Abstract
Background and Purpose
Diabetes mellitus is a high risk factor for ischemic stroke. Diabetic stroke patients suffer worse outcomes, poor long term recovery, risk of recurrent strokes and extensive vascular damage. We investigated the neurorestorative effects and the underlying mechanisms of stroke treatment with human umbilical cord blood cells (HUCBCs) in Type two diabetes mellitus (T2DM) rats.
Methods
Adult male T2DM rats were subjected to 2 h of middle cerebral artery occlusion (MCAo). Three days after MCAo, rats were treated via tail-vein injection with: 1) phosphate-buffered-saline (PBS); 2) HUCBCs (5×106); n=10/group.
Results
HUCBC stroke treatment initiated 3 days after MCAo in T2DM rats did not significantly decrease blood-brain-barrier (BBB) leakage (p=0.1) and lesion volume (p=0.078), but significantly improved long term functional outcome and decreased brain hemorrhage (p<0.05) when compared to the PBS-treated T2DM-MCAo control group. HUCBC treatment significantly promoted white matter (WM) remodeling as indicated by increased expression of Bielschowsky silver (axons marker), Luxol fast blue (myelin marker), SMI-31 (neurofilament) and Synaptophysin in the ischemic border zone (IBZ). HUCBC promoted vascular remodeling, and significantly increased arterial and vascular density. HUCBC treatment of stroke in T2DM rats significantly increased M2 macrophage polarization (increased M2 macrophage CD163, CD 206; decreased M1 macrophage ED1 and iNOS expression) in the ischemic brain compared to PBS-treated T2DM-MCAo controls (p<0.05). HUCBC also significantly decreased pro-inflammatory factors i.e., matrix metalloproteinase 9 (MMP9), receptor for advanced glycation end-products (RAGE) and toll like receptor 4 (TLR4) expression in the ischemic brain.
Conclusion
HUCBC treatment initiated 3 days after stroke significantly increased WM and vascular remodeling in the ischemic brain as well as decreased neuroinflammatory factor expression in the ischemic brain in T2DM rats and promoted M2 macrophage polarization. HUCBC reduction of neuroinflammation and increased vascular and WM-axonal remodeling may contribute to the HUCBC induced beneficial effects in T2DM stroke rats.
Keywords: HUCBC, neurorestorative therapy, stroke, T2DM, vascular remodeling, white matter remodeling
Introduction
The global concern for stroke, a cardiovascular disease that can lead to long term disability or even death, is rapidly growing. Among the prominent risk factors for ischemic stroke is Diabetes Mellitus (DM). The combination of diabetes and stroke leads to extensive brain damage and worse post stroke outcomes1. The T2DM-stroke combination puts the patient at a risk of recurrent strokes2 and massive vascular damage leading to poor long term functional recovery and even lifelong paralysis. While the only FDA approved drug for ischemic stroke is tPA (tissue plasminogen activator) that can breakdown the blood clot and restore blood flow to the brain, the treatment is challenged by practicality because of its narrow treatment window (3–4.5 hrs after stroke onset). Thus, there is a compelling need to develop and test neurorestorative therapeutic approaches for stroke, with treatment initiated days after stroke3. One such therapy employs HUCBCs to induce neurorestorative effects post ischemic stroke.
HUCBCs therapy administered via tail vein injection within 24–72 hrs post an ischemic insult in non diabetic stroke rats has significant functional benefits associated with the regulation of inflammatory and immune responses4, 5. In T1DM rats, initiation of HUCBC treatment (5×106 cells) via tail vein injection 24 hrs post stroke had several beneficial effects including improved functional outcome, enhanced vascular and WM remodeling6. The primary mechanism of action was attributed to increasing angiopoetin-1 (Ang-1) and decreasing inflammatory factor RAGE (receptor for advanced glycation end products) expression in the ischemic border zone (IBZ) in T1DM rats6. T2DM mice that underwent stroke suffered from worse functional outcome, increased brain hemorrhage, severe WM damage and increased inflammatory factor MMP9 (matrix mettaloproteinase-9) expression1. Mortality rates in diabetic stroke mice were significantly greater than in wild type mice7. With T2DM being the most common form of diabetes and the success of HUCBC treatment in non diabetic and T1DM stroke animals, our aim in this study is to evaluate the therapeutic efficiency and underlying mechanism of delayed HUCBC treatment in T2DM rats when treatment is initiated 3 days after stroke.
Materials and Methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Diabetes induction
T2DM was induced using a combination of 2 weeks high fat diet followed by low dose of streptozotocin (STZ, 35 mg/kg, Sigma Chemical Co., St. Louis, MO) intraperitoneal injection in adult Male Wistar rats and a continued high fat diet for another 2 weeks. Two weeks after STZ injection, the fasting blood glucose level was tested using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN) and animals with fasting blood glucose >300 mg/dl underwent 2 hrs of transient MCAo.
Middle cerebral artery occlusion (MCAo) model and experiment groups
Twenty four T2DM rats were subjected to transient (2 hrs) right MCAo via intraluminal vascular occlusion, as previously described8, 9. The exclusion criteria were rats with modified neurological severity score (mNSS) less than 6 (possibly small to no lesion) or over 13 (poor survival) at 24 hrs after MCAo (prior to treatment). Accordingly, 4 rats were excluded from this study. The rats were randomly assigned to different groups and treated with 1) PBS as vehicle control (n=10); 2) HUCBCs (5×106, n=10) (Saneron CCEL Therapeutics) via tail-vein injection starting at 3 days post MCAo. The treatment time point of 72 hrs after stroke in T2DM rats was employed to investigate HUCBC induced neurorestorative effects as well as to accommodate a wide treatment window. Rats were sacrificed 28 days after MCAo for immunostaining quantification analysis.
Neurological functional tests
An investigator was blinded to the experimental groups to perform a battery of functional tests including foot-fault10, adhesive removal8 and evaluation of mNSS8 before MCAo, and after MCAo on days 1, 7, 14, 21 and 28.
Histological and Immunohistochemical assessment
Brains were fixed using transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde. Then the brains were embedded in paraffin and a standard block was obtained from the center of the lesion (bregma −2 mm~+2 mm). A series of 6μm thick sections were cut from the block. Hematoxylin and eosin (H&E) stained seven coronal sections of tissue were used for lesion volume calculation and presented as a percentage of lesion compared to the contralateral hemisphere.
Brain coronal tissue sections were prepared and antibody against α-smooth muscle actin (α-SMA, mouse monoclonal IgG, 1:800; Dako); Von Willebrand Factor (vWF, 1:400; Dako); SMI-31 (Neurofilaments, phosphorylated monoclonal, 1:1000, Covance), Synaptophysin (monoclonal; 1:500, Millipore); RAGE (1:400; Dako), TLR4 (goat polyclonal IgG; dilution 1:100; Santa Cruz Biotechnology); MMP9 (1:500, Santa Cruz Biotechnology); CD163 (1:500, Abcam Cambridge, MA); ED1 (a mouse mAb against rat microglia/macrophages, monoclonal, 1:30; AbD Serotec); CD 206 (1:3000, Abcam), iNOS (1:200, Millipore) were employed.
Antibody against albumin (albumin-FITC, polyclonal, 1:500, Abcam) was used to demonstrate BBB leakage and Prussian blue staining used to evaluate hemorrhage. Bielschowsky-silver (BS) immunostaining was used to demonstrate axons; luxol fast blue (LFB) staining was used to demonstrate myelin. Control experiments consisted of staining brain coronal tissue sections as outlined above, but non-immune serum was substituted for the primary antibody.
Quantification analysis
All the immunostaining quantification analysis was performed by an investigator who was blinded to the experimental groups. Five slides from each brain, with each slide containing 8 fields from striatum of the IBZ were digitized under a 20x objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada). For BS and LFB measurements positive areas of immunoreactive cells were measured in the WM bundles of the striatum in the IBZ11. For other immunostaining (albumin-FITC, Prussian blue, Synaptophysin, SMI-31, RAGE, MMP9 and TLR4), positive areas of immunoreactive cells were measured in the IBZ and for CD163, iNOS, ED1 and CD206 positive cell number was measured in the IBZ12.
Vascular density measurement
To measure the vascular density in the IBZ, 8 fields of view of vWF immunostaining from the IBZ were digitized using a 20×objective via the MCID software13. The α-SMA stained arteries were analyzed with regard to small and large vessels (≥10μm diameter). The arterial density in the IBZ was measured14.
Statistical Analysis
One-way Analysis of Variance (ANOVA) was used for the evaluation of functional outcome and histology, respectively. “Contract/estimate” statement was used to test the group difference. If an overall treatment group effect was detected at p<0.05, pair-wise comparisons were made. All data are presented as mean ± standard error (SE).
Results
HUCBC treatment significantly improved long term functional outcome but did not significantly decrease BBB leakage and lesion volume
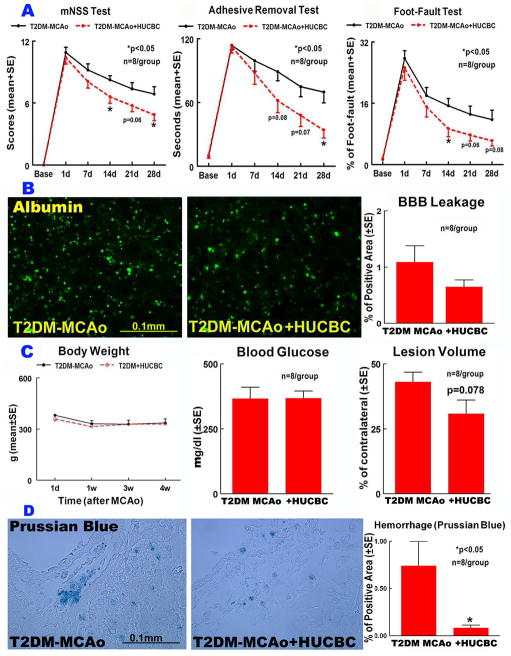
Long term functional benefit derived from HUCBC treatment initiated at 3 days after MCAo in T2DM rats was assessed using a battery of behavioral tests. N=10/group was employed and with a mortality rate of 20% (between days 7–14 after MCAo), 8 rats/group survived at the end of experiments in both treatment and control groups. Figure 1A presents mNSS, adhesive removal and Foot fault data for 28 days after stroke that indicate significantly improved functional outcome in T2DM MCAo rats treated with HUCBCs compared to PBS treated control group (p<0.05).
Figure 1.
HUCBC treatment 3d post stroke in T2DM-MCAo rats significantly improved functional outcome A) mNSS, Adhesive removal test, Foot-fault; B) did not significantly decrease BBB leakage, C) body weight, blood glucose or lesion volume but significantly decreased D) brain hemorrhage compared to PBS treated T2DM MCAo rats.
HUCBC treatment significantly decreased hemorrhage in the brain identified by Prussian blue staining (p<0.05, Fig. 1D) compared to PBS-treated T2DM stroke rats. HUCBC treatment in T2DM rats did not alter body weight, blood glucose level, did not significantly decrease lesion volume (p=0.078, Fig. 1C) and BBB leakage as indicated by FITC-albumin immunostaining (p=0.1; Fig. 1B), compared to PBS-treated T2DM-MCAo rats.
HUCBC stroke treatment significantly promoted vascular remodeling
To test the beneficial effects of HUCBC treatment, vascular remodeling was evaluated using α-SMA and vWF immunostaining. Figure 2 indicates that HUCBC significantly enhanced cerebral artery density (α-SMA, Fig. 2A) and vascular density (vWF, Fig. 2B) compared to PBS treated control T2DM stroke rats (p<0.05).
Figure 2.
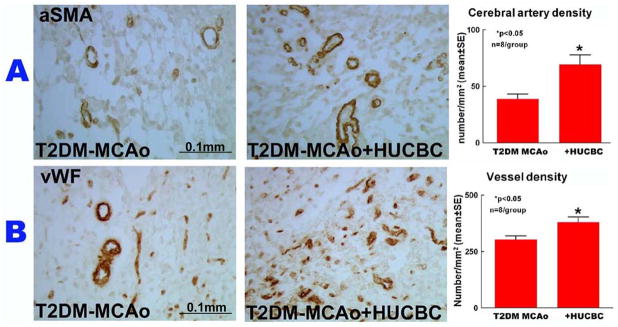
HUCBC treatment 3d post stroke in T2DM-MCAo rats significantly improved vascular remodeling as indicated by A) a-SMA and B) vWF immunostaining and quantification data in the IBZ compared to PBS treated T2DM MCAo rats.
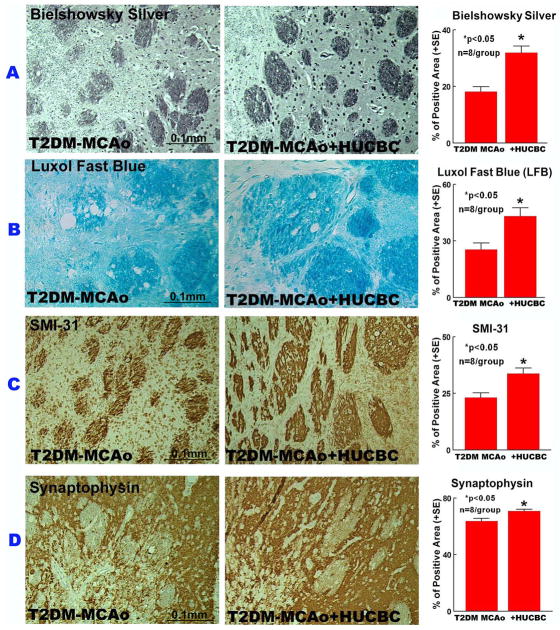
HUCBC stroke treatment promoted white matter remodeling and increased axonal and synaptic plasticity
BS and LFB staining were used to evaluate the beneficial effects of HUCBC treatment on WM remodeling. HUCBC treatment significantly increased BS and LFB expression (Fig. 3A–B) in the WM bundles compared to PBS-treated T2DM-MCAo rats (p<0.05). HUCBC treatment also regulated axonal and synaptic plasticity, indicated by significantly increased SMI-31 (Fig. 3C) and Synaptophysin (Fig. 3D) expression levels in the IBZ compared to PBS-treated T2DM-MCAo rats (p<0.05).
Figure 3.
HUCBC treatment 3d post stroke in T2DM-MCAo rats significantly increased WM remodeling, axon density and synaptic protein expression in the ischemic brain. A) Immunostaining with Bielschowsky silver, B) Luxol fast blue, C) SMI-31, D) Synaptophysin and quantification data in the IBZ compared to PBS treated T2DM MCAo rats.
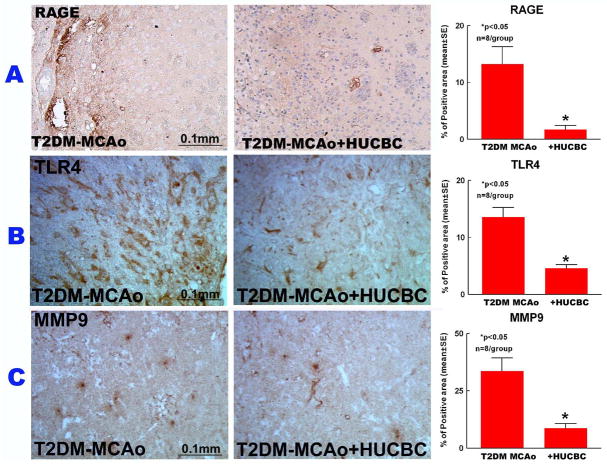
HUCBC stroke treatment significantly decreased neuroinflammatory factor expression and increased M2 macrophage polarization in the IBZ
To understand the mechanisms of HUCBC therapy derived benefits, neuroinflammation was evaluated in the ischemic brain. Expression levels of inflammatory factors TLR4, MMP9 and RAGE were measured in the IBZ. As indicated in Figure 4, HUCBC treatment significantly decreased TLR4, MMP9 and RAGE (p<0.05) expression compared to PBS-treated T2DM stroke rats (p<0.05).
Figure 4.
HUCBC treatment 3d post stroke in T2DM-MCAo rats significantly decreased inflammatory factors expression in the ischemic brain. A) Immunostaining with Rage, B) TLR4, C) MMP9 and quantification data in the IBZ compared to PBS treated T2DM MCAo rats.
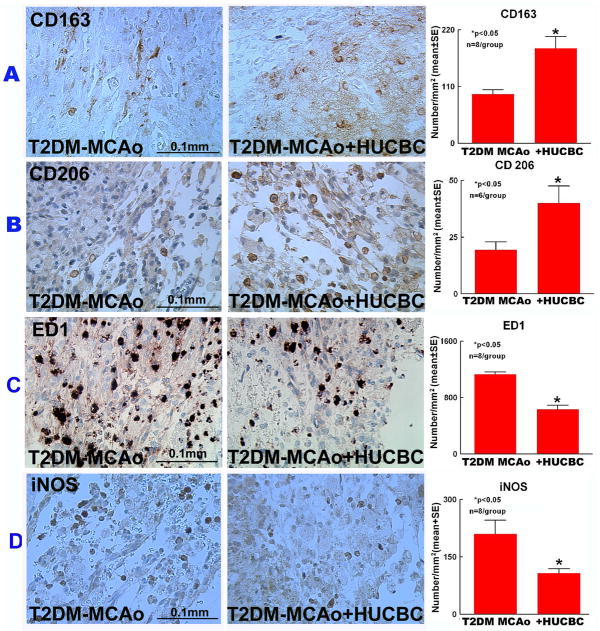
To test the effect of HUCBC treatment on macrophage polarization, expression levels of M2 macrophage markers CD163, CD206 and M1 macrophage markers ED1, iNOS were measured. Figure 5 shows that HUCBC treatment in T2DM MCAo rats significantly increased M2 and decreased M1 macrophage expression in the ischemic brain in comparison to PBS-treated T2DM stroke rats (p<0.05).
Figure 5.
HUCBC treatment 3d post stroke in T2DM-MCAo rats significantly increased M2 macrophage polarization in the ischemic brain. A) Immunostaining with CD 163, B) CD 206, C) ED1, D) iNOS and quantification data in the IBZ compared to PBS treated T2DM MCAo rats.
Discussion
HUCBCs have received a great deal of attention as a treatment option for hematological disorders and malignancies as they are a rich source of hematopoietic stem cells. The driving advantages in using HUCBCs include easy availability as it is discarded post birth, lack of ethical conflicts and none to low severity effects like GVHD (graft versus host disease) from donor-recipient HLA (human leukocyte antigen) mismatch15. This tolerance of HUCBC therapy to donor recipient HLA mismatch aided by cord blood’s naïve and immature immune function15 play a key role in translating this therapy to the clinic. We found that HUCBC therapy in T2DM rats initiated intravenously at 3 days post stroke, did not significantly decrease BBB leakage and lesion volume, but significantly decreased brain hemorrhagic transformation and significantly increased vascular and WM remodeling and improved functional recovery. The underlying mechanisms of HUCBC-induced benefits may be decreased neuroinflammatory effects and promotion of M2 macrophage polarization.
Several stroke treatments in the diabetic rat population have failed including bone marrow stromal cells16 and tPA17. These failures were associated with increased BBB leakage, brain hemorrhagic transformation and inflammation in T1DM16, 17 and T2DM 18 rats. In this study, significant functional benefit was derived upon HUCBC therapy, although we found that HUCBC treatment initiated 3 days after stroke in T2DM did not significantly decrease BBB leakage and lesion volume compared to T2DM PBS-treated stroke rats. These data suggest that HUCBC induced therapeutic and functional benefits in T2DM stroke rats may primarily be derived from the regulation of vascular and WM remodeling. While in non diabetic rats, hemorrhagic transformation and BBB leakage occur early after stroke, in diabetic rats these events have an extended time window and are present at 14 days after stroke12, 16. Although the 28 day time point is not ideal for studying BBB permeability and hemorrhagic transformation which occur acutely after stroke19, 20, since we observed HUCBC treatment induced functional benefits starting at 14 days after stroke, we elected not to perform these measurements at an early time point.
A multifaceted mechanism of action has been suggested by several studies employing cell based therapies to promote post stroke long term beneficial effects; minor benefit derived from a small portion of infused cells migrating to the brain and differentiating into neuronal cells, and major benefit derived by promoting various aspects of neurorestoration like WM remodeling, vascular remodeling, synaptogenesis and neurogenesis by: a) enhancing endogenous brain repair mechanisms, and b) secreting trophic and growth factors. Stroke decreases cerebral blood flow and triggers vascular remodeling to improve blood supply via angiogenesis and arteriogenesis21. Increasing angiogenesis and arteriogenesis are correlated with neurological functional outcome after stroke22. In T2DM mice, poor functional outcome, compared with non diabetic stroke mice was correlated with exacerbated WM and vascular damage1. HUCBC treatment increased cerebral arterial and vascular density in the IBZ. These results indicate that HUCBC stroke treatment induces an increase in cerebral vascular remodeling in T2DM rats.
WM of brain is highly susceptible to ischemic stress primarily because of its relatively limited blood supply. Hence, after a stroke, WM recovery is critical for sustained long term functional recovery. HUCBC treatment enhanced the expression of Bielschowsky silver, SMI-31 and Luxol fast blue in the IBZ indicative of enhanced axonal plasticity and myelin regeneration. Several studies point towards axonal remodeling to improve brain repair, attenuate stroke induced neurological deficits as well as contribute to long term benefits in functional improvement23–25. At the same time, worse outcomes in DM stroke subjects have been associated with defective or restricted axonal regeneration26, 27. The importance of enhanced axonal myelination centers on faster communication and sensory/motor reflexes which helps restore lost neurological function and decreases stroke induced paralytic symptoms. Stroke leads to loss of myelin in the peri infarct area at 48 hrs after onset, but also stimulates the formation of new myelin around the damaged and sprouting axons28. This endogenous brain repair process is significantly enhanced by HUCBC treatment to promote the expression of LFB, a myelin marker, in the ischemic brain regions. HUCBC treatment also enhanced synaptic plasticity, indicated by increased Synaptophysin expression in the IBZ. Intercellular communication between neurons and other neurons/cells is facilitated via a synapse in the central nervous system, and improved synaptic plasticity indicated by enhanced Synaptophysin expression has been reported to mediate stroke treatment benefits29. Enhanced axonal and myelin remodeling, and axonal and synaptic plasticity may contribute to the observed HUCBC treatment induced post stroke recovery in T2DM rats.
Regulation of the inflammatory responses induced by stroke early after onset lasting up to weeks later, is crucial for effectiveness of stroke treatments. While mild inflammation can be favorable for brain repair in a chronic stage30, in the acute phase, upon uncontrolled inflammation the activated microglia, astrocytes and macrophages can exacerbate damage and/or death to the injured brain by releasing pro-inflammatory factors and by creating an inhospitable environment for neurovascular plasticity31, 32. MMP9 has been implicated in enhancing T2DM induced WM and axonal damage1, 33. TLR4 and RAGE are both inflammatory factors typically increased in diabetic stroke animals and have been implicated in exacerbating brain damage34, 35. HMGB1 (high-mobility group box 1) is an inflammatory mediator secreted upon injury by immune cells or injured cells. HMGB1 release can trigger an inflammatory cascade and binds to its receptors TLR4 and RAGE. It has been reported that in cerebral ischemia HMGB1 triggers MMP9 increase in neurons and astrocytes mainly through TLR436. Hence, treatments that can regulate the HMGB1/TLR4 signaling pathway can potentially decrease tissue damage by controlling post ischemic inflammatory responses. HUCBC treatment significantly decreased the expression levels of these detrimental inflammatory factors (TLR4, RAGE, and MMP9) in the IBZ and induces restorative effects in T2DM stroke rats.
M2 macrophage polarization has been associated with decreased neuroinflammation and enhanced axon growth in injured mouse spinal cord37. Our data show that M2 macrophage polarization was significantly increased by HUCBC treatment in the IBZ of T2DM MCAo rats. M2 macrophage polarization, marked by increased M2 macrophage CD163 and decreased M1 macrophage ED1 expression, was evident with HUCBC treatment. The M2 macrophage polarization mechanism can improve functional outcome post stroke38. Microglia and macrophages upon ischemic insult can assume an anti inflammatory M2 activation and protect neurons; which is a potential target for neurorestorative therapies39, 40. Soon after focal cerebral ischemia, the local and infiltrating macrophages assume M2 phenotype and decrease the expression of inflammatory factors thereby extending a protective effect to the neurons and improving their survival in the ischemic environment39. Extending the M2 phase of these macrophages and microglia and delaying their transit into M1 phenotype which is detrimental to the ischemic brain due to increased pro-inflammatory factor production, is a desirable effect. HUCBC treatment promotes M2 macrophage polarization which may contribute to improved neurological outcome. While it is common knowledge that macrophage invasion starts around 24 hrs after stroke and increases by 3 to 7 days after stroke; recent studies have revealed that the increased level of macrophage accumulation in the brain persists to at least 28 days after stroke41, lasting up to 1 year after stroke42. The links between M2 polarization and HUCBC induced regulation of neuroinflammation, WM and vascular remodeling leading to beneficial effects are not clear and further studies are warranted.
Conclusions
Treatment of stroke initiated 3 days post the ischemic insult via intravenous administration of HUCBCs in T2DM rats significantly improves functional recovery by enhancing WM-axonal and vascular remodeling in the ischemic brain. Our data suggest that decreasing neuroinflammatory factors and increasing M2 macrophage polarization may be contributing mechanisms underlying HUCBC treatment derived beneficial effects.
Supplementary Material
Acknowledgments
The authors wish to thank Qinge Lu and Sutapa Santra for the technical assistance.
Sources of Funding
This work was supported by National Institute on Aging RO1 AG031811 (JC), National institute of neurological disorder and stroke (NINDS) R01NS083078-01A1 (JC), National Institute on Aging RO1 AG 037506 (MC), R41NS080329-01A1 and American Heart Association grant 14GRNT20460026 (J.C.).
Footnotes
Disclosures
JC is a consultant to Saneron CCEL Therapeutics, Inc. Also, CDS & NKN are inventors on cord blood patents/applications. CDS is Sr. VP of R&D, and NKN is the President & COO at Saneron CCEL Therapeutics, Inc.
References
- 1.Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan A, Amarenco P, Goldstein LB, Sillesen H, Messig M, Samsa GP, et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: Secondary analysis of the stroke prevention by aggressive reduction in cholesterol levels (sparcl) trial. Arch Neurol. 2011;68:1245–1251. doi: 10.1001/archneurol.2011.146. [DOI] [PubMed] [Google Scholar]
- 3.Hurtado O, Pradillo JM, Alonso-Escolano D, Lorenzo P, Sobrino T, Castillo J, et al. Neurorepair versus neuroprotection in stroke. Cerebrovasc Dis. 2006;2:54–63. doi: 10.1159/000091704. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 5.Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: Time course and cytokines. Stem Cells Dev. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- 6.Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, et al. Hucbcs increase angiopoietin 1 and induce neurorestorative effects after stroke in t1dm rats. CNS Neurosci Ther. 2014;20:935–944. doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–628. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- 10.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Yan T, Chopp M, Zacharek A, Ning R, Venkat P, et al. Combination bmsc and niaspan treatment of stroke enhances white matter remodeling and synaptic protein expression in diabetic rats. Int J Mol Sci. 2013;14:22221–22232. doi: 10.3390/ijms141122221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan T, Ye X, Chopp M, Zacharek A, Ning R, Venkat P, et al. Niaspan attenuates the adverse effects of bone marrow stromal cell treatment of stroke in type one diabetic rats. PLoS One. 2013;8:e81199. doi: 10.1371/journal.pone.0081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 14.Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb JD, Sanberg PR, Klasko SK, Willing AE. Umbilical cord blood research: Current and future perspectives. Cell Transplant. 2007;16:151–158. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ning R, Chopp M, Yan T, Zacharek A, Zhang C, Roberts C, et al. Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience. 2012;222:326–332. doi: 10.1016/j.neuroscience.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding G, Yan T, Chen J, Chopp M, Li L, Li Q, et al. Persistent cerebrovascular damage after stroke in type two diabetic rats measured by magnetic resonance imaging. Stroke. 2015;46:507–512. doi: 10.1161/STROKEAHA.114.007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuntz M, Mysiorek C, Petrault O, Petrault M, Uzbekov R, Bordet R, et al. Stroke-induced brain parenchymal injury drives blood-brain barrier early leakage kinetics: A combined in vivo/in vitro study. J Cereb Blood Flow Metab. 2014;34:95–107. doi: 10.1038/jcbfm.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, et al. Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X, Chopp M, Zacharek A, Dai J, Zhang C, Yan T, et al. Combination treatment of stroke with sub-therapeutic doses of simvastatin and human umbilical cord blood cells enhances vascular remodeling and improves functional outcome. Neuroscience. 2012;227:223–231. doi: 10.1016/j.neuroscience.2012.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, et al. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43:2221–2228. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan T, Chopp M, Ye X, Liu Z, Zacharek A, Cui Y, et al. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46:157–164. doi: 10.1016/j.nbd.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol. 2002;50:257–292. doi: 10.1016/s0074-7742(02)50080-5. [DOI] [PubMed] [Google Scholar]
- 27.Walmsley AR, Mir AK. Targeting the nogo-a signalling pathway to promote recovery following acute cns injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Zacharek A, Cui X, Shehadah A, Jiang H, Roberts C, et al. Treatment of stroke with a synthetic liver x receptor agonist, to901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30:102–109. doi: 10.1038/jcbfm.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriz J. Inflammation in ischemic brain injury: Timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 33.Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem. 2011;119:1029–1040. doi: 10.1111/j.1471-4159.2011.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, et al. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor rage as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, et al. High-mobility group box 1 promotes metalloproteinase-9 upregulation through toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41:2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;12:00069–00065. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 40.Perego C, Fumagalli S, De Simoni M-G. Three-dimensional confocal analysis of microglia/macrophage markers of polarization in experimental brain injury. 2013:e50605. doi: 10.3791/50605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalski D, Heindl M, Kacza J, Laignel F, Kuppers-Tiedt L, Schneider D, et al. Spatio-temporal course of macrophage-like cell accumulation after experimental embolic stroke depending on treatment with tissue plasminogen activator and its combination with hyperbaric oxygenation. Eur J Histochem. 2012;56:e14. doi: 10.4081/ejh.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karki K, Knight RA, Shen LH, Kapke A, Lu M, Li Y, et al. Chronic brain tissue remodeling after stroke in rat: A 1-year multiparametric magnetic resonance imaging study. Brain Res. 2010;11:168–176. doi: 10.1016/j.brainres.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.