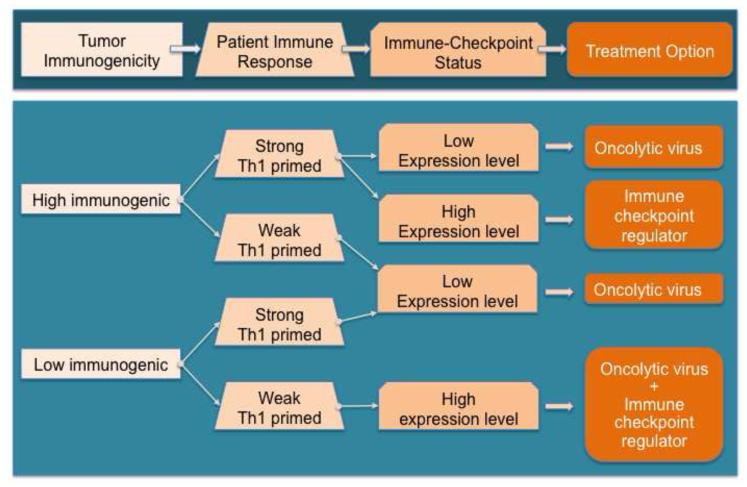
Figure 2. Rationale for the combination of oncolytic adenoviruses with immune checkpoint modulators.
Future personalized medicine in the field of oncolytic virotherapy might depend on the degree of tumor immunogenicity, the status of the host immune response with respect to tumor cells, and the levels of expression of immune checkpoints and other positive and negative regulators of anergy. Combination therapy might be required particularly for patients with weak Th1 primed lymphocytes, and high levels of expression of immune checkpoints in the tumor infiltrative T cells, and with a low degree of immunogenicity. Further preclinical studies would be necessary to buttress these algorithms.
Future Directions
The focus of oncolytic virotherapy has shifted from direct oncolysis of the tumor by the virus to suppressing the tumor via virus-mediated immune response. During virotherapy, the host immunity will eventually clear the virus, which is necessary for the safety of cancer patients, but anti-tumor immunity is ideally elicited before the adenovirus is cleared. The immunosuppressive tumor microenvironment could give a window of opportunity, albeit short, for viral propagation. Thus, strategies should be developed to make tumor-associated antigens more visible to the immune system afterwards. Although targeting T cells with antagonist antibodies of immune checkpoint inhibitors avoids some of the toxicity generated by cytokines, expressing immune co-stimulators by the virus on the tumor cell surface should confine the effect more to the tumor cells. Strategies that target the immune inhibitors on tumor cells could also be a safer approach.
It is almost impossible to test every virotherapy regimen directly in cancer patients although it is the optimal setting for the task. The animal models are far from ideal, but are less expensive and more accessible to test some basic aspects of the therapeutic effect, including survival and immune cell activation. Oncolytic adenoviruses are mainly derived from human adenoviruses and most mouse cells are not permissive for producing progeny virions. But these viruses can express viral early genes and cause autophagy and cell lysis in mouse cancer cells efficiently [45,46]. The autophagic cell death induced by the virus releases cellular content that functions as PAMPs and DAMPs to induce inflammation at the infection site, just as in humans. In an immunocompetent glioma mouse model, three injections of Delta-24-RGD partially compensated its replication deficiency and achieved therapeutic effect [37]. Although the Syrian hamster has been used for evaluating the therapeutic effect of oncolytic adenoviruses for several cancers [47], it is only semi-permissive for adenoviral replication, and only a few models are available for a small group of tumor types.
We believe that the paradigm shift in oncolytic virotherapy (from virus-mediated oncolysis as the exclusive anti-tumor mechanism to the predominant role of the virus in anti-tumor immune response) will spur rapid development in this field and that the translation of these discoveries should improve the prognosis of cancer patients significantly during the next decade.
Highlights.
Oncolytic adenoviruses should not be attenuated viruses
In a small subset of patients, treatment of tumors with oncolytic viruses leads to tumor regression
The main barriers to oncolytic virotherapy are both the host and the tumor immune systems
Virotherapy combined with immune checkpoint modulators represents a logic avenue of progress
Acknowledgements
We thank Dr. Jill R. Delsigne (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, Houston, TX) for editorial assistance. This work has been partially supported by the National Institutes of Health (P50CA127001; R01NS069964), the Marnie Rose Foundation, the Will Power Foundation, and the Broach Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. CGM, CAC, and JF are shareholders of DNAtrix, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, McCormick F, Lang FF, Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, McCormick F, Gomez-Manzano C, Curiel DT, Lang FF, Yung WKA, Fueyo J. In: Replicating viruses for brain tumor treatment. Castro M, Lowenstein P, editors. Taylor & Francis; New York: 2006. [Google Scholar]
- 4.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 5.Alemany R. Chapter four--Design of improved oncolytic adenoviruses. Adv Cancer Res. 2012;115:93–114. doi: 10.1016/B978-0-12-398342-8.00004-5. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 7.Frew SE, Sammut SM, Shore AF, Ramjist JK, Al-Bader S, Rezaie R, Daar AS, Singer PA. Chinese health biotech and the billion-patient market. Nat Biotechnol. 2008;26:37–53. doi: 10.1038/nbt0108-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 9.McCormick F. Cancer-specific viruses and the development of ONYX-015. Cancer Biology & Therapy. 2003;2:S157–160. [PubMed] [Google Scholar]
- 10.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, Zhang Y, Hu XH, Wu GH, Wang HQ, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus.] Aizheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 11.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature Medicine. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 12.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Molecular Therapy. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 14.Babiss LE, Ginsberg HS, Darnell JE., Jr. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leppard KN, Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Medicine. 1997;3:639–645. doi: 10.1038/nm0697-639. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 20.Whyte P, Ruley HE, Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988;62:257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whyte P, Williamson NM, Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989;56:67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- 22.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 23.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 24.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 25.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 26.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 28.Seth P. Adenoviruses: Basic Biology to Gene Therapy. R.G. Landes Company; Austin, Texas: 1999. [Google Scholar]
- 29.Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. Advances in Pharmacology. 1997;40:137–206. doi: 10.1016/s1054-3589(08)60140-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, Raben D, Curiel DT. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Research. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 31.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 32.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. discussion 390. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 34.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 36.Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- 37.Jiang H, Clise-Dwyer K, Ruisaard KE, Fan X, Tian W, Gumin J, Lamfers ML, Kleijn A, Lang FF, Yung WK, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS One. 2014;9:e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Fueyo J. Healing after death: anti-tumor immunity induced by oncolytic adenoviral therapy. OncoImmunology. 2014;3:e947872. doi: 10.4161/21624011.2014.947872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720. doi: 10.4161/onci.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008095. 226ra232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 43.Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Loskog A, et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–998. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 44.Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee MG, Kim H, Yun CO. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther. 2010;18:264–274. doi: 10.1038/mt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H, White EJ, Rios-Vicil CI, Xu J, Gomez-Manzano C, Fueyo J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J Virol. 2011;86:4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein SR, Piya S, Lu Z, Xia Y, Alonso MM, White EJ, Wei J, Gomez-Manzano C, Jiang H, Fueyo J. C-Jun N-terminal kinases are required for oncolytic adenovirus-mediated autophagy. Oncogene. 2015 doi: 10.1038/onc.2014.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wold WS, Toth K. Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res. 2012;115:69–92. doi: 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]