Abstract
Background
The total cholesterol to high-density lipoprotein cholesterol (TC/HDL-C) ratio, estimated low-density lipoprotein cholesterol (LDL-C), and non-HDL-C are routinely available from the standard lipid profile. We aimed to assess the extent of patient-level discordance of TC/HDL-C with LDL-C and non-HDL-C because discordance suggests the possibility of additional information.
Methods and Results
We compared population percentiles of TC/HDL-C, Friedewald-estimated LDL-C, and non-HDL-C in 1,310,432 U.S. adults from the Very Large Database of Lipids. Lipid testing was performed by ultracentrifugation (VAP, Atherotech, AL). One in three patients had ≥25 percentile units discordance between TC/HDL-C and LDL-C while one in four had ≥25 percentile units discordance between TC/HDL-C and non-HDL-C. The proportion of patients with TC/HDL-C > LDL-C by ≥25 percentile units increased from 3% at triglycerides <100 mg/dL to 51% at triglycerides 200–399 mg/dL. On a smaller scale, TC/HDL-C > non-HDL-C discordance by ≥25 percentile units increased from 6% to 21%. In those with <15th percentile levels of LDL-C (<70 mg/dL) or non-HDL-C (<93 mg/dL), a respective 58% and 46% were above the percentile-equivalent TC/HDL-C of 2.6. Age, sex, and directly measured components of the standard lipid profile explained >86% of the variance in percentile discordance between TC/HDL-C vs. LDL-C and non-HDL-C.
Conclusions
In this contemporary, cross-sectional, big data analysis of U.S. adults who underwent advanced lipid testing, the extent of patient-level discordance suggests that TC/HDL-C may offer potential additional information to LDL-C and non-HDL-C. Future studies are required to determine the clinical implications of this observation.
Clinical Trial Registration Information
www.clinicaltrials.gov. Identifier: NCT01698489.
Keywords: Lipids, Lipoproteins, cholesterol, prevention
INTRODUCTION
Approximately 100 million cholesterol tests are performed annually in U.S. ambulatory clinics alone.1 Controlling cholesterol is one of the American Heart Association’s Life’s Simple 7 and a central aspect of atherosclerotic cardiovascular disease (ASCVD) prevention in the U.S. and abroad.2 Guidelines recommend using the standard lipid profile in several ways.3–7 On initial patient evaluation, estimating a 10-year ASCVD risk score using the 2013 U.S. pooled cohort equations, the Framingham risk score or European systemic coronary risk estimation score is one of the components for eligibility for primary prevention statin therapy.3–7 These risk scores include total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) as individual variables. Baseline LDL-C is also used to define treatment eligibility, and after intervention on-treatment LDL-C levels are compared to baseline and monitored over time, as are non-HDL-C and apolipoprotein B (apoB) levels in some guidelines.4–7
Current guidelines do not recommend using the TC/HDL-C ratio. It remains uncertain what information the ratio may add given that TC and HDL-C are already used in risk estimation, in estimating LDL-C by the Friedewald formula [LDL-C = TC – HDL-C – (triglycerides/5)],8 and in calculating non-HDL-C. Moreover, Mendelian randomization and HDL-C raising trials argue against a causal role of HDL-C in ASCVD.9–11 However, to some extent, it has been suggested that TC/HDL-C may be a marker of atherogenic particle burden.12 Prior studies have shown TC/HDL-C’s tracking with LDL particle concentration (LDL-P) and its association with risk for cardiovascular events.13–16
Before considering additional tests (e.g., LDL-P, apoB), it may be desirable to extract as much information as possible from the standard lipid profile. We have previously shown significant patient-level percentile discordance between LDL-C and non-HDL-C suggesting additional information carried by non-HDL-C.17 Likewise, TC/HDL-C may offer potential additional clinical information to LDL-C and non-HDL-C if it is significantly discordant with them within individuals. Therefore, the primary aim of this study was to examine the extent of patient-level percentile TC/HDL-C discordance.
METHODS
Study population and lipid testing
We examined consecutive lipid profiles from 1,310,432 U.S. adults ≥18 years of age with triglycerides (TG) <400 mg/dL from the Very Large Database of Lipids (VLDL).18 This study is part B of the VLDL-2 study that specifically aims to assess discordance between lipid parameters. In VLDL-2A,17 we examined discordance between LDL-C and non-HDL-C and in this study (VLDL-2B) we examine discordance between TC/HDL-C vs LDL-C and non-HDL-C. Lipid profiles were measured using direct ultracentrifugation by the Vertical Auto Profile (VAP) test (Atherotech Diagnostics Laboratory, Birmingham, Alabama).18,19 The accuracy and precision of VAP lipid parameters have been validated, as previously described.18,19 Lipid distributions in the VLDL population were nearly superimposable with lipid distributions from the National Health and Nutrition Examination Survey (NHANES) (Supplemental Figure 1).18
LDL-C in the main analyses was estimated by the Friedewald formula given its longstanding use in clinical practice worldwide.8 In order to address bias associated with the Friedewald LDL-C estimation method, we performed supplemental analyses using LDL-C estimated by our recently described novel method20 as well as VAP-measured direct LDL-C.
The Johns Hopkins Institutional Review Board declared our study exempt and further information regarding data extraction and management has been previously described.18
Statistical analysis
We assigned population percentiles to TC/HDL-C, LDL-C, and non-HDL-C, and also determined the TC/HDL-C percentiles corresponding to LDL-C and non-HDL-C cut-points still used in some current worldwide guidelines such as the Canadian Cardiovascular Society, European Society of Cardiology and European Atherosclerosis Society, National Lipid Association and International Society of Atherosclerosis cholesterol guidelines (Supplemental Table 1).4–7
We used pseudocolor-encoded density scatter plots to visually assess discordance between TC/HDL-C, LDL-C, and non-HDL-C percentiles in the whole population and across TG categories of <100, 100–149, 150–199, and 200–399 mg/dL. In order to quantify the magnitude of discordance, we calculated the difference between TC/HDL-C percentile, LDL-C percentile, and non-HDL-C percentile for every patient as follows: [TC/HDL-C percentile minus LDL-C percentile] and [TC/HDL-C percentile minus non-HDL-C percentile]. We calculated the median with 1st to 3rd quartiles (Q1–Q3) of discordance. In supplemental analyses, the same calculations were performed to study discordance of TC/HDL-C with direct LDL-C and LDL-C estimated by the novel method.20
After considering the heterogeneous definitions of discordance in the literature,17,21–25 we quantified discordance at the 4 arbitrary thresholds of ≥5, ≥10, ≥25, and ≥50 percentile units discordance and chose the 10th and 25th percentile units cut-points for further analyses. For each percentile unit (x) cut-point, the population was divided into patients with TC/HDL-C percentile > LDL-C percentile by ≥(x) percentile units, patients with TC/HDL-C percentile < LDL-C percentile by ≤(x) percentile units and patients with concordant TC/HDL-C and LDL-C percentiles within +/− (x) percentile units. The same method was used in all other discordance analyses.
In patients with Friedewald LDL-C or non-HDL-C <15th population percentile (Friedewald LDL-C <70 mg/dL, non-HDL-C <93 mg/dL), we examined the proportion of discordant patients above the percentile-equivalent TC/HDL-C of 2.6 across TG categories. Similarly in patients with direct and novel method LDL-C <12th percentile (70 mg/dL), we examined the proportion of discordant patients above the percentile-equivalent TC/HDL-C of 2.5.
Next, we compared age, sex, and multiple lipid parameters derived from the standard lipid profile between the 2 discordant (TC/HDL-C > LDL-C or non-HDL-C and TC/HDL-C < LDL-C or non-HDL-C) and concordant patient populations. This analysis was performed using a discordance definition of ≥10 and ≥25 percentile units. Subsequently, linear regression models of multiple variables were utilized to determine the strength of association (R2) with discordance. TC/HDL-C – LDL-C percentile discordance and TC/HDL-C – non-HDL-C percentile discordance followed a normal distribution and were used as continuous outcomes. The natural log of TG (ln(TG)) was used in the model given that TG levels followed a log normal distribution. We initially forced in age, sex and ln(TG) since TG is not involved in calculating TC/HDL-C or non-HDL-C. We then sequentially added TC followed by HDL-C. Subsequently, we used various combinations of lipid parameters including HDL-C subfractions (HDL2-C, HDL3-C) and logarithmic LDL density ratio (LLDR).26 Additionally, we standardized continuous predictor variables (per 1 standard deviation) in order to make them more comparable.
Statistical analyses and logarithmically scaled pseudocolor encoded density plots were generated using R Version 2.15.1 (Vienna, Austria), Stata Version 11.0 (College Station, TX) and Microsoft Excel 2010 (Redmond, WA).
RESULTS
TC/HDL-C discordance with LDL-C and non-HDL-C
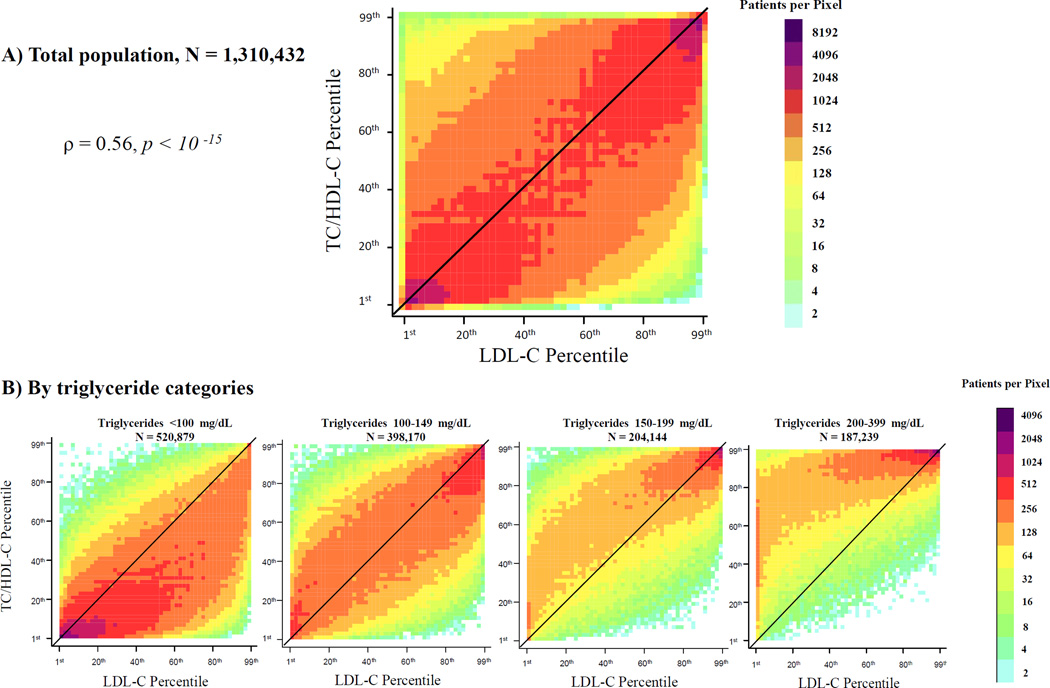
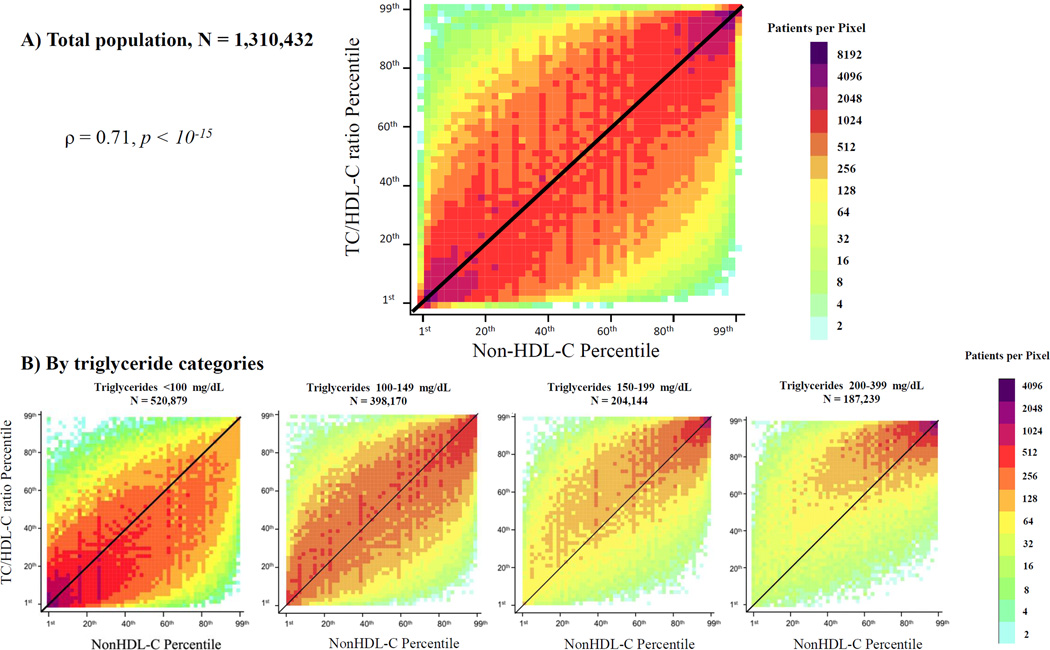
We visually observed significant patient-level percentile discordance in TC/HDL-C vs. LDL-C (Figure 1A) and non-HDL-C (Figure 2A) in the whole population and across TG categories (Figures 1B and 2B). Correlation coefficients of TC/HDL-C with LDL-C were moderate (Spearman rho =0.56, r =0.55, p <10−15), and higher with non-HDL-C (Spearman rho = 0.72, r =0.70, p <10−15). At TG levels <100 mg/dL, discordance was largely characterized by TC/HDL-C < LDL-C or non-HDL-C percentiles. At higher TG levels ≥150 mg/dL, discordance shifted towards TC/HDL-C > LDL-C or non-HDL-C percentiles (Figures 1B and 2B).
Figure 1.
Patient-level discordance between population percentiles of TC/HDL-C and LDL-C. Population percentiles of TC/HDL-C and LDL-C are presented on this plot for the whole population (A) and for four different triglyceride categories (B). Points to the left of the diagonal line represent individuals with TC/HDL-C percentile > LDL-C percentile and points to the right of the diagonal line represent individuals with TC/HDL-C percentile < LDL-C percentile. The density of data is expressed by different shades of color, which represent increasing densities of patients per pixel, from light blue to purple. The number next to each color on the color axis represents the maximum number of patients per pixel of this color. ρ is Spearman correlation coefficient. TC/HDL-C (Total Cholesterol to HDL-C ratio); LDL-C (Low-density Lipoprotein Cholesterol).
Figure 2.
Patient-level discordance between population percentiles of TC/HDL-C and non-HDL-C. Population percentiles of TC/HDL-C and non-HDL-C are presented on this plot for the whole population (A) and for four different triglyceride categories (B). Points to the left of the diagonal line represent individuals with TC/HDL-C percentile > non-HDL-C percentile and points to the right of the diagonal line represent individuals with TC/HDL-C percentile < non-HDL-C percentile. The density of data is expressed by different shades of color, which represent increasing densities of patients per pixel, from light blue to purple. The number next to each color on the color axis represents the maximum number of patients per pixel of this color. ρ is Spearman correlation coefficient. TC/HDL-C (Total Cholesterol to HDL-C ratio); non-HDL-C (Non-High-density Lipoprotein Cholesterol).
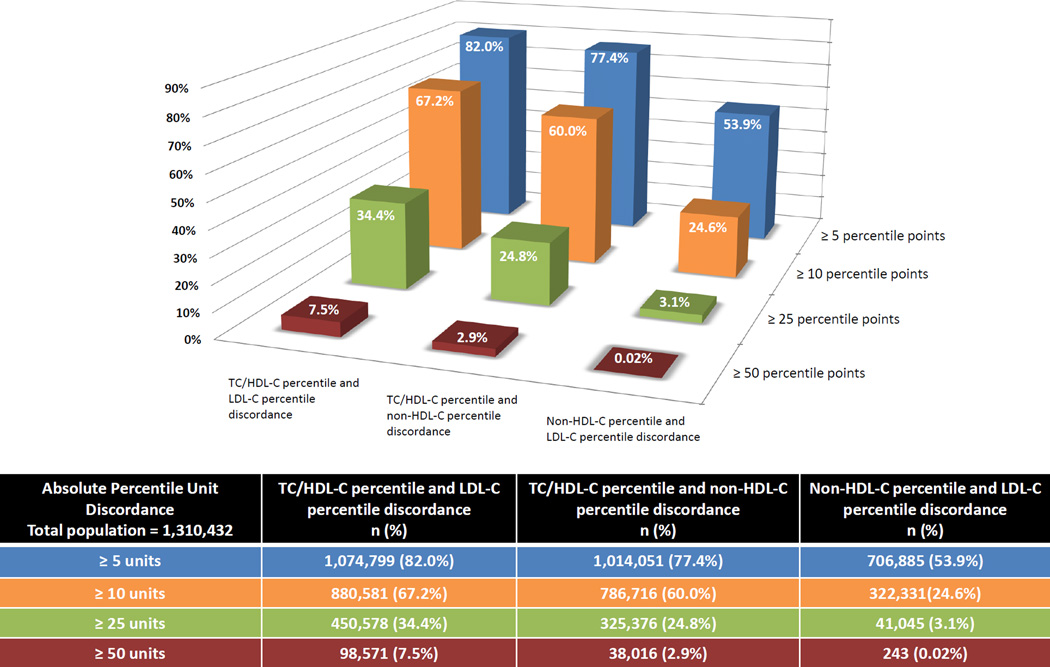
In Figure 3, we observed that 67% and 34% of patients had ≥10 percentile units and ≥25 percentile units discordance between TC/HDL-C and LDL-C, respectively. On a smaller scale, 60% and 25% of patients had ≥10 percentile units and ≥25 percentile units discordance between TC/HDL-C and non-HDL-C, respectively. In contrast to TC/HDL-C discordance, non-HDL-C vs. LDL-C percentile discordance was relatively small with only 3% having ≥25 percentile units discordance.
Figure 3.
3-D plot of the extent of discordance between TC/HDL-C, LDL-C and non-HDL-C percentiles across different percentile units thresholds. On the X-axis, we present discordance between TC/HDL-C and LDL-C percentiles, TC/HDL-C and non-HDL-C percentiles and LDL-C and non-HDL-C percentiles from left to right, respectively. On the Y-axis, we represent the magnitude (%) of patient-level discordance at thresholds of ≥5, ≥10, ≥25, and ≥50 percentile units. TC/HDL-C (Total Cholesterol to HDL-C ratio); LDL-C (Low-density Lipoprotein Cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol).
Discordance of ≥10 and ≥25 percentile units between TC/HDL-C and LDL-C estimated by the novel method was also significant, occurring in 64% and 30% of patients, respectively (Supplemental Table 2). Discordance of ≥10 and ≥25 percentile units between TC/HDL-C and direct LDL-C occurred in 65% and 31% of patients, respectively (Supplemental Table 2).
Examining TC/HDL-C – LDL-C percentile discordance, the median (Q1–Q3) discordance in percentile units was −13.3 (−29.8 to 0.1), 0.1 (−14.2 to14.9), 10.6 (−2.6 to 27.4), and 25.7 (7.2 to 46.1) in patients with TG levels <100, 100–149, 150–199, and 200–399 mg/dL, respectively (Table 1). To a smaller extent, TC/HDL-C – non-HDL-C percentile discordance was −5.1 (−20.3 to 6.2), 1.0 (−12.7 to 15.2), 4.9 (−6.5 to 19.4), and 7.2 (−1.5 to 22.0), respectively (Table 1).
Table 1.
Patient-level percentile discordance between TC/HDL-C compared to LDL-C and non-HDL-C across triglyceride categories.
| Triglyceride categories (mg/dL) | |||||
|---|---|---|---|---|---|
| <100 N = 520,879 |
100–149 N = 398,170 |
150–199 N = 204,144 |
200–399 N = 187,239 |
Total N = 1,310,432 |
|
| A) TC/HDL-C and LDL-C percentile discordance | |||||
| TC/HLD-C percentile minus LDL-C percentile median (Q1–Q3) | −13.3 (−29.8, 0.1) | 0.1 (−14.2, 14.9) | 10.6 (−2.6, 27.4) | 25.7 (7.2, 46.1) | −0.73 (−17.5, 16.5) |
| ≥10 percentile units discordance | |||||
| TC/HDL-C < LDL-C, n (%) | 290,999 (55.9) | 124,294 (31.2) | 29,621 (14.5) | 8,158 (4.4) | 453,072 (34.6) |
| Concordant, n (%) | 167,655 (32.2) | 145,922 (36.7) | 70,338 (34.5) | 45,936 (24.5) | 429,851 (32.8) |
| TC/HDL-C > LDL-C, n (%) | 62,225 (11.9) | 127,954 (32.1) | 104,185 (51.0) | 133,145 (71.1) | 427,509 (32.6) |
| ≥25 percentile units discordance | |||||
| TC/HDL-C < LDL-C, n (%) | 164,103 (31.5) | 51,827 (13.0) | 9,008 (4.4) | 1,879 (1.0) | 226,727 (17.3) |
| Concordant, n (%) | 339,495 (65.2) | 293,620 (73.5) | 137,744 (67.5) | 89,995 (48.1) | 859,854 (65.6) |
| TC/HDL-C > LDL-C, n (%) | 17,371 (3.3) | 53,723 (13.5) | 57,392 (28.1) | 95,365 (50.9) | 223,851 (17.1) |
| B) TC/HDL-C and Non-HDL-C percentile discordance | |||||
| TC/HDL-C percentile minus non-HDL-C percentile median (Q1–Q3) | −5.1 (−20.3, 6.2) | 1.0 (−12.7, 15.2) | 4.9 (−6.5, 19.4) | 7.2 (−1.5, 22.0) | 0.17 (−13.2, 13.6) |
| ≥10 percentile units discordance | |||||
| TC/HDL-C < non-HDL-C, n (%) | 212,270 (40.8) | 115,724 (29.0) | 40,003 (19.6) | 21,191 (11.3) | 398,188 (29.7) |
| Concordant, n (%) | 207,514 (39.8) | 150,783 (37.9) | 82,340 (40.3) | 83,079 (44.4) | 523,716 (40.0) |
| TC/HDL-C > non-HDL-C, n (%) | 101,095 (19.4) | 131,663 (33.1) | 81,801 (40.1) | 82,969 (44.3) | 397,528 (30.3) |
| ≥25 percentile units discordance | |||||
| TC/HDL-C < non-HDL-C, n (%) | 100,396 (19.3) | 45,139 (11.3) | 12,766 (6.3) | 5,559 (3.0) | 163,860 (12.5) |
| Concordant, n (%) | 389,154 (74.7) | 299,857 (75.3) | 154,397 (75.6) | 141,648 (75.6) | 985,056 (75.2) |
| TC/HDL-C > non-HDL-C, n (%) | 31,329 (6.0) | 53,174 (13.4) | 36,981(18.1) | 40,032 (21.4) | 161,516 (12.3) |
TC/HDL-C (Total Cholesterol to HDL-C ratio); LDL-C (Low-density Lipoprotein Cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol); Q1–Q3 (1st to 3rd quartile)
The proportion of patients with TC/HDL-C > LDL-C by ≥25 percentile units increased gradually from 3% in the TG <100 mg/dL group to as high as 51% in the TG 200–399 mg/dL group (Table 1). This was much larger than TC/HDL-C > non-HDL-C discordance where the proportion of patients increased from 6% to 21% across the respective TG groups (Table 1). On the other hand, the proportion of patients with TC/HDL-C < LDL-C by ≥25 percentile units decreased gradually with increasing TG levels and was much larger than TC/HDL-C < non-HDL-C discordance (Table 1). TC/HDL-C discordance with direct LDL-C and LDL-C estimated by the novel method was less dramatic at higher TG levels compared to Friedewald LDL-C (Supplemental Table 2).
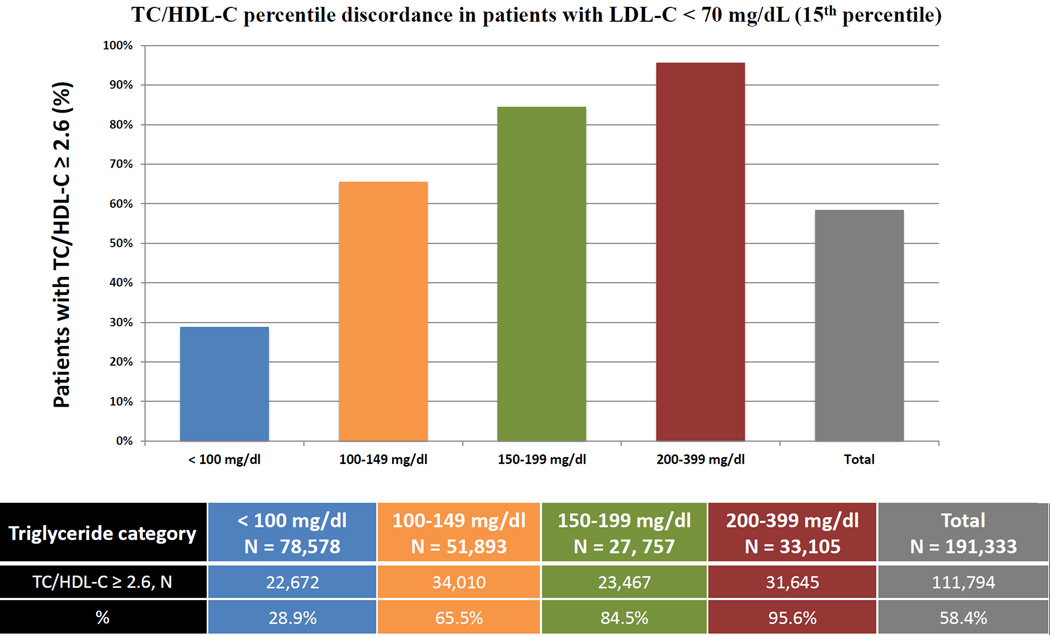
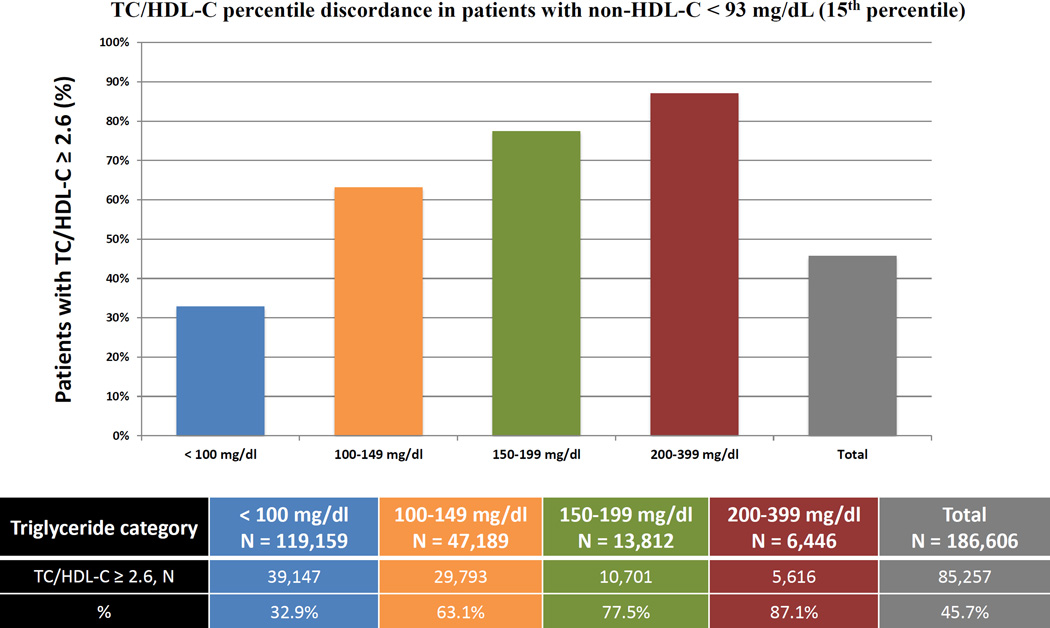
We also assessed TC/HDL-C discordance with LDL-C and non-HDL-C at the LDL-C goal <70 mg/dL recommended by multiple guidelines.4–7 In our population, LDL-C of 70 mg/dL was the percentile-equivalent of non-HDL-C of 93 mg/dL and TC/HDL-C of 2.6 (15th percentile) (Supplemental Table 1). In patients with <15th percentile levels of Friedewald LDL-C or non-HDL-C, a respective 58% and 46% were at or above the percentile-equivalent TC/HDL-C of 2.6. When studied across TG categories, the percentage of patients with LDL-C <15th percentile and TC/HDL-C ≥15th percentile, increased from 29% at TG levels <100 mg/dL to 96% at TG levels 200–399 mg/dL (Figure 4A). A similar analysis showed that the percentage of discordant patients with non-HDL-C <15th percentile and TC/HDL-C ≥15th percentile increased from 33% at TG levels <100 mg/dL to 87% at TG levels 200–399 mg/dL (Figure 4B). Similar analyses revealed that 57% of patients with direct LDL-C <12th percentile (70 mg/dL) and 56% with novel method LDL-C <12th percentile (70 mg/dL) were above the percentile-equivalent TC/HDL-C ratio of 2.5 (Supplemental Figure 2).
Figure 4.
Proportions with LDL-C or non-HDL-C <15th population percentile but discordantly high TC/HDL-C. A) The proportion of patients with LDL-C < 70 mg/dL and TC/HDL-C ≥ 2.6 (15th percentile equivalent cut-points) across various triglyceride categories B) The proportion of patients with non-HDL-C < 93 mg/dL and TC/HDL-C ≥ 2.6 (15th percentile equivalent cut-points) across various triglyceride categories. TC/HDL-C (Total Cholesterol to HDL-C ratio); LDL-C (Low-density Lipoprotein Cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol).
Characteristics of discordant vs. concordant patient populations
Using discordance definitions of ≥10 and ≥25 percentile units, we compared three groups of patients as follows: TC/HDL-C > LDL-C percentile, concordant percentiles and TC/HDL-C < LDL-C percentile (Table 2). Age was similar between the three groups of patients at both levels of discordance (≥10 percentile units and ≥25 percentile units discordance). Patients with TC/HDL-C > LDL-C, compared to concordant and TC/HDL-C < LDL-C, were more commonly male (approximately two thirds) with a more atherogenic lipid phenotype characterized by lower HDL-C and its subfractions, and higher TG, TG/HDL-C ratio, and LDL density (LLDR).26 However, TC and LDL-C levels were lower in these patients. We observed similar results when comparing the 3 TC/HDL-C vs. non-HDL-C groups (Table 3).
Table 2.
Characteristics of patients with TC/HDL-C > LDL-C percentile discordance and TC/HDL-C < LDL-C percentile discordance versus percentile concordance.
| ≥10 percentile units discordance | ≥25 percentile units discordance | |||||
|---|---|---|---|---|---|---|
| Variables | TC/HDL-C > LDL-C |
Concordant | TC/HDL-C < LDL-C |
TC/HDL-C > LDL-C |
Concordant | TC/HDL-C < LDL-C |
| N (%) | 427,509 (32.6%) | 429,851 (32.8%) | 453,072 (34.6%) | 223,851 (17.1%) | 859,854 (65.6%) | 226,727 (17.3%) |
| Age | 60 (49–70) | 60 (49–70) | 59 (50–69) | 60 (49–70) | 59 (49–70) | 59 (51–68) |
| Male, n (%) | 283,680 (66.4%) | 214,974 (50.0%) | 121,479 (26.8%) | 157,428 (70.3%) | 417,561 (48.6%) | 45,144 (19.9%) |
| HDL-C | 40 (36–44) | 51 (47–56) | 68 (61–78) | 37 (33–41) | 52 (46–59) | 75 (69–85) |
| TG | 159 (118–217) | 113 (83–154) | 86 (67–113) | 184 (137–246) | 112 (83–153) | 80 (64–103) |
| TG/HDL-C | 3.92 (2.88–5.54) | 2.19 (1.59–3.04) | 1.26 (0.91–1.71) | 4.9 (3.71–6.66) | 2.17 (1.53–3.07) | 1.06 (0.80–1.39) |
| TC | 163 (141–187) | 182 (153–221) | 212 (190–238) | 158 (139–178) | 185 (159–215) | 224 (205–246) |
| LDL-C | 89 (70–109) | 108 (77–143) | 123 (102–146) | 83 (67–99) | 107 (81–136) | 129 (112–148) |
| Non-HDL-C | 122 (101–146) | 131 (96–173) | 142 (119–167) | 120 (102–140) | 130 (101–166) | 146 (127–167) |
| TC/HDL-C | 4.02 (3.41–4.80) | 3.52 (2.73–4.64) | 3.05 (2.61–3.57) | 4.2 (3.67–4.90) | 3.49 (2.82–4.37) | 2.93 (2.58–3.31) |
| HDL2-C | 9 (7–10) | 12 (10–15) | 19 (15–23) | 8 (6–9) | 12 (10–15) | 22 (18–26) |
| HDL3-C | 31 (28–35) | 39 (36–42) | 50 (45–56) | 29 (26–32) | 39 (35–44) | 54 (50–60) |
| LLDR | 1.0 (0.56 – 1.41) | 0.66 (0.24 – 1.08) | 0.25 (−0.17 – 0.69) | 1.11 (0.68 – 1.50) | 0.64 (0.19 – 1.08) | 0.13 (−0.83 – 1.24) |
All values reported other than n (%) are median (Q1–Q3)
HDL-C (high-density lipoprotein cholesterol); TG (triglycerides); TC (total cholesterol); LDL-C (low-density lipoprotein cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol); LLDR (logarithmic LDL-C density ratio); Q1–Q3 (1st to 3rd quartile)
Table 3.
Characteristics of patients with TC/HDL-C > non-HDL-C percentile discordance and TC/HDL-C < non-HDL-C percentile discordance versus percentile concordance.
| ≥10 percentile units discordance | ≥25 percentile units discordance | |||||
|---|---|---|---|---|---|---|
| Variables | TC/HDL-C > Non- HDL-C |
Concordant | TC/HDL-C < Non- HDL-C |
TC/HDL-C > Non- HDL-C |
Concordant | TC/HDL-C < Non- HDL-C |
| N (%) | 397,528 (30.3) | 523,716 (40.0%) | 389,188 (29.7%) | 161,516 (12.3%) | 985,056 (75.2%) | 163,860 (12.5%) |
| Age | 60 (49–70) | 59 (48–70) | 59 (51–69) | 61 (50–71) | 59 (49–70) | 60 (52–69) |
| Male, n (%) | 277,138 (69.7%) | 252,153 (48.1%) | 90,842 (23.3%) | 122,789 (76.0%) | 470,354 (47.7%) | 26,990 (16.5%) |
| HDL-C | 40 (36–44) | 52 (48–57) | 69 (62–79) | 36 (32–39) | 52 (45–60) | 78 (72–88) |
| TG | 135 (99–186) | 115 (80–167) | 95 (72–128) | 146 (108–199) | 114 (81–162) | 89 (69–117) |
| TG/HDL-C | 3.40 (2.39–5.00) | 2.19 (1.44–3.43) | 1.34 (0.95–1.92) | 4.13 (2.97–5.90) | 2.18 (1.44–3.36) | 1.12 (0.82–1.54) |
| TC | 157 (138–177) | 186 (161–219) | 219 (199–243) | 147 (131–162) | 188 (163–213) | 234 (218–254) |
| LDL-C | 87 (69–105) | 108 (79–140) | 127 (107–149) | 79 (64–93) | 106 (83–134) | 134 (118–153) |
| Non-HDL-C | 116 (97–137) | 133 (99–173) | 148 (126–172) | 111 (96–126) | 132 (104–164) | 154 (137–174) |
| TC/HDL-C | 3.89 (3.33–4.62) | 3.53 (2.70–4.68) | 3.10 (2.66–3.62) | 4.06 (3.60–4.68) | 3.50 (2.83–4.40) | 2.96 (2.62–3.33) |
| HDL2-C | 8 (7–10) | 12 (10–15) | 19 (16–24) | 7 (6–9) | 12 (10–16) | 23 (19–28) |
| HDL3-C | 31 (28–34) | 40 (36–43) | 50 (46–56) | 28 (26–31) | 39 (35–45) | 56 (52–61) |
| LLDR | 0.99 (0.54 – 1.42) | 0.67 (0.23 – 1.09) | 0.22 (−0.19 – 0.66) | 1.12 (0.67 – 1.54) | 0.65 (0.19 – 1.10) | 0.07 (−0.33 – 0.49) |
All values reported other than n (%) are median (Q1–Q3)
HDL-C (high-density lipoprotein cholesterol); TG (triglycerides); TC (total cholesterol); LDL-C (low-density lipoprotein cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol); LLDR (logarithmic LDL-C density ratio); Q1–Q3 (1st to 3rd quartile)
Explaining discordance
In a linear regression model using TC/HDL-C – LDL-C percentile discordance as a continuous outcome, age, sex and ln(TG) (Model A) explained 40% of discordance (R2 0.4). Adding TC to the model increased R2 to 0.74 (Model B) and consecutively adding HDL-C increased R2 to 0.88 (Model C). For each 1 standard deviation (SD) increment in ln(TG), discordance increased by 20 and 12 percentile units in models B and C, respectively (Table 4A). For TC/HDL-C – non-HDL-C percentile discordance, age, sex and ln(TG) explained 21% of discordance (R2 0.21) which increased to 0.64 and 0.86 by adding TC then consecutively HDL-C in models B and C, respectively (Table 4B). For each 1 SD increment in ln(TG), discordance increased by 10 and 2 percentile units in models B and C, respectively, a smaller change compared to TC/HDL-C vs. LDL-C discordance.
Table 4.
Multivariable linear regression analysis to predict percentile discordance between TC/HDL-C versus LDL-C and non-HDL-C.
| A) TC/HDL-C – LDL-C percentile discordance |
B) TC/HDL-C – non-HDL-C percentile discordance |
|||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| Constant | −7.66 (0.03); −301.07 | −3.83 (0.02); −234.36 | −0.91 (0.01); −76.48 | −7.32 (0.02); −312.3 | −3.96 (0.02); −246.56 | −0.78 (0.01); −76.11 |
| ln(TG)* | 16.07 (0.02); 787.95 | 20.03 (0.01); 1513.74 | 12.46 (0.01); 1086.47 | 6.39 (0.02); 339.97 | 9.87 (0.01); 759.52 | 1.64 (0.01); 165.56 |
| Age | 0.17 (0.02); 9.01 | −1.76 (0.01); −150.99 | −0.32 (0.01); −38.23 | −0.04 (0.02); −2.09 | −1.73 (0.01); −150.83 | −0.16 (0.01); −22.27 |
| Gender (male) | 16.41 (0.04); 446.19 | 8.38 (0.02); 349.42 | 2.03 (0.02); 113.89 | 15.65 (0.03); 461.78 | 8.60 (0.02); 364.92 | 1.70 (0.02); 110.00 |
| HDL-C | −13.24 (0.01); −1140.88 | −14.39 (0.01); −1434.58 | ||||
| TC | −17.27 (0.01); −1393.68 | −11.52 (0.01); −1142.03 | −15.18 (0.01); −1247.17 | −8.94 (0.01); −1022.61 | ||
| R2 | 0.4025 | 0.7601 | 0.8801 | 0.2138 | 0.6417 | 0.8611 |
Natural log of triglycerides due to log normal distribution of triglycerides
Results shown are: coefficient (Standard Error); T statistic
All covariates, except gender, were standardized by their standard deviations.
All values are statistically significant (p<0.0001)
HDL-C (high-density lipoprotein cholesterol); TG (triglycerides); TC (total cholesterol); LDL-C (low-density lipoprotein cholesterol); non-HDL-C (Non-High-density Lipoprotein Cholesterol)
More regression models using HDL subfractions and LLDR are shown in Supplemental Table 3. Models incorporating HDL3-C were better at explaining discordance than HDL2-C, while LLDR added minimally to the prediction of discordance.
DISCUSSION
Our cross-sectional study of 1.3 million patients shows the existence of significant patient-level TC/HDL-C discordance in relation to LDL-C and non-HDL-C. Patients with a disproportionately high TC/HDL-C do not differ in age, but tend to be male and have a more atherogenic lipid phenotype with lower HDL-C and higher TG while patients with disproportionately low TC/HDL-C have a less atherogenic phenotype. Discordance is largely explained by age, sex, and levels of standard lipid parameters, predominantly the latter. Overall, the finding of significant TC/HDL-C discordance may suggest potential additional information in TC/HDL-C not available in LDL-C or non-HDL-C alone.
Perhaps the most striking and original finding in our big data analysis is the sizable discordance between TC/HDL-C and non-HDL-C. TC/HDL-C is calculated from the same two data points as non-HDL-C, with the only difference being the mathematical operation of division, rather than subtraction. Although one might intuit that there is no additional information to extract from dividing rather than subtracting TC and HDL-C, this question requires careful attention and empirical evidence.
We document considerable TC/HDL-C discordance with non-HDL-C. We found only one previous study examining patient-level TC/HDL-C and non-HDL-C discordance. In 692 severely hypercholesterolemic patients, TC/HDL-C was only modestly correlated with non-HDL-C (r = 0.39),27 compared with r = 0.70 in our study. The difference in correlation may be due to the relatively small size and high cholesterol levels in the prior study population with mean non-HDL-C and TC/HDL-C of 192 mg/dL and 6.7, respectively, compared to our larger population with means of 136 mg/dL and 3.7, respectively. In the prior study, among low-risk patients with a non-HDL-C <190 mg/dL, only 8% had TC/HDL-C ≥6.0 but among high-risk patients with non-HDL-C <130 mg/dL, 58% had a TC/HDL-C ≥3.5 consistent with findings in our study.
Prior studies have shown that particle-based measures such as LDL-P or apoB are discordantly greater than LDL-C more frequently in patients with insulin resistance, lower HDL-C, lower LDL-C, higher TG, and those on statins.22,25,28,29 To our knowledge, our study is the first and largest to evaluate characteristics of patients with TC/HDL-C discordance. We found that those with disproportionately high TC/HDL-C were most commonly men and had a generally more atherogenic lipid phenotype characterized by lower HDL-C and its subfractions, higher TG, and higher LDL density.21,22,26,28 TG/HDL-C, an important marker associated with insulin resistance and inversely associated with LDL particle size,30 was also higher. Our findings suggest that the lipid phenotype of these patients is, generally, comparable to those with obesity, diabetes and metabolic syndrome who have a prevalence of triglyceride-rich remnant lipoproteins and cholesterol-depleted apoB particles.22,28 This phenotype may be associated with a higher risk of coronary events compared to patients with cholesterol-rich apoB particles.31 Using linear regression, we have also shown that >86% of the variance in discordance is fundamentally explained by age, sex and the three directly measured standard lipid parameters, a finding that in the future may help clinicians focus attention on certain patient clusters, such as those with low TC and HDL-C, where significant discordance exists and risk may track more closely to TC/HDL-C.
By inversely integrating HDL-C, a higher TC/HDL-C ratio may reflect, to some extent, discordance between particle cholesterol content and concentration that tends to occur in patients with insulin resistance and low HDL-C levels.28 This novel concept suggests that potential additional information contained in TC/HDL-C may not be due to the contentious conviction of an inverse relationship between HDL-C and CVD9 but instead, TC/HDL-C might provide a partial gateway to lipoprotein particle concentration and size information from the standard lipid profile. A recent analysis showed that TC/HDL-C ratio of <3 was the standard lipid profile measure that was most correlated with a LDL-P of <1000 nmol/L.12 In another study, the significant difference in LDL size between patients with coronary artery disease and controls became non-significant after adjusting for TC/HDL-C.32 While more study is needed, this initial evidence indicates that TC/HDL-C may carry information related to particle concentration and size.
If viewed in this way, as a marker of atherogenic lipoprotein burden, TC/HDL-C may be more acceptable for clinical use with focus shifted away from the lack of proven HDL-C raising strategies. By such a view, lowering a discordantly elevated TC/HDL-C may be desirable, to achieve a further lowering in atherogenic lipoproteins. Retention of apoB containing lipoproteins is the fundamental event leading to subendothelial accumulation of cholesterol and atherosclerosis.22,28,33 However, both non-HDL-C and LDL-C are inherently cholesterol, not particle, focused measures. When small, dense lipoproteins predominate, non-HDL-C and LDL-C may underestimate the burden of circulating atherogenic particles. Particle burden can be measured with an added test, or, for no additional cost, perhaps TC/HDL-C could be first considered.
At present, we can only comment on overall population-level risk signals for TC/HDL-C compared with non-HDL-C and LDL-C. The TC/HDL-C ratio has been strongly associated with cardiovascular risk.14–16,34–36 In the Women’s Health Study, TC/HDL-C was better than LDL-C and as good as or better than non-HDL-C and apolipoprotein fractions in the prediction of future cardiovascular events.15 In another Women’s Health Study, the net reclassification index for adding either apoB or LDL-P to TC/HDL-C was only 2%.16 Similar results were demonstrated in the Framingham population,35 Physicians Health Study,37 and in statin-treated patients.34 In a meta-analysis of approximately 900,000 patients with 55,000 vascular deaths, TC/HDL-C was suggested to provide 40% more risk information than non-HDL-C.38
Rather than the question of the general population risk information in a given lipid parameter, the most clinically-relevant question when considering additional parameters would seem to be: in those who have discordance, does discordance relate to greater atherosclerosis or greater risk of events? That is, related lipid parameters should be compared for risk signals when they disagree, not when they agree. This is a relatively new approach to epidemiologic analysis. Two studies have examined discordance between particle-based measures such as LDL-P and apoB vs. non-HDL-C in this way.23,39 In these studies, discordance was sizeable and cardiovascular outcomes, including events, coronary artery calcium and carotid intimal medial thickness, tracked more closely with LDL-P and apoB. However, there is no conclusive outcome data to suggest that an advantage lies in the direction of the TC/HDL-C ratio in instances of discordance; thus, additional clinical studies using the patient-level discordance approach are warranted.
Study limitations
Our study limitations have been described in detail.18 Although we lack important clinical characteristics, such as statin use, our population represents a contemporary population of 1.3 million patients with parallel age, sex, and lipid distributions to the NHANES population (Supplemental Figure 1). As a cross-sectional study, we cannot determine if TC/HDL-C discordance relates to risk for cardiovascular events, and if so, what magnitude of discordance is clinically significant. We note, however, that discordance between apoB and non-HDL-C of >5 percentiles was clinically significant23 while another study showed that >12 percentile discordance between LDL-P and LDL-C was clinically significant.25
Conclusions
Our contemporary, big data analysis demonstrates that a substantial proportion of patients have significant discordance of TC/HDL-C with LDL-C and non-HDL-C. Therefore, the fundamental criterion for potential additional information – existence of discordance – is met. TC/HDL-C, available at no extra cost, warrants continued investigation of its potential clinical importance through discordance analyses in studies with longitudinal follow-up for clinical events.
Supplementary Material
Acknowledgements
SSM is supported by the Pollin Cardiovascular Prevention Fellowship, Marie-Josee and Henry R. Kravis endowed fellowship, and a National Institutes of Health training grant (T32HL07024).
Funding Sources: This study was initiated by the investigators and did not receive any outside funding. Atherotech provided the investigators with de-identified data generated from commercial lipid analyses and did not provide payments or participate in data analysis or influence the conclusions. MBE, RQ, SRJ and SSM take responsibility for the accuracy of the statistical analyses and had the sole authority on manuscript preparation and submission for publication.
SSM and SRJ are listed as co-inventors on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. PPT serves on the medical advisory board for Atherotech Diagnostic Lab; has received compensation for consultancy and lecturers from Abbvie, Aegerion, Amgen, AstraZeneca, Glaxo-SmithKline, Kowa, and Merck & Co, Novartis, and Regeneron. MB is a member of International Advisory Board of Amgen, and Sanofi and has given talks, attended conferences and participated in studies sponsored by MSD, Abbott, Sanofi and Amgen. KRK is an employee of Atherotech Diagnostics Laboratory and receives modest royalty from the University of Alabama in Birmingham, AL. SRJ serves on the medical advisory board for Atherotech Diagnostic Lab and as an advisor to Sanofi and Regeneron.
Footnotes
Disclosures: MBE, RQ, EDM, ADS, JC and RSB have no disclosures.
References
- 1.National Ambulatory Medical Care Survey. 2009 Summary Tables. [Accessed February 2, 2015]; http://www.cdc.gov/nchs/data/ahcd/namcs_summary/2009_namcs_web_tables.pdf. Published in December 2009.
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;12:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W-K, Smith SC, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 4.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. Bax J, Vahanian A, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Filippatos G, Funck-Brentano C, Hasdai D, Hobbs R, Hoes A, Kearney P, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vardas P, Widimsky P, Windecker S, Reviewers D, Funck-Brentano C, Poldermans D, Berkenboom G, de Graaf J, Descamps O, Gotcheva N, Griffith K, Guida GF, Gulec S, Henkin Y, Huber K, Kesaniemi YA, Lekakis J, Manolis AJ, Marques-Vidal P, Masana L, McMurray J, Mendes M, Pagava Z, Pedersen T, Prescott E, Rato Q, Rosano G, Sans S, Stalenhoef A, Tokgozoglu L, Viigimaa M, Wittekoek ME, Zamorano JL. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 5.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GBJ, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 Update of the Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – executive summary. J Clin Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Arai H, Barter P, Bersot TP, Betteridge DJ, Carmena R, Cuevas A, Davidson MH, Genest J, Kesäniemi YA, Sadikot S, Santos RD, Susekov AV, Sy RG, LaleTokgözoglu S, Watts GF, Zhao D. An International Atherosclerosis Society Position Paper: Global recommendations for the management of dyslipidemia—Full report. J Clin Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, Li Y, Spertus JA, Jones SR on behalf of the Lipoprotein Investigators Collaborative (LIC) HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the lipoprotein investigators collaborative. Eur Heart J. 2015;36:22–30. doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 11.Toth PP, Barylski M, Nikolic D, Rizzo M, Montalto G, Banach M. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract Res Clin Endocrinol Metab. 2014;28:353–368. doi: 10.1016/j.beem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Mathews SC, Mallidi J, Kulkarni K, Toth PP, Jones SR. Achieving Secondary Prevention Low-Density Lipoprotein Particle Concentration Goals Using Lipoprotein Cholesterol-Based Data. In: Wang G, editor. PLoS ONE. Vol. 7. 2012. p. e33692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castelli WP. Cholesterol and lipids in the risk of coronary artery disease--the Framingham Heart Study. Can J Cardiol. 1988;4:5A–10A. [PubMed] [Google Scholar]
- 14.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non–HDL Cholesterol, Apolipoproteins A-I and B 100, Standard Lipid Measures, Lipid Ratios, and CRP as Risk Factors for Cardiovascular Disease in Women. JAMA. 2005;294:326. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein Particle Profiles by Nuclear Magnetic Resonance Compared With Standard Lipids and Apolipoproteins in Predicting Incident Cardiovascular Disease in Women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elshazly MB, Martin SS, Blaha MJ, Joshi PH, Toth PP, McEvoy JW, Al-Hijji MA, Kulkarni KR, Kwiterovich PO, Blumenthal RS, Jones SR. Non-high-density lipoprotein cholesterol, guideline targets, and population percentiles for secondary prevention in 1.3 million adults: the VLDL-2 study (very large database of lipids) J Am Coll Cardiol. 2013;62:1960–1965. doi: 10.1016/j.jacc.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Martin SS, Blaha MJ, Toth PP, Joshi PH, McEvoy JW, Ahmed HM, Elshazly MB, Swiger KJ, Michos ED, Kwiterovich PO, Kulkarni KR, Chimera J, Cannon CP, Blumenthal RS, Jones SR. Very large database of lipids: rationale and design. Clin Cardiol. 2013;36:641–648. doi: 10.1002/clc.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni KR. Cholesterol Profile Measurement by Vertical Auto Profile Method. Clin Lab Med. 2006;26:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin SS, Michos ED. Are We Moving Towards Concordance on the Principle That Lipid Discordance Matters? Circulation. 2014;129:539–541. doi: 10.1161/CIRCULATIONAHA.113.007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sniderman AD, Lamarche B, Contois JH, De Graaf J. Discordance analysis and the Gordian Knot of LDL and non-HDL cholesterol versus apoB. Curr Opin Lipidol. 2014;25:461–467. doi: 10.1097/MOL.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 23.Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–449. doi: 10.1016/j.atherosclerosis.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. doi: 10.1016/j.jacl.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed H, Elshazly M, Martin S, Blaha M, Kulkarni K, Jones S. Ratio of Dense to Buoyant LDL Subclass is Associated with LDL Density Phenotype (VLDL-5) The Open Chemical and Biomedical Methods Journal. 2013;6:1–5. [Google Scholar]
- 27.Vodnala D, Bard RL, Krishnan SM, Jackson EA, Rubenfire M, Brook RD. Potential effects on clinical management of treatment algorithms on the basis of apolipoprotein-B/A-1 and total/high-density lipoprotein-cholesterol ratios. J Clin Lipidol. 2011;5:159–165. doi: 10.1016/j.jacl.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Reddy KS, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KMS. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 29.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PWF, D'Agostino RB. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—Implications for LDL management. J Clin Lipidol. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D'Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, Peterson ED, Vasan RS, Sniderman AD. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. 2015 doi: 10.1177/2047487315569411. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 33.Mora S. Advanced Lipoprotein Testing and Subfractionation Are Not (Yet) Ready for Routine Clinical Use. Circulation. 2009;119:2396–2404. doi: 10.1161/CIRCULATIONAHA.108.819359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastelein JJP, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, Deedwania P, Olsson AG, Boekholdt SM, Demicco DA, Szarek M, LaRosa JC, Pedersen TR, Grundy SM for the TNT and IDEAL Study Groups. Lipids, Apolipoproteins, and Their Ratios in Relation to Cardiovascular Events With Statin Treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
- 35.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PWF, D'Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 36.Manickam P, Rathod A, Panaich S, Hari P, Veeranna V, Badheka A, Jacob S, Afonso L. Comparative prognostic utility of conventional and novel lipid parameters for cardiovascular disease risk prediction: Do novel lipid parameters offer an advantage? J Clin Lipidol. 2011;5:82–90. doi: 10.1016/j.jacl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 38.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 39.DeGoma EM, Davis MD, Dunbar RL, Mohler ER, III, Greenland P, French B. Discordance between non-HDL-cholesterol and LDL-particle measurements: Results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;229:517–523. doi: 10.1016/j.atherosclerosis.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.