Abstract
An oncolytic virus (OV) based on poliovirus (PV), the highly attenuated polio-/rhinovirus recombinant PVSRIPO, may deliver targeted inflammatory cancer cell killing; a principle that is showing promise in clinical trials for recurrent glioblastoma (GBM). The two decisive factors in PVSRIPO anti-tumor efficacy are selective cytotoxicity and its in situ immunogenic imprint. While our work is focused on what constitutes PVSRIPO cancer cytotoxicity, we are also studying how this engenders host immune responses that are vital to tumor regression. We hypothesize that PVSRIPO cytotoxicity and immunogenicity are inextricably linked in essential, complimentary roles that define the anti-neoplastic response. Herein we delineate mechanisms we unraveled to decipher the basis for PVSRIPO cytotoxicity and its immunotherapeutic potential.
Introduction
Immunotherapy approaches that bolster immune effector responses against cancer have gained traction after demonstrating significant clinical responses in several indications [1]. These therapies reverse tumor-induced blockades that skew inflammatory responses to favor tumor expansion and persistence and thereby unmask the tumor to the host immune system. Efficacious immunotherapy approaches rely upon the immune system’s ability to recognize and react to the distress ligands and neo-antigens present in all cancer cells. Viruses offer a unique advantage for immunologically ‘revealing’ tumors, having co-evolved with mammalian immune systems for millennia and training our immune system to recognize and kill infected and/or damaged cells.
OVs may recruit immune effector responses through a two-pronged mechanism: infecting and directly lysing cancer cells while simultaneously activating inflammatory anti-viral pathways (Figure 1). Among the major requisite attributes for OVs is documented affinity and specificity for malignant tissue in patients. Our group has developed an attenuated recombinant oncolytic PV that relies on a confluence of many factors to deliver inflammatory cytotoxicity specifically to cancer cells (Figure 1). This strategy was inspired by founding work, demonstrating that replacement of the cognate PV internal ribosomal entry site (IRES), essential for driving translation initiation at the PV RNA genome, with that of the human rhinovirus 2 (HRV2) completely abolishes the inherent, grim neurovirulence of PV [2]. The recombinant, called PVSRIPO for PV (Sabin)-Rhinovirus IRES PV Open reading frame, is derived from the (Sabin) live-attenuated type 1 vaccine strain of PV [3]. PVSRIPO is currently being evaluated in a Phase-I clinical trial against recurrent GBM, where it has shown durable complete radiographic and clinical responses in several patients [4]. Critical mediators of PVSRIPO’s clinical efficacy are its uniquely simple, swift and violent cytotoxicity and its unique relation to Mda5, an intriguing cytosolic pattern recognition receptor (PRR) with a powerful immunogenic range [5] (Figure 1). We are working fervently to identify mechanisms mediating both of these aspects of PVSRIPO oncolytic immunotherapy, as they will likely open opportunities to broaden and enhance clinical application.
Figure 1.
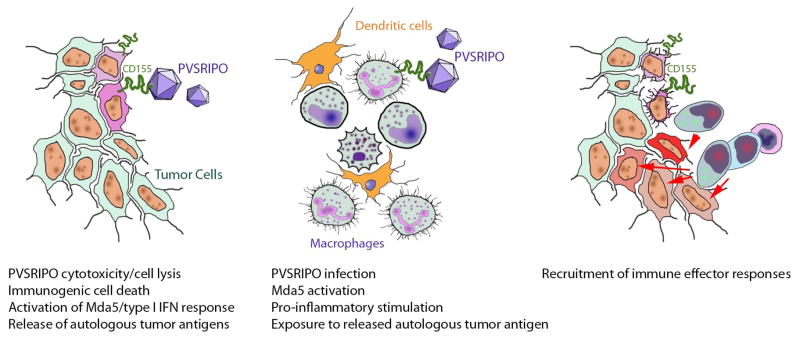
Hypothetical model of PVSRIPO oncolytic immunotherapy mechanisms. A combination of (left) direct viral tumor cytotoxicity and engagement of Mda5/the anti-viral IFN response; and (middle) PVSRIPO non-lethal infection and pro-inflammatory stimulation of tumor-associated macrophages (TAM) and/or dendritic cells; (right) recruits immune effector responses directed against tumor neo-antigens.
The PV receptor CD155 in cancer
To be successful, OVs must have tropism for tumor tissue and/or stromal components, usually determined by host cell surface entities (receptors) participating in virus attachment and entry functions. PV tropism is exceedingly simple, because all attachment and entry events are mediated by a single molecule, necessary and sufficient for PV host cell entry, the Ig-superfamily cell adhesion molecule CD155 (a.k.a. PVR, Necl5) [6]. Although the physiologic roles of CD155 remain poorly defined, its arguably most intriguing attribute is near-universal ectopic upregulation in solid neoplasia [7]. This is certainly true for GBM [8], as demonstrated in immunoblots from a panel of primary patient explant GBM xenotransplantation lines with diverse molecular signatures (e.g. with regard to PTEN and AKT status; Figure 2). CD155 has been shown to enhance cancer cell motility and invasiveness [9], regulate NK cell activity [10], and become transcriptionally up-regulated during DNA damage signaling [11]. It is plausible that near-ubiquitous CD155 expression in cancer is due to selective pressure from one or more of these functions.
Figure 2.
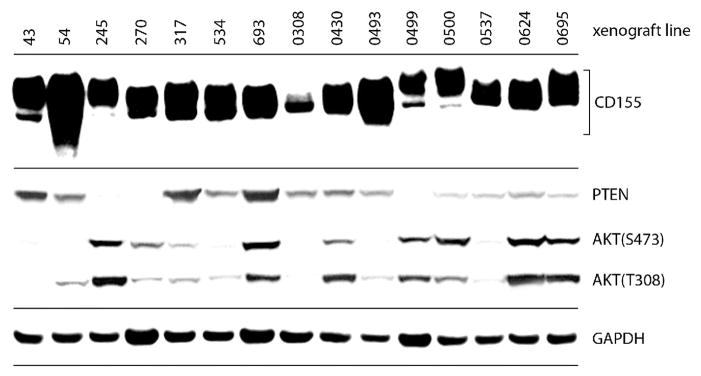
CD155 expression is common in GBM. Primary patient explant GBM xenografts (passaged exclusively in mice) were harvested and lysed for immunoblot analysis. CD155 (variable electrophoretic mobility is due to distinct glycosylation patterns); PTEN; p-AKT(S473) and (T308); and GAPDH were analyzed by immunoblot.
Of interest, e.g. in the context of GBM where macrophages and myeloid-derived suppressor cells (MDSCs) comprise a significant portion of tumors, CD155 is also expressed on antigen-presenting cells (APCs; explaining its designation as a cluster of differentiation molecule; [12,13]; Figure 1). Wild-type PV infection of these cells leads to their pro-inflammatory activation and facilitates antigen presentation/immune effector functions [13]. We confirmed these findings for PVSRIPO and are working to assess how APC infection by PVSRIPO may contribute to antitumor efficacy in murine models.
Upon binding CD155, the PV capsid undergoes a conformational expansion, extruding the myristoylated capsid protein VP4 and externalizing the N-terminus of VP1. These events may enable association of the disintegrating viral capsid with the cell membrane and mediated trans-membrane cytosolic transfer of the viral RNA genome [14]. Once arrived within the cytoplasm, viral RNA genomes are immediately translated, initiating the next important step in mediating oncolytic efficacy.
PVSRIPO targets neoplasia-specific conditions for translation initiation
In addition to expression of CD155 in cancerous cells, PVSRIPO cytotoxicity and inflammation is specific to cancer cells due to advantages for unorthodox, alternative translation initiation employed by PV (and all picornaviruses). In essence, PV is a sophisticated mechanism to propagate a highly toxic mRNA. The PVSRIPO +strand RNA genome, ~7.3Kb in length, is 5′ tethered to a viral protein (VPg) in lieu of the canonical 5′ 7-methyl-guanosine (m7G) cap on eukaryotic mRNAs [15,16]. Approximately 7.5% of the genetically extremely austere viral RNA is devoted to a structurally complex 5′ UTR element mediating m7G-cap-independent translation initiation: the IRES. Exemplified by the live-attenuated PV (Sabin) vaccines, it is clear that the IRES is a major factor in PV neuropathogenicity. The Sabin serotypes each carry critical attenuating point mutations in stem-loop domain V of the IRES [nt positions 480 (type 1); 481 (type 2); 472 (type 3)] [17]. These affect an IRES region coordinating assembly and/or function of ribonucleoprotein complexes (RNPs) containing canonical translation factors (eukaryotic initiation factor (eIF) 4G, eIF4A, eIF4B; [18,19]) and, possibly, accessory IRES trans-acting factors (ITAFs; e.g. poly(rC) binding protein 2 (PCBP2); Ser-Arg (SR) rich protein 20 (SRp20); [18–22]) [23,24]. The IRES RNP recruits 40S ribosomal subunits (e.g. via eIF4G and eIF3; or -alternatively- via a mechanism involving ITAFs) and the Sabin point mutations likely disrupt IRES structure, the IRES RNP, or dynamic events in ribosome recruitment in ways that interfere with this process specifically in neurons [18,23–25].
We believe that the unprecedented, profound neuro-attenuation of PVSRIPO [26] with retention of full IRES competence in malignant cells [27] is due to combination of both (i) neuron-specific functional deficits of the HRV2 IRES; and (ii) globally permissive conditions for m7G-cap-independent translation initiation in cancer, including initiation at the HRV2 IRES. With regard to (i): we identified a neuron-specific IRES RNP that, due to interactions with host RNA-binding proteins, is incompatible with ribosome recruitment at the HRV2 IRES [28–30]. The double stranded RNA binding protein 76 (DRBP76; a.k.a. NF90) displays fundamentally distinct isoform expression, subcellular partitioning and, thus, RNA-binding properties in normal neuronal cells compared to malignancies [30]. In neuronal cells, DRBP76 is predominantly cytoplasmic [30]. It associates with the HRV2 IRES in such cells [28] and interferes with PVSRIPO ribosome recruitment [29]. In fact, DRBP76 has been reported to act as an RNA-binding protein with broad antiviral properties, precisely due to its (potential) roles in translation control [31]. In neoplastic cells, DRBP76 is restricted to the nuclear compartment, excluding it as a factor in PVSRIPO translation [30].
With regard to (ii): the absence of cytoplasmic DRBP76 in neoplastic cells suggests that there may be fewer obstacles to viral IRES competence in such cells. Rather then just tolerating viral translation, our studies indicate that neoplastic cells exhibit relaxed conditions for translation initiation that actually favor viral, m7G-cap independent translation. This is due to broadly de-regulated mitogenic signal transduction cascades that converge on translation machinery. For example, oncogenic H-Ras transduction of neuron-like HEK293 cells, which are resistant to PVSRIPO translation and cytotoxicity [32], fully restores HRV2 IRES competence and PVSRIPO translation/propagation [33]. This effect is in large part due to the activation of the downstream ERK1/2 substrate, MAPK-interacting kinase (MNK) [33]. MNK is mainly known for phosphorylation of its signature substrate, the m7G-cap-binding protein eIF4E [34,35], but the functional consequences of this event for translation control remains obscure. We recently identified a novel connection between MNK and mTOR signaling, that has profound implications for the homeostatic balance of mitogenic signaling in cancer cells and is liable to exert important influence on post-transcriptional gene regulatory systems, e.g. translation control [23,24]. We believe that our observations explain the remarkably specific and efficient translation, propagation and cytotoxicity of PVSRIPO in malignant cells [4]. We found that a major effect of ERK1/2-MNK signaling is to temper (potentially toxic) runaway AKT activity via stimulation of mTORC1/concomitant repression of mTORC2 (the kinase for AKT(S473); [36]) [24]. This balancing between Ras-MEK-ERK1/2 and PI3K-AKT signaling, centered on the functions of MNK, relieves constitutive AKT-mediated activation of the Ser-Arg rich protein kinase (SRPK) to enhance PVSRIPO IRES-mediated translation and cytotoxicity [24]. Indeed, in vivo studies using a GBM xenograft model revealed that upstream AKT inhibition (with PI3K inhibitors) enhances tumor regression when combined with PVSRIPO [33]. While the mechanism by which SRPK activity restricts viral translation is currently under investigation, it is likely that nucleo-cytoplasmic shuttling SR proteins, principal substrates of SRPK, play a role. One SR protein in particular, SRp20, is a confirmed ITAF [20]. We hypothesize that active SRPK-mediated phosphorylation of SRp20 (or other SR proteins) may favor cytoplasmic accumulation and translation involvement of shuttling SR proteins, a scenario that was documented for the shuttling SR protein ASF/SF2 [37]. In cancerous cells, SRPK activity/SRp20 phosphorylation occurs at an equilibrium that facilitates IRES mediated translation [24]. Of course it is likely that other events converging on translation machinery in transformed cells, e.g. those affecting eIF4G and its many binding partners in the translation initiation scaffold, also play a role in enabling unfettered PVSRIPO translation competence.
Future perspectives
To fully grasp the immunotherapeutic potential of PVSRIPO oncolysis, we have developed robust syngeneic murine models of GBM and other cancer types. These models will enable us discern how immunological events shape PVSRIPO cancer immunotherapy (see Figure 1) and to build a platform to test how PVSRIPO therapy may be broadened, enhanced or optimized. While another oncolytic PV construct was shown to produce efficacious anti-tumor CD8 T cell responses in a syngeneic murine cancer model [38], resolving issues such as: (i) does PVSRIPO oncolytic efficacy entirely rely on such responses? (ii) what is the mechanism linking viral cytotoxicity and innate anti-viral immunity to the adaptive anti-tumor immune response? (iii) are there tumor-specific or patient-specific determinants for efficacy? are of high priority.
Although the exact host immune response to PVSRIPO oncolysis in patients remains unclear, we hypothesize that the multiplex direct viral effects on the host cell and the host innate anti-viral defense conspire to engage effector antitumor immune responses directed against the target tumor (Figure 1). Of particular interest in this context is recent insight that implicate PV-related enteroviruses (Coxsackie B viruses), their cytotoxic effects on pancreatic insulin-producing beta cells, and their relation to Mda5 and the innate anti-viral interferon response, in the pathogenesis of type 1 diabetes [39,40]. In this scenario, a convergence of targeted enteroviral cytotoxicity and Mda5 engagement result in recruiting cytotoxic T cell responses against beta cell antigens [41]. The immunogenic potential implicit in such enterovirus-induced autoimmune pathogenesis is evident as the power to breach self-tolerance, a property most desirable for overcoming the notorious suppression of immune effector responses inherent to cancer.
Conclusions
Our approach of using an attenuated PV recombinant for the treatment of cancer was founded on the exquisite specificity of the virus for malignancy, both tumor cells and stromal APCs. This begins at the earliest stage with the virus’ relation to its receptor (CD155), continues with factors that govern IRES competence in cancerous cells and (simultaneously) engage the cytosolic pattern recognition receptor MDA5. Perhaps the most exciting answers on how PVSRIPO efficacy is achieved will come from future studies focused on identifying the role(s) of the host immune system’s response to loco-regional virus tumor cell killing and inflammation. Defining such roles is of utmost importance, because it will guide future efforts to optimize OV therapy in the clinic and extend the clinical spectrum of indications suitable to intervention with PVSRIPO.
Highlights.
PVSRIPO targets expression of CD155 on tumor and antigen-presenting cells
PVSRIPO is categorically neuron-incompetent, explaining its attenuation phenotype
Tumor-specific cytotoxicity is due to unfettered IRES activity in malignant cells
In situ vaccine effects of viral tumor cytotoxicity and pro-inflammatory activation
Acknowledgments
This work was supported by PHS service grants R01 CA124756 and P50 CA190991 (M.G.). We thank all present and past members of our group for their many contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. NEJM. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrikova EY, Broadt T, Poiley-Nelson J, Yang X, Soman G, Giardina S, Harris R, Gromeier M. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol Ther. 2008;16:1865–1872. doi: 10.1038/mt.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MC, Dobrikova EY, Dobrikov MI, Walton RW, Gemberling SL, Nair SK, Desjardins A, Sampson JH, Friedman HS, Friedman AH, et al. Oncolytic polio virotherapy of cancer. Cancer. 2014;120:3277–3286. doi: 10.1002/cncr.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 7.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 8.Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro-oncology. 2004;6:208–217. doi: 10.1215/S1152851703000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73–82. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 11.Fionda C, Abruzzese M, Zingoni A, Soriani A, Ricci B, Molfetta R, Paolini R, Santoni A, Cippitelli M. Nitric oxide donors increase PVR/CD155 DNAM-1 ligand expression in multiple myeloma cells: role of DNA damage response activation. BMC Cancer. 2009;15:17–28. doi: 10.1186/s12885-015-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freistadt MS, Fleit HB, Wimmer E. Poliovirus receptor on human blood cells: a possible extraneural site of poliovirus replication. Virology. 1993;195:798–803. doi: 10.1006/viro.1993.1433. [DOI] [PubMed] [Google Scholar]
- 13.Wahid R, Cannon MJ, Chow M. Dendritic cells and macrophages are productively infected by poliovirus. J Virol. 2005;79:401–409. doi: 10.1128/JVI.79.1.401-409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss M, Filman DJ, Belnap DM, Cheng N, Noel RT, Hogle JM. Nectin-like interactions between poliovirus and its receptor trigger conformational changes associated with cell entry. J Virol. 2015;89:4143–4157. doi: 10.1128/JVI.03101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci USA. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomoto A, Lee YF, Wimmer E. The 5′ end of poliovirus mRNA is not capped with m7G(5′)ppp(5′)Np. Proc Natl Acad Sci USA. 2000;73:375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans DM, Dunn G, Minor PD, Schild GC, Cann AJ, Stanway G, Almond JW, Currey K, Maizel JV. Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985;314:548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- 18.de Breyne S, Yu Y, Unbehaun A, Pestova TV, Hellen CU. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc Natl Acad Sci USA. 2009;106:9197–9202. doi: 10.1073/pnas.0900153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Sweeney TR, Abaeva IS, Pestova TV, Hellen CU. The mechanism of translation initiation on Type 1 picornavirus IRESs. EMBO J. 2014;33:76–92. doi: 10.1002/embj.201386124. This thorough and technically sophisticated study provides a detailed, comprehensive analysis of the IRES ribonucleoprotein complex and its mechanistic implications for viral, m7G-cap-independent translation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard KM, Daijogo S, Semler BL. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J. 2007;26:459–467. doi: 10.1038/sj.emboj.7601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald KD, Semler BL. Re-localization of cellular protein SRp20 during poliovirus infection: bridging a viral IRES to the host cell translation apparatus. PLoS Pathogens. 2011;7:e1002127. doi: 10.1371/journal.ppat.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Brown MC, Bryant JD, Dobrikova EY, Shveygert M, Bradrick SS, Chandramohan V, Bigner DD, Gromeier M. Induction of viral, 7-methyl-guanosine cap-independent translation and oncolysis by mitogen-activated protein kinase-interacting kinase-mediated effects on the serine/arginine-rich protein kinase. J Virol. 2014;88:188, 13135–13148. doi: 10.1128/JVI.01883-14. This paper deciphers the effects of MAPK signaling cascades on protein synthesis machinery, reveals SRPK as a major factor in cap-independent translation, and suggests important roles for the SR proteins/SRp20 in translation regulation. It thus provides crucial novel insight into the molecular mechanisms responsible for signal-responsive translation adapation in cells (using a viral template) [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Brown MC, Dobrikov MI, Gromeier M. Mitogen-activated protein kinase-interacting kinase regulates mTOR/AKT signaling and controls the serine/arginine-rich protein kinase-responsive type 1 internal ribosome entry site-mediated translation and viral oncolysis. J Virol. 2014;88:13149–13160. doi: 10.1128/JVI.01884-14. This manuscript establishes a direct functional relationship of the downstream ERK1/2 and p38 MAPK substrate, MNK, and mTORC1. These studies document the intricate homeostatic balance forming between distinct mitogenic signal transduction pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochs K, Zeller A, Saleh L, Bassili G, Song Y, Sonntag A, Niepmann M. Impaired binding of standard initiation factors mediates poliovirus translation attenuation. J Virol. 2003;77:115–122. doi: 10.1128/JVI.77.1.115-122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Dobrikova EY, Goetz C, Walters RW, Lawson SK, Peggins JO, Muszynski K, Ruppel S, Poole K, Giardina SL, Vela EM, et al. Attenuation of neurovirulence, biodistribution, and shedding of a poliovirus:rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis. J Virol. 2012;86:2750–2759. doi: 10.1128/JVI.06427-11. This study supplies definitive evidence for the unprecedented level of neuro-attenuation achieved with IRES recombination in PVSRIPO. It provides seminal support for current clinical trials of PVSRIPO in cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrill MK, Dobrikova EY, Gromeier M. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J Virol. 2006;80:3147–3156. doi: 10.1128/JVI.80.7.3147-3156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J Virol. 2006;80:6936–6942. doi: 10.1128/JVI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neplioueva V, Dobrikova EY, Mukherjee N, Keene JD, Gromeier M. Tissue type-specific expression of the dsRNA-binding protein 76 and genome-wide elucidation of its target mRNAs. PloS One. 2010;5:e11710. doi: 10.1371/journal.pone.0011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harashima A, Guettouche T, Barber GN. Phosphorylation of the NFAR proteins by the dsRNA-dependent protein kinase PKR constitutes a novel mechanism of translational regulation and cellular defense. Genes & Dev. 2010;24:2640–2653. doi: 10.1101/gad.1965010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell SA, Lin J, Dobrikova EY, Gromeier M. Genetic determinants of cell type-specific poliovirus propagation in HEK 293 cells. J Virol. 2005;79:6281–6290. doi: 10.1128/JVI.79.10.6281-6290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetz C, Everson RG, Zhang LC, Gromeier M. MAPK signal-integrating kinase controls cap-independent translation and cell type-specific cytotoxicity of an oncolytic poliovirus. Mol Ther. 2010;18:1937–1946. doi: 10.1038/mt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Sanford JR, Ellis JD, Cazalla D, Caceres JF. Reversible phosphorylation differentially affect nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. Proc Natl Acad Sci USA. 2005;102:15042–15047. doi: 10.1073/pnas.0507827102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyoda H, Yin J, Mueller S, Wimmer E, Cello J. Oncolytic treatment and cure of neuroblastoma by a novel attenuated poliovirus in a novel poliovirus-susceptible animal model. Cancer Res. 2007;67:2857–2864. doi: 10.1158/0008-5472.CAN-06-3713. [DOI] [PubMed] [Google Scholar]
- **39.Lincez PJ, Shanina I, Horwitz MS. Reduced expression of the MDA5 gene IFIH1 prevents autoimmune diabetes. Diabetes. 2015 doi: 10.2337/db14-1223. epub ahead of print. This interesting paper links combined activation of cytoplasmic viral RNA sensors/viral cytotoxicity to generation of immune effector responses in autoimmunity. [DOI] [PubMed] [Google Scholar]
- 40.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson SJ, Horwitz MS. Is type 1 diabetes “going viral”? Diabetes. 2014;63:2203–2205. doi: 10.2337/db14-0510. [DOI] [PubMed] [Google Scholar]


