Abstract
Background and Purpose
The Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke (CLEAR) and CLEAR-Enhanced Regimen (CLEAR-ER) trials demonstrated safety of reduced dose recombinant tissue plasminogen activator (rt-PA) plus the glycoprotein 2b/3a inhibitor, eptifibatide, in acute ischemic stroke (AIS) compared to rt-PA alone. The objective of the CLEAR-Full Dose Regimen (CLEAR-FDR) trial was to estimate the rate of symptomatic intracerebral hemorrhage (sICH) in AIS patients treated with the combination of full dose rt-PA plus eptifibatide.
Methods
CLEAR-FDR was a single-arm, prospective, open-label, multi-site study. Patients aged 18-85 years treated with 0.9 mg/kg IV rt-PA within three hours of symptom onset were enrolled. After obtaining consent, eptifibatide (135 mcg/kg bolus and 2 hour infusion at 0.75 mcg/kg/min) was administered. The primary endpoint was the proportion of patients who experienced sICH within 36 hours. An independent clinical monitor adjudicated if a sICH had occurred and an independent neuroradiologist reviewed all images. The stopping rule was three sICHs within the first 19 patients or four sICHs within 29 patients.
Results
From October 2013 to December 2014, 27 AIS patients were enrolled. Median age was 73 years (Range 34-85, IQR 65-80) and median NIHSS was 12 (Range 6-26, IQR 9-16). One sICH (3.7%, 95% CI 0.7%-18%) was observed.
Conclusion
These results demonstrate comparable safety of full dose rt-PA plus eptifibatide with historical rates of sICH with rt-PA alone and support proceeding with a phase 3 trial evaluating full dose rt-PA combined with eptifibatide to improve outcomes after AIS.
Keywords: ischemic stroke, tissue plasminogen activator, eptifibatide, clinical trial
Introduction
Intravenous (IV) recombinant tissue plasminogen activator (rt-PA) remains the only proven medical therapy for improving functional outcomes after acute ischemic stroke (AIS). Unfortunately, rt-PA alone is often inadequate for opening occluded intracranial arteries with recanalization of large arterial occlusions in only ∼50% with subsequent re-occlusion of 14-34% of initially recanalized arteries.1-8 Recent trials of endovascular therapy found that carefully selected patients treated with endovascular and IV rt-PA have improved outcomes compared with rt-PA alone,9-13 but half of all rt-PA treated patients do not have a proximal arterial occlusion.10 Further, access of stroke patients to centers capable of delivering endovascular therapy is limited.14 Thus, intravenous medical treatments that augment reperfusion and improve functional outcomes beyond that seen with rt-PA alone remain sorely needed.
We have previously conducted two randomized phase 2 clinical trials of escalating doses of IV rt-PA plus IV eptifibatide, a platelet glycoprotein 2b/3a inhibitor that prevents platelet aggregation,15 versus rt-PA alone in AIS patients treated with rt-PA within three hours of symptom onset. The 94-patient Combined approach to Lysis utilizing Eptifibatide And Recombinant tissue-type plasminogen activator (CLEAR) stroke trial randomized AIS patients to low-dose rt-PA (tier 1=0.3 mg/kg, tier 2=0.45 mg/kg) plus eptifibatide (75 μg/kg bolus followed by 0.75 μg/kg per min infusion for 2 hours) or standard-dose rt-PA (0.9 mg/kg). The symptomatic intracerebral hemorrhage (sICH) rate in the combination arm was 1.4% and there was no signal of improved efficacy over rt-PA alone.16 The follow up 126-patient CLEAR - Enhanced Regimen (CLEAR-ER) trial found that a slightly higher dose of rt-PA (0.6 mg/kg) plus a higher eptifibatide bolus (135 μg/kg) followed by the two-hour infusion at 0.75 μg/kg/min had a sICH rate of 2% (95% CI 0.5, 6.9%) and a direction of effect in favor of the combination therapy over IV rt-PA with a non-significant, unadjusted increase in the proportion of patients with modified Rankin scores (mRS) of 0-1 or return to baseline of 13.5% (95% CI -7.7-34.7%).17
Given the safety of reduced dose rt-PA combined with eptifibatide, the primary objective of the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke-Full Dose Regimen (CLEAR-FDR) Stroke Trial was to estimate the rate of sICH, as defined in the NINDS rt-PA Stroke Trials,18 in AIS patients treated with the combination of full dose rt-PA (0.9 mg/kg) plus eptifibatide when rt-PA is initiated within three hours of symptom onset. We hypothesized that the sICH rate would be less than 8%. An evaluation of the safety of the full dose combination therapy is required prior to proceeding with a phase 3 trial.
Methods
This was a single-arm, prospective, open-label, eight-site study within a single metropolitan area served by one stroke team. The primary outcome was the proportion of patients who experienced sICH as defined in the NINDS rt-PA Stroke Trials within 36 hours of rt-PA initiation. An independent neuroradiologist reviewed all images and determined if any hemorrhage was present. All cases for which a hemorrhage occurred were then reviewed by an independent clinical monitor who classified the hemorrhage as symptomatic or not using the NINDS rt-PA trial definition. Secondary outcomes included: the proportion of patients who developed parenchymal hemorrhage types 1 (PH-1) and 2 (PH-2); serious systemic bleeding (defined as requiring transfusion of three or more units of blood); and, 90-day outcomes as measured by the modified Rankin score (mRS). Ninety day mRS was determined by in-person or phone interview with the patient and/or surrogate using validated methods.19 Institutional review board (IRB) approval was obtained for all participating sites and no study procedures occurred prior to IRB and hospital approval. Key inclusion and exclusion criteria beyond eligibility for IV rt-PA are shown in Table 1.
Table 1. Key Inclusion and Exclusion Criteria.
| Inclusion Criteria |
|---|
| Subjects must have had a serious measurable neurological deficit on the NIH Stroke Scale due to focal brain ischemia. |
| An NIH Stroke Scale score >5 at the time the rt-PA was begun. |
| Age: 18 through 85 years (i.e. candidates must have had their 18th birthday, but not had their 86th birthday). |
| Intravenous rt-PA therapy must have been initiated within 3 hours of onset of stroke symptoms. |
| Exclusion Criteria |
| History of stroke in the past 3 months. |
| Previous intra-cranial hemorrhage, neoplasm, subarachnoid hemorrhage, or arterial venous malformation. |
| Clinical presentation suggested a subarachnoid hemorrhage, even if initial CT scan was normal. |
| Hypertension at time of treatment; systolic BP > 185 or diastolic > 110 mmHg or aggressive measures (requirement for AND repeated titrations of a continuous infusion medication) to lower blood pressure to below these limits were needed. |
| Known hereditary or acquired hemorrhagic diathesis, coagulation factor deficiency, or oral anticoagulant therapy with INR > 1.7. |
| Baseline lab values: positive urine pregnancy test, glucose < 50 or > 400 mg/dl, platelets <100,000/mm3, Hct<25 %, or creatinine > 4 mg/dl. |
| Ongoing renal dialysis, regardless of creatinine. |
| Informed consent was not or could not be obtained. |
Interventions
After 0.9 mg/kg IV rt-PA was started in eligible ischemic stroke patients per standard of care, the patient or surrogate was approached for participation in the study and consent obtained in eligible patients. Open label eptifibatide (bolus 135 mcg/kg and 2 hour infusion at 0.75 mcg/kg/min) was started as soon as possible after consent was obtained. The goal was to start eptifibatide within 40 minutes of initiation of rt-PA. A repeat NIH stroke scale was performed at the end of the two-hour eptifibatide infusion and at 24 (+/- 6) hours after initiation of rt-PA.
Stopping rules
The primary goal was to ensure with high probability, which we defined as 80%, that the rate of sICH did not exceed 8%. The 8% expected sICH rate was based on the observed rate in NINDS rt-PA trial patients with baseline NIHSS ≥6. The stopping rule was three sICH cases within the first 19 patients enrolled or four sICH cases within 29 patients enrolled. Assuming the sICH rate was 8% then the probability of observing three or more events out of 19, or four or more events out of 29, would be less than 20%. If the study was stopped due to observing more than three events out of 19 or four events out of 29, then the hypothesis that the sICH rate is less than 8% would be rejected. Otherwise, it would be accepted. The probability was computed assuming a binomial distribution with a mean of 0.08 for sICH rate. We planned to enroll up to 30 patients as long as there was at least an 80% probability that the sICH rate was no more than 8% given the observed data. The study is registered at clinicaltrials.gov (NCT01977456).
Results
From October 2013 to December 2014, we enrolled 27 patients. The study was stopped after 27 enrollments due to the fact that the primary safety threshold had been crossed. With only one sICH out of 27, four sICHs out of 29 is impossible. Figure 1 shows screening and eligibility data. Patient characteristics are shown in Table 2. The median age for enrolled patients was 73 years (range 34 to 85, IQR 65 to 80). Median NIH stroke scale score (NIHSS) for all patients was 12 (range 6 to 26, IQR 9 to 16).
Figure 1. Screening and Eligibility of Enrolled Patients.

Table 2. Patient Characteristics (N=27).
| Age, median (range) | 73.4 (34.0, 85.7) |
|---|---|
| Male, N (%) | 13 (48%) |
| Black, N (%) | 4 (15%) |
| Hispanic (%) | 1 (4%) |
| Baseline NIHSS, median (range) | 12 (6, 26) |
| Pre-stroke mRS, N (%) | |
| 0 | 17 (63%) |
| 1 | 1 (4%) |
| 2 | 1 (4%) |
| 3 | 7 (26%) |
| 4 | 1 (4%) |
| Time from stroke onset to IV rt-PA (minutes), median (range) | 118 (71, 182) |
| Time from start of IV rt-PA to start of eptifibatide (minutes), median (range) | 37 (15, 57) |
| Medical History, N (%) | |
| Atrial Fibrillation | 12 (44%) |
| Hypertension | 20 (74%) |
| Diabetes | 11 (41%) |
| Congestive heart failure | 4 (15%) |
| Myocardial infarction | 6 (22%) |
| Hyperlipidemia | 18 (67%) |
| Prior stroke | 7 (26%) |
Data presented as Median (minimum, maximum) or n (%)
The primary outcome of sICH was observed in one out of 27 patients (3.7%, 95% CI 0.7%-18%). Figure 2 shows the independent monitor adjudicated sICH. The pre-specified secondary outcomes included rates of PH-1 (n=1, 3.7%) at 24 hours and PH-2 (n=0, 0%). Serious systemic bleeding occurred in one patient (3.7%). Table 3 shows the mRS distribution at 90-days for all patients. Of all enrolled patients, 17 (63%, 95% CI 44%-78%) had an mRS 0-1 OR return to pre-stroke function at 90 days; 12 (44%, 95% CI 28%-63%) had mRS 0-1, 13 (48%, 95% CI 31%-66%) had mRS 0-2, and 5 (19%, 95% CI 8%-37%) were dead at 90 days. mRS assessments were done in person for 24 subjects and by phone for three. At 2 and 24 hours, the median NIHSS for all enrolled patients was 8 (0-25) and 5 (0-24), respectively.
Figure 2. Case of Symptomatic Intracerebral Hemorrhage.
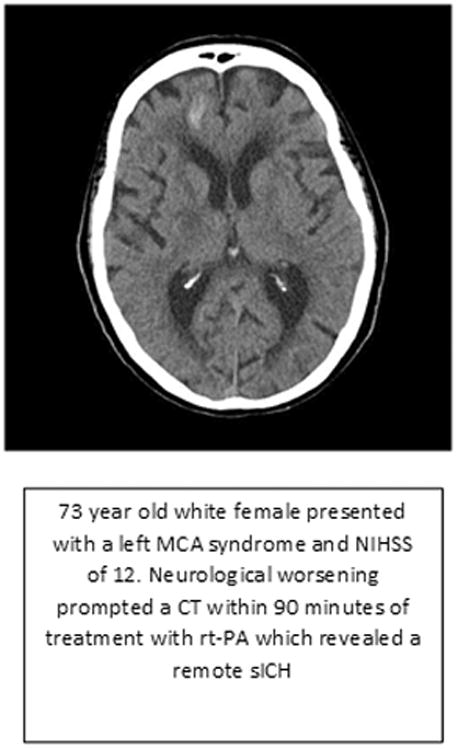
Table 3. 90-day mRS Scores.
| (n=27) | |
|---|---|
| 90-day mRS | |
| 0 | 9 (33%) |
| 1 | 3 (11%) |
| 2 | 1 (4%) |
| 3 | 5 (19%) |
| 4 | 4 (15%) |
| 5 | 0 (0%) |
| 6 | 5 (19%) |
Of the 19 patients with a pre-stroke mRS 0-2, median age (range) was 67.5 years (34.0, 85.7) and median NIHSS (range) was 11 (6, 22). Of the eight patients with a pre-stroke mRS of 3 or 4, median age (range) was 75.9 years (69.1, 84.8) and median NIHSS (range) was 13 (7, 26). Among patients with a pre-stroke mRS of 0-2 (n=19), 13 (68%) had mRS 0-2 at 90 days. Among patients with pre-stroke mRS 3 or 4 (n=8), 5 (62.5%) had mRS 3 or 4 at 90 days. The analyses by pre-stroke mRS were performed based on observed data and not pre-specified. A summary of the CLEAR, CLEAR-ER and CLEAR-FDR trials is shown in Table 4.
Table 4. Summary of CLEAR, CLEAR-ER and CLEAR-FDR Trials.
| CLEAR Trial | CLEAR-ER Trial | CLEAR-FDR Trial | |
|---|---|---|---|
| Intervention | 0.3 mg/kg and 0.45 mg/kg rt-PA + eptifibatidea | 0.6 mg/kg rt-PA + eptifibatideb | 0.9 mg/kg rt-PA + eptifibatideb |
| Randomized | Yes | Yes | No |
| Study Size, N (total) | 94 | 126 | 27 |
| - Combination arm, N | 69 | 101 | NA |
| - rt-PA arm, N | 25 | 25 | NA |
| sICH rate in combination arm only, N (%) | 1 (1.4%) | 2 (2%) | 1 (3.7%) |
| Unadjusted mRS 0-1 or return to baseline at 90 days in combination arm, N (%) | 21 (30%) | 50 (49.5%) | 17 (62.9%) |
Eptifibatide dose = 75 μg/kg bolus followed by 0.75 μg/kg/min infusion for 2 hours
Eptifibatide dose = 135 μg/kg bolus followed by 0.75 μg/kg/min infusion for 2 hours
Discussion
Our findings support the hypothesis that combining eptifibatide in the dose studied with full dose intravenous rt-PA administered within three hours of symptom onset in AIS patients is associated with a sICH rate of less than 8%. This represents the culmination of three phase 2 clinical trials conducted over an 11-year span and combining gradually increasing doses of rt-PA and eptifibatide to estimate the safety of this combination prior to proceeding with an efficacy trial. In all, 247 ischemic stroke patients have been enrolled in prospective clinical trials studying the combination of rt-PA plus eptifibatide by our group.16,17 Given the potential treatment effect observed in the previously published double-blind randomized CLEAR-ER stroke trial,17 the results of the CLEAR-FDR trial provide justification for the safety of proceeding with a phase 3 trial evaluating 0.9 mg/kg of intravenous rt-PA combined with eptifibatide to improve outcomes after AIS.
Of 27 AIS patients with a median age of 73 years and median NIHSS of 12 treated with full dose rt-PA plus eptifibatide, we observed one case of sICH (Figure 1). The age, severity and observed sICH rate are similar to those of the 101 patients treated with the combination of 0.6 mg/kg of rt-PA and the same dose of eptifibatide in CLEAR-ER.17 Notably, 30% of patients enrolled in CLEAR-FDR had a pre-stroke mRS>2. As such our estimate of the safety of the combination accounts for inclusion of patients with poor pre-stroke function who may end up receiving the treatment in routine clinical practice should it be proven effective in a larger clinical trial.
Given the single-arm design, CLEAR-FDR was not intended to evaluate the impact of the trial intervention on functional outcomes. We note that 63% (95% CI 44%-78%) of all enrolled patients had mRS 0-1 OR return to pre-stroke function at 90 days but this must be taken with caution given broad confidence intervals around this point estimate. Since about one third of enrolled patients had a pre-stroke mRS greater than 1 (Table 1), a dichotomized mRS 0-1 is inadequate for evaluation of potential efficacy.
We acknowledge limitations including the small sample size, single-arm non-blinded design, lack of vascular imaging and data on large vessel occlusion, and enrollment by a single regional stroke team. However, the primary objective of the trial was to estimate the sICH rate in AIS patients with moderate to severe stroke who were treated with the combination.
Strengths of the study include the inclusion of patients with pre-stroke disability who would end up receiving any proven intervention in the real world of acute stroke care, use of the standard dose of rt-PA which would allow ready translation to a larger trial, and a sensitive definition of sICH, the NINDS rt-PA trial definition, which ensures the estimation of safety is not based on an overly restrictive definition of sICH.
We conclude that full dose rt-PA combined with eptifibatide at the dose studied is sufficiently safe to proceed with a phase 3 trial evaluating the combination for improving outcomes in otherwise eligible AIS patients. Prior to such a trial, we plan to investigate the dose-response of rt-PA plus eptifibatide via a pooled analyses of all three completed trials, as well as a comparison of outcomes between CLEAR-FDR patients and contemporaneously enrolled IV rt-PA only patients in other trials matched on important baseline demographic and clinical characteristics.
Acknowledgments
Funding Sources: NIH/National Institutes of Neurological Disorders and Stroke - P50 NS044283
Footnotes
Disclosures: Dr. Kleindorfer receives honorarium from Genentech Speakers' Bureau
References
- 1.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke; a journal of cerebral circulation. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 2.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. Ajnr. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. The New England journal of medicine. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 5.Rubiera M, Alvarez-Sabin J, Ribo M, Montaner J, Santamarina E, Arenillas JF, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:1452–1456. doi: 10.1161/01.STR.0000170711.43405.81. [DOI] [PubMed] [Google Scholar]
- 6.Muchada M, Rodriguez-Luna D, Pagola J, Flores A, Sanjuan E, Meler P, et al. Impact of Time to Treatment on Tissue-Type Plasminogen Activator-Induced Recanalization in Acute Ischemic Stroke. Stroke; a journal of cerebral circulation. 2014;45:2734–8. doi: 10.1161/STROKEAHA.114.006222. [DOI] [PubMed] [Google Scholar]
- 7.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 8.Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II) Stroke; a journal of cerebral circulation. 2010;41:461–465. doi: 10.1161/STROKEAHA.109.573477. [DOI] [PubMed] [Google Scholar]
- 9.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England journal of medicine. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, et al. SWIFT PRIME Investigators. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 13.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 14.Adeoye O, Albright KC, Carr BG, Wolff C, Mullen MT, Abruzzo T, et al. Geographic access to acute stroke care in the United States. Stroke; a journal of cerebral circulation. 2014;45:3019–3024. doi: 10.1161/STROKEAHA.114.006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarborough RM. Development of eptifibatide. Am Heart J. 1999;138:1093–1104. doi: 10.1016/s0002-8703(99)70075-x. [DOI] [PubMed] [Google Scholar]
- 16.Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, et al. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke; a journal of cerebral circulation. 2008;39:3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pancioli AM, Adeoye O, Schmit PA, Khoury J, Levine SR, Tomsick TA, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen stroke trial. Stroke; a journal of cerebral circulation. 2013;44:2381–2387. doi: 10.1161/STROKEAHA.113.001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The New England journal of medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 19.Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis. 2010;29:137–139. doi: 10.1159/000262309. [DOI] [PubMed] [Google Scholar]


