Abstract
Background
Increased attention to gastrointestinal symptoms and disease-specific contexts may play an important role in the enhanced perception of visceral stimuli frequently reported in patients with irritable bowel syndrome (IBS). In the present study, we test the hypothesis that altered attentional mechanisms underlie central pain amplification in IBS.
Methods
To evaluate brain networks that support alerting, orienting, and executive attention, we employed the attention network test (ANT), a modified flanker task which measures the efficiency of functioning of core attentional networks, during functional MRI (fMRI) in 15 IBS patients [mean age = 31(11.96)] and 14 healthy controls [HCs; mean age = 31(10.91)].
Key Results
IBS patients, compared to HCs, showed shorter reaction times during the alerting and orienting conditions which were associated with greater activation of anterior midcingulate and insular cortices, as well as, decreased activity in the right inferior frontal junction and supplementary motor cortex. Patients also showed activation in the dorsal medial prefrontal cortex and concurrent thalamic deactivation during the executive control portion of the ANT relative to HCs, but no group difference in reaction times were found. The activity in brain regions showing group differences during the ANT were associated with measures of gastrointestinal-specific anxiety, pain catastrophizing and fear of uncertainty. In IBS, activity in the anterior midcingulate during alerting correlated with duration of GI-symptoms and overall symptom severity.
Conclusion & Inferences
Together, these results suggest that IBS patients have specific abnormalities in attentional network functioning and these deficits may underlie symptom-related anxiety, hypervigilance, and visceral hypersensitivity.
Keywords: attention network test, chronic pain, fMRI, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a common visceral pain disorder characterized by chronically recurring abdominal pain and discomfort associated with altered bowel habits, and enhanced visceral sensitivity1,2. Symptom-related fears and associated hypervigilance toward gut-related stimuli [e.g., gastrointestinal (GI) sensations, symptoms, contexts] are also hallmarks of this condition, and may play an important role in triggering central pain amplification in these patients3–5. For example, negative cognitions about IBS symptoms, including pain catastrophizing and somatization, are important factors in determining symptom severity6,7. Previous studies have shown that patients with IBS show an attentional bias toward negatively-valenced words, as well as words describing pain and GI-related sensations, compared to words with neutral connotations8–13. Furthermore, IBS patients also show up-regulation in attentional networks during expectation and experience of aversive visceral stimuli14,15. Together, these findings suggest an attentional bias towards symptom-related stimuli, possibly attributable to dysregulation in general attentional processes, which may play a role in central pain amplification and in the chronicity of IBS symptoms.
Attention is a process involving allocation of mental resources, and the selection and prioritization of competing sensory information for optimization of behavioral responses to specific stimuli that are biologically relevant to an organism16–20. Three types of general attentional processes with distinct underlying neural networks have been identified: an alerting network, an orienting network and an executive control network. The alerting network [thalamus, prefrontal cortex (PFC), and posterior parietal cortex] helps achieve and maintain a high state of sensitivity to incoming stimuli. The orienting network (pulvinar, temporal parietal junction, superior parietal cortex, and frontal eye fields) selects information from competing sensory input to attend to, and the executive control network [dorsolateral PFC, anterior cingulate cortex (ACC), and medial PFC (mPFC)] exerts top-down control in an effort to allocate attentional resources and resolve conflict among thoughts, feelings, and behavioral responses17,19,21–22. Brain regions subserving attentional functions operate on identified salience, biologically and cognitively relevant stimuli16–20 and are often coactivated with regions of the salience network whose core regions are the dorsal ACC and the anterior insula23.
Cognitive control strategies24 such as distraction and focused attention influence activity of cortical and subcortical brain networks implicated in the experience and expression of pain25–29 and efficient functioning of these networks is essential to proper inhibitory cognitive control. Moreover, selective attention and hypervigilance to bodily or visceral sensations and associated symptom-related exogenous cues in IBS patients30–32 depend on these brain processes. As such, we aimed to test the hypothesis that these core attentional networks are dysregulated in IBS patients.
To determine how having a chronic abdominal pain condition such as IBS impacts general attentional processing and brain function16,23, we administered the attention network test (ANT) to IBS patients and healthy controls (HCs) during fMRI to examine differences in functioning of these networks (alerting, orienting, executive control). The ANT is a modified flanker task developed by Fan and colleagues23 that tests the efficiency of functioning of each of the three core attentional networks23. The administration of the ANT in other clinical populations, including fibromyalgia, has revealed disease-specific alterations in functioning of attention networks that support alerting, orienting, and executive control33–37.
Studying general attentional processes in IBS patients compared to HCs can facilitate our understanding of the behavioral and functional brain changes associated with chronic visceral pain conditions, such as IBS38–40. We hypothesized that 1) the orienting and alerting functions of attention as measured by both behavioral performance (accuracy and reaction times) and brain activity during the ANT is heightened in IBS patients compared to HCs, and that 2) IBS patients demonstrate less efficient executive control functioning. In addition to the primary aim, we sought to evaluate whether altered attentional network functioning was associated with IBS symptom expression and related clinical constructs such as intolerance of uncertainty, pain catastrophizing, and persistent worry about GI symptoms.
Material and Methods
Participants
Our sample consisted of 29 right-handed females, 14 HCs [mean age (SD) = 31(10.91), range 20 to 49 yrs], and 15 patients with IBS [mean age (SD) = 31(11.96), range 20 to 55 yrs]. Participants were recruited from multiple clinical sites, all part of the clinical research network of the Oppenheimer Center for Neurobiology of Stress, at the University of California, Los Angeles (UCLA), and through community advertisements. Upon arrival to the clinic, each participant read and signed informed consent and underwent a history and physical exam. Diagnosis of IBS was made by a gastroenterologist experienced in the diagnosis of functional bowel disease using Rome III criteria1. IBS patients with all types of predominant bowel habits (i.e., constipation = IBS-C, diarrhea = IBS-D, mixed = IBS-M, unspecified) were included in this study. All participants were administered the Hospital Anxiety and Depression Scales41 (HADS) to screen for the presence of mood disorders. Participants were excluded from this study if they reported 1) a serious medical condition with the exception of IBS diagnosis for the patient group, 2) were currently taking any medications with CNS effects or 3) had a positive symptom score (> 11) on either the anxiety or depression subscale of the HADS. All procedures were approved by the UCLA Medical Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Power analysis
We selected our sample size for this study based on the desire to detect a large effect size difference in the fMRI region of interest analysis, which is a linear contrast analysis based on estimates from a general linear model. Using G*Power v 3.1.542–43, a priori power analysis was specified along with a one-tailed independent t-test model, an alpha of .05, a Cohen’s d = .95, and desired power set at 80% with an allocation ratio of 1. Analysis indicated that 15 subjects per group were required to detect an effect size difference as small as .95 (actual power = .81). Imaging data from one subject was lost due to imaging artifacts. Post-hoc power analysis in G*power confirmed that with a sample size of with 15 IBS and 14 HCs we had greater than adequate power (greater than 80%) to detect an effect size difference > Cohen’s d = .95. For the two-tailed tests of behavioral and clinical parameters we had adequate power to detect an effect size difference greater than d = 1.08 in this same sample.
Self-report measures
All participants were a given a series of self-administered questionnaires to complete, including the Visceral Sensitivity Index (VSI)44, the Perceived Stress Questionnaire (PSQ)45, the harm avoidance subscale of the revised Temperament and Character Inventory (TCI-R)45, the Patient Health Questionnaire (PHQ-15)47, the HADS41 and the State-Trait Anxiety Inventory (STAI)48. The HADS was used to measure state anxiety and depression whereas the STAI was used to assess state and trait anxiety. GI-specific anxiety as measured by the VSI includes hypervigilance to, and fear, worry, and avoidance of, GI sensations and contexts. The VSI consists of 15-items that reliably assess GI symptom-specific anxiety related to pain, diarrhea, constipation, bloating, and a sense of urgency in the belly or lower abdomen44. The items are general enough to be applicable to IBS and non-IBS samples. The PSQ is a brief, 30-item, validated questionnaire designed to assess perceived stress over the past month45. The PHQ-15 consists of 15 somatic symptoms taken from the original PHQ47, including 14 of the 15 most prevalent DSM-IV somatization disorder somatic symptoms. In IBS only, overall GI symptom severity and abdominal pain were assessed using a 21-point Numerical Rating Scale (ranging from 0 – 20, with 0 representing no pain and 20, representing the most intense symptoms imaginable). Usual symptom severity was assessed on an ordinal scale where 1 = None, 2 = Mild, 3 = Moderate, 4 = Severe, and 5 = Very Severe.
Experimental paradigm
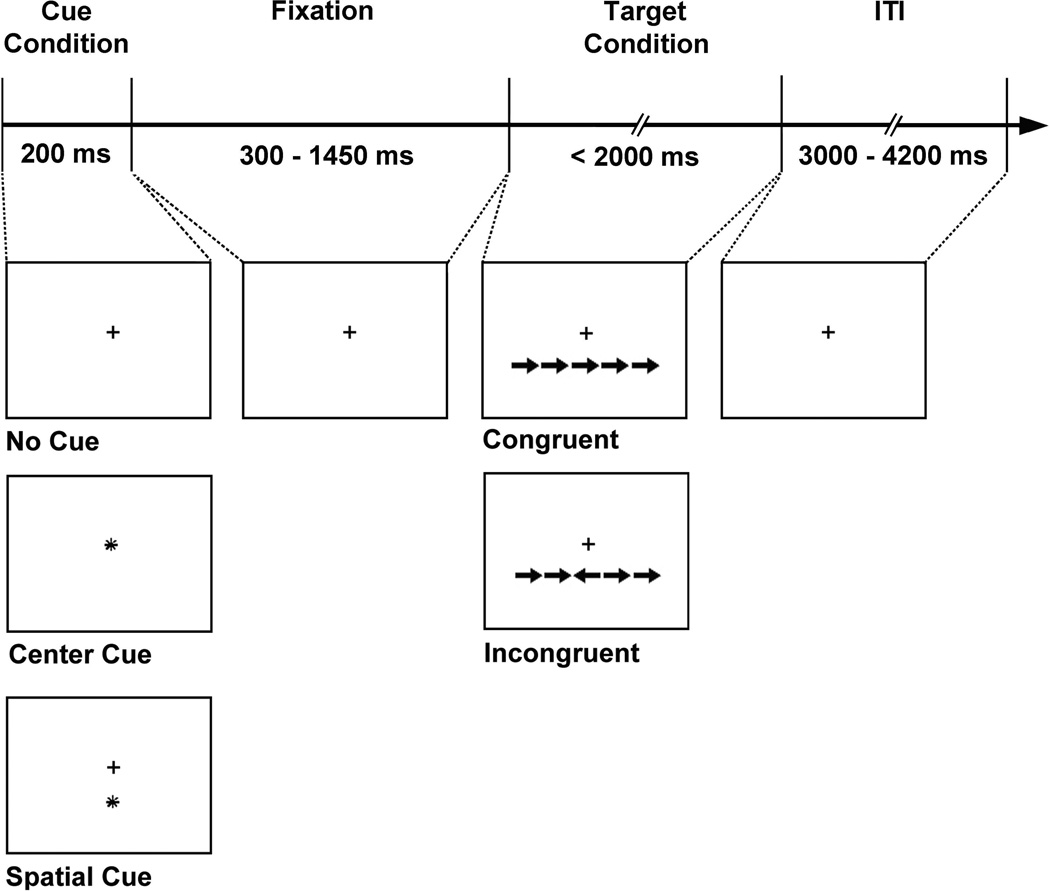
The ANT (Fig. 1) was applied to assess the efficiency of specific brain networks involved in alerting, orienting, and executive control of attention16,19. Each participant was fitted with a pair of goggles (VisuaStim Digital, Resonance Technology, Inc.) that displayed the visual task stimuli, which were delivered digitally via a laptop computer (Windows XP SP2) running E-Prime 2.0 software (Psychology Software Tools, Pittsburg, PA). The experimental design, stimulus parameters, and task stimuli were adapted from Fan et al. (2005). To assess alerting and orienting efficiency, there were three conditions, a no cue condition which served as a baseline and a center cue or a spatial cue condition which prompted the participant with regard to timing and spatial location of the target (Fig. 1). To assess executive control efficiency, there were two conditions, a congruent target condition wherein the center arrow pointed in the same direction as flanking arrows or an incongruent target condition in which the center arrow pointed in the opposite direction of the flankers (Fig. 1). For the three cue conditions, stimuli were displayed for 200 ms followed by presentation of the target and flanking arrows, with timings between the cue and target stimulus jittered for a variable duration (300–1450 ms; mean interstimulus interval = 550ms). Each target was displayed for up to 2000 ms and was followed by a jittered intertrial interval ranging from 3000 to 4200 ms (mean ITI = 3300ms). Participants were instructed to respond by pressing a button on a fiber optic MRI-compatible response box (Current Design, Philadelphia, PA) with their index finger (for left direction) or middle finger (for right direction) if the center target arrow pointed to the left or to the right, respectively. Participants were told to respond as quickly and accurately as possible following the presentation of each target arrow. Responses and reaction times (RT) were recorded on the presentation computer running E-Prime in the scanner console room. Prior to the start of the experiment, a short practice session consisting of six trials was given to familiarize each participant with the response box and the ANT task.
Figure 1.
The schematic summarizes the Attention Network Test (ANT; Fan et al., 2005). The ANT consisted of two target conditions (congruent and incongruent) and three cue conditions (no cue, center cue, and spatial cue). For each trial, a row of five arrows was presented either above or below a central fixation cross, which always remained on the screen. To assess alerting and orienting efficiency, there were three conditions, a no cue condition which served as a baseline and a center cue or a spatial cue condition which prompted the participant with regard to timing and spatial location of the target, respectively. To assess executive control efficiency, there were two target conditions, a congruent target condition wherein the center arrow was pointing in the same direction as flanking arrows or an incongruent target condition in which the center arrow pointed in the opposite direction of the flankers. For the cue conditions, stimuli were displayed for 200 ms followed by presentation of the target and flanking arrows, with timings between the cue and target stimulus jittered for a variable duration. Each target was displayed for up to 2000 ms and was followed by a jittered intertrial interval (ITI). Participants were instructed to respond as quickly and accurately as possible if the center target arrow pointed to the left or to the right using a response button box.
Efficiency scores for each attentional network, including alerting, orienting, and executive control were calculated using the same procedures reported by Fan et al. (2005). Briefly, efficiency of the alerting network was computed by subtracting RT for the center cue condition from RT scores for the no cue condition (Alerting efficiency = RTno cue – RTcenter cue). Orienting network efficiency was calculated by subtracting RT scores for the spatial cue condition from RT for the center cue condition (Orienting efficiency = RTcenter cue – RTspatial cue). The efficiency of the executive control network was quantified by subtracting RT scores for the congruent trials from RT scores for incongruent trials (Executive control efficiency = RTincongruent – RTcongruent).
An event-related fMRI design was used to study brain activation of the attentional networks during the ANT. For the experiment, there were a total of six BOLD runs with 114 trials in each run (38 trials per cue condition per run; 19 congruent and 19 incongruent target conditions), with each run lasting about 8 minutes. As described above, intervals between cues and target and target and the next trial were jittered in time. The order of the trials was counterbalanced. In addition to brain activity, mean RT during the three cue (no cue, center cue, spatial cue) and two target conditions (e.g., congruent, incongruent) were subtracted to yield behavioral measures of alertness, orienting and executive control16.
fMRI acquisition and image processing
All brain imaging was conducted with a Siemens 3T Trio MRI scanner equipped with a 12-channel head coil at Staglin IMHRO Center for Cognitive Neuroscience. For each subject, six functional BOLD runs were acquired during the ANT (echo-planar T2-weighted gradient-echo, TR = 2000 ms, TE = 28 ms, flip angle = 77°, matrix size 64 × 64, 40 axial slices, FOV = 220 mm; 4-mm thick, skip 1-mm), each lasting approximately 8 min. A total of 232 BOLD volumes were collected during each functional run and the first two images of each run were discarded to account for instability of signal in these early scans. A high-resolution T1-weighted MP-RAGE MRI was acquired to locate gross anatomical abnormalities (TR = 20 ms, TE = 3 ms, flip angle = 25°, FOV = 256 mm, slice thickness = 1 mm).
All imaging analyses and summaries were generated using Statistical Parametric Mapping version 8 (SPM8; Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) and Statistical Package for the Social Sciences (SPSS) software (version 17). Images were converted from DICOM into NIFTI format, adjusted for slice timing, and realigned to control for superfluous motion. The motion correction parameters in each degree were examined for excessive motion. No volume-to-volume motion correction parameter was above 2 mm translation or 2° rotation. The average of all realigned fMRI images for each subject was co-registered with the participant’s high-resolution T1-weighted MP-RAGE image, and then transformed into standard Montreal Neurological Institute (MNI) stereotactic coordinates (resolution = 2 mm isotropic) and smoothed with an 8 mm isotropic Gaussian kernel.
Data Analysis
Analysis of fMRI data
Identical statistical procedures for fMRI analysis previously described by Fan et al. (2005) were employed. We applied the general linear model (GLM) in SPM8 to preprocessed data to test the hypotheses regarding group differences in brain activity during alerting, orienting, and executive control conditions. Regressors in the subject level model were created by convolving a train of dirac delta functions representing individual trial events (e.g., fixation with no cue, central cue, spatial cue, targets with congruent flankers, and targets with incongruent flankers) with a hemodynamic response function comprising two gamma functions and their derivatives. We also included motion realignment parameters as covariates. Group level analyses were also performed using the GLM specifying contrast images represent alerting, orienting, and executive control as dependent variables and group as an independent regressor. ROI analyses were performed using small volume correction in SPM8 that controls for the effective number of voxels with an ROI. We set a stringent cluster-defining threshold of p = 0.001 and cluster level significance was set at p < 0.05 corrected for multiple comparisons using family wise error (FWE) rate49. We examined a priori ROIs based on hypothesized interaction of attention and central pain circuits in IBS patients. Our ROIs included the anterior insula (aINS), posterior insula (pINS), anterior midcingulate (aMCC), pregenual ACC, medial (Brodmann Area; BA 9/10/46), superior (BA 4/6/8) and inferior frontal gyrus (BA 11/47). In addition, to look at reliability and validity of the task we examined ROIs reported by Fan and colleagues16, including the thalamus, superior (BA 22) and inferior (BA 40) temporal gyri, superior parietal gyrus (BA 7), fusiform gyrus (BA 37), cerebellar vermis, supplementary motor area (SMA; BA6/32), and pre- (BA 4/6) and postcentral (BA 2) gyri. All ROIs were generated using the automated anatomical labeling (AAL) atlas and the SPM8 Wake Forest University PickAtlas extension toolbox. Global conjunction analysis was also performed to validate the ANT and highlight similarities in attentional network functioning across groups (IBS + HCs), thresholding the images at p < 0.05 uncorrected.
Mean comparisons of behavioral data
The sample size (14 HCs, 15 IBS patients) only provided adequate power for detection of a large effect sizes (d = 1.10), thus our behavioral analyses are underpowered in terms of finding small effect size differences. To avoid Type I and Type II errors in assessing group differences in self-report measures of affect and cognitive functioning, and network efficiency, we emphasize estimation of effect sizes and precision or certainty of the estimates, rather than significance testing50–53. However, we also performed significant testing using independent samples t-tests to evaluate group differences in self-report measures and reaction times, and to provide p values for readers who are accustomed to seeing them, but as mentioned previously we interpret effect sizes, rather than p values. Effect size difference were calculated using Hedges' g, which adjusts the pooled standard deviation for sample size54. Effect sizes reflect differences between groups in units of standard deviations. Historically, an effect size of 0.80 is interpreted as large (14% explained variance), 0.50 as medium (6% variance) and 0.20 as small (1% of the variance explained)55. In addition, we calculated 95% confidence intervals to quantify the precision of the estimated effect sizes and provide an estimated range of the true population parameter. The correct interpretation of a confidence interval is that if we were to repeat the experiment 100 times, 95% of the time the true estimate would lie within the computed interval. Although confidence intervals that do not contain zeroes suggest significance56, our focus for the analysis of the behavioral performance data was on discovery and hypothesis generation. As a reporting threshold, we considered a medium effect size difference, Hedge’s g ≥ .30, valuable evidence to report.
Correlation al analysis
Pearson’s R was applied to test the hypothesis that attentional network efficiency scores and brain activity in regions showing group differences during the ANT were correlated with self-report measures of affect, stress and cognitive functioning. Specifically, we calculated the correlation between brain activity and 1) fear of uncertainty (TCI) and pain catastrophizing (PCS) and 2) persistent worry about GI symptoms (VSI), across all subjects. Furthermore, we examined the association between brain activity and overall severity and chronicity in IBS subjects only. Regional brain activity (as represented by the first eigenvariate of the peak voxel in the cluster) showing group differences were extracted for analysis in SPSS. We only report medium-sized effects (r = .30, r2 = .09) or greater55. Again, we emphasize the magnitude of effect rather than significance testing to minimize the potential for Type I and II errors. However, we also performed significance testing with Pearson’s R bivariate correlational analyses for those readers interested in seeing these results.
Results
Self-report measures and clinical characteristics
Table 1 shows the descriptive and inferential statistics for demographic and self-report measures in both IBS patients and HCs. Patients (IBS-C = 7, IBS-D = 4, IBS-M = 1, unspecified = 3) reported an average duration of IBS symptoms extending over the past 10.4 years (SD = 6.2). Mean (SD) patient ratings for overall GI symptom severity for the past week was 9.33 (4.7) and ratings for abdominal pain was 8.87 (4.47). Patients reported their usual symptom severity as moderate, 3.07 (0.70).
Table 1.
Demographic and self-report measures.
| IBS (n = 15) | HCs (n = 14) | T value |
P value |
Hedge’s g |
Confidence Intervals |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower | Upper | ||||
| Age | 31.90 | 10.70 | 32.00 | 10.80 | 0.038 | 0.970 | −0.08 | −0.79 | 0.62 |
| HADS (anxiety) | 5.33 | 3.94 | 3.07 | 2.87 | −1.76 | 0.090 | 0.65 | −0.07 | 1.38 |
| HADS (depression) | 2.07 | 2.12 | 1.50 | 1.95 | −0.73 | 0.470 | 0.28 | −0.43 | 0.99 |
| PHQ-no IBS (somatic) | 5.23 | 2.74 | 2.80 | 2.30 | −2.26 | 0.035 | 0.96 | 0.21 | 1.70 |
| VSI | 31.93 | 14.52 | 1.36 | 2.85 | −7.73 | <0.001 | 2.87 | 1.85 | 3.89 |
| PSQ | 0.30 | 0.17 | 0.19 | 0.11 | −1.90 | 0.069 | 0.76 | 0.03 | 1.49 |
| Fear of uncertaintya | 4.04 | 1.32 | 2.86 | 1.75 | −2.07 | 0.048 | 0.77 | 0.04 | 1.50 |
| PCS | 13.93 | 7.67 | 5.47 | 1.46 | −4.07 | <0.001 | 1.51 | 0.70 | 2.31 |
| STAI-state | 46.87 | 5.71 | 44.93 | 7.86 | −0.76 | 0.452 | 0.28 | −.042 | 0.99 |
| STAI-trait | 48.20 | 6.93 | 42.36 | 9.40 | −1.91 | 0.066 | 0.71 | −0.02 | 1.44 |
Fear of uncertainty from the Revised Temperament Characteristics Inventory (TCI-R).
Abbreviations: IBS = irritable bowel syndrome; HCs = healthy controls; HADS = Hospital Anxiety and Depression Scale; PHQ = Patient Health Questionnaire; VSI = Visceral Sensitivity Index; PSQ = Perceived Stress Questionnaire; PCS = Pain Catastrophizing Scale; STAI = State-Trait Anxiety Inventory; SD = standard deviation. Effect size (g) reflects differences between groups in units of standard deviations. An effect size of 0.80 is interpreted as large (14% explained variance), 0.50 as medium (6% variance) and 0.20 as small (1% of the variance explained)54. An effect size difference of g ≥ .30 is considered evidence of an effect.
Comparison of network efficiency scores between IBS and HCs
Scores indexing the efficiency of the alerting, orienting, and executive control networks within groups can be seen in Table 2. IBS had greater efficiency in the alerting network than HCs as evidenced by a medium effect size difference, g = −0.34 (−1.04, 0.38). Evidence supporting more efficient orienting network functioning in IBS compared to HCs was also observed, g = −0.91(−1.62,−0.14). . However, no behavioral evidence for group differences in executive control network functioning was found, g = −0.20 (−0.87, 0.52).
Table 2.
The efficiency scores for the alerting, orienting, and executive control networks in IBS patients and HCs.
| Alerting | Orienting | Executive Control | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| IBS | 21.61 | 14.01 | 59.99 | 29.32 | 85.47 | 30.39 |
| HCs | 28.93 | 27.49 | 85.45 | 28.77 | 90.59 | 25.89 |
Abbreviations: IBS = irritable bowel syndrome; HCs = healthy controls; SD = standard deviation.
Correlates of network efficiency scores
Across all subjects, fear of uncertainty was negatively associated with the behavioral efficiency scores for the orienting network, r(29)= −.30. Furthermore, scores on the VSI were negatively correlated with the efficiency scores for the alerting network, r(29) = −.31. We found no evidence for correlations between network efficiency scores and depression, state and trait anxiety, or perceived stress measures.
For IBS patients, greater efficiency in alerting was associated with greater abdominal pain in the past week, [r(15) = −.53, p = 0.042)], and greater overall usual symptom severity, [r(15)= −.67, p = 0.006)]. There was also evidence for an association between executive control efficiency scores and greater overall usual symptom severity [r(15)= −.51, p = 0.054]. In addition, when restricting the correlational analysis between GI-specific anxiety and alerting efficiency to just IBS, an even greater negative magnitude of association was observed, r(15) = −.64.
Group differences in brain activity during alerting, orienting and executive control
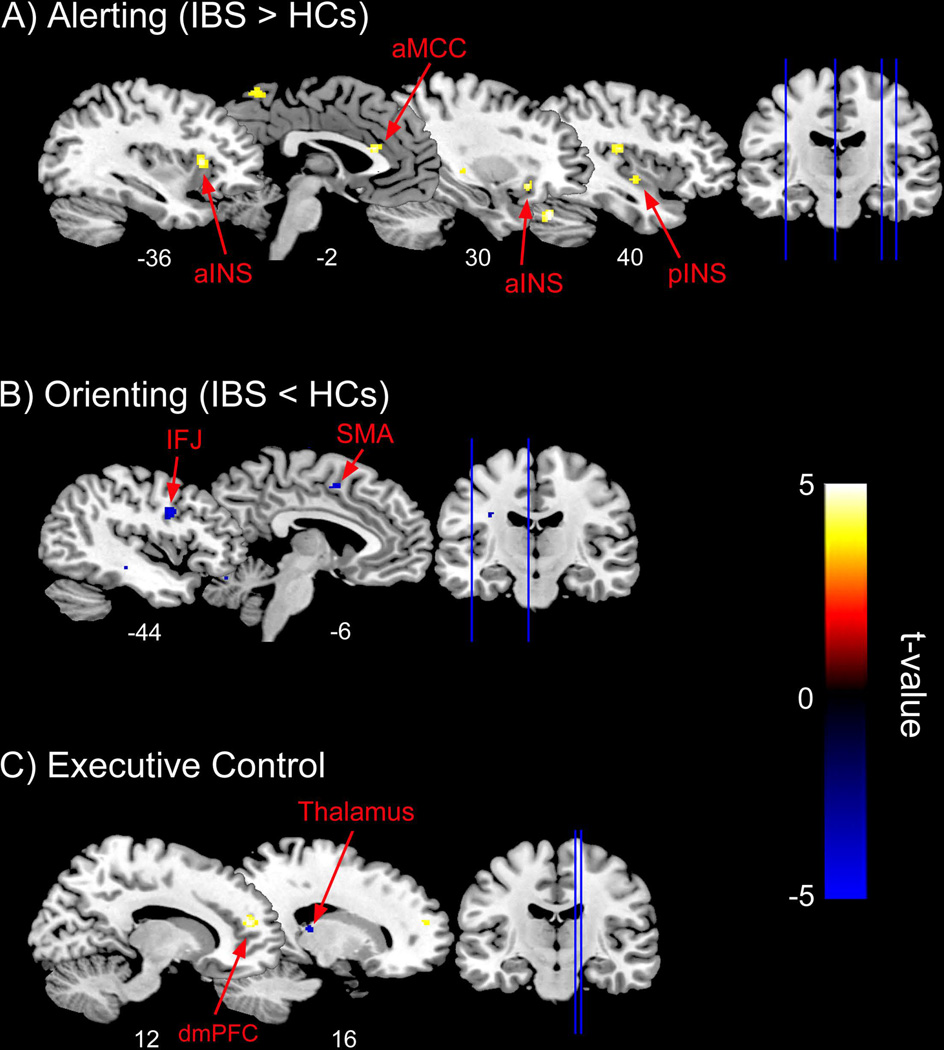
IBS patients showed significantly greater activations compared to HCs during alerting in the L aMCC, bilateral aINS, and R pINS (Table 3 and Figure 2A). For orienting, IBS patients showed significant deactivations compared to HCs in the left precentral gyrus. This deactivation is spatially consistent with a part of the inferior frontal junction (IFJ), which is located between the inferior frontal sulcus and inferior precentral sulcus, designated as BA 6, 9 and 4457. In addition, patients showed significant suppression of activity in L superior frontal gyrus, corresponding to the SMA (BA 6/32)(Table 3 and Figure 2B). No other significant group differences were found. For the executive control task, IBS patients relative to HCs showed significant deactivation in the R thalamus and activation in the R dorsal medial prefrontal cortex (dmPFC), corresponding to the pre-SMA (Table 3 and Figure 2C).
Table 3.
Brain regions showing group differences for the alerting, orienting, and executive control networks in IBS patients versus HCs.
| Region | Side | Cluster P (FWE) |
Cluster extent k |
Peak Z |
MNI coordinates |
|||
|---|---|---|---|---|---|---|---|---|
| x | Y | z | ||||||
| Alerting | ||||||||
| IBS > HCs | aMCC | L | 0.006 | 55 | 4.23 | −2 | 26 | 18 |
| aINS | L | 0.012 | 39 | 4.00 | −36 | 18 | 6 | |
| aINS | R | 0.037 | 17 | 3.87 | 30 | 22 | −10 | |
| pINS | R | 0.031 | 18 | 3.65 | 40 | −8 | −6 | |
| Orienting | ||||||||
| HCs > IBS | IFJ | L | 0.037 | 37 | 4.06 | −44 | 4 | 32 |
| SMA | L | 0.026 | 37 | 3.71 | −8 | 8 | 52 | |
| Executive Control | ||||||||
| HCs > IBS | Thalamus | R | 0.037 | 12 | 3.62 | 16 | −26 | 12 |
| IBS > HCs | dmPFC | R | 0.017 | 58 | 4.32 | 12 | 48 | 16 |
Abbreviations: IBS = irritable bowel syndrome; HCs = healthy controls; L = left; R = right; aMCC = anterior midcingulate; aINS = anterior insula; pINS = posterior insula; IFJ = inferior frontal junction; SMA = supplementary motor area; dmPFC = dorsal medial prefrontal cortex; FWE = family-wise error; MNI = Montreal Neurological Institute. Group level analyses were performed using the general linear model specifying contrast images representing alerting, orienting, and executive control as dependent variables and group as a factor. Region of interest analyses were performed using small volume correction in SPM8, which controls for the effective number of voxels with a region of interest. We set a stringent cluster-defining threshold of p = 0.001 and cluster level significance was set at p < 0.05 corrected for multiple comparisons using family wise error rate.
Figure 2.
Statistical T maps for the ROI analyses for the altering, orienting, and executive control conditions of the Attention Network Test (ANT) overlaid onto the ch2better brain template in MRIcron. (A) For the alerting condition, IBS patients showed greater activation (warm voxels) in the left anterior midcingulate (aMCC), bilateral anterior insulae (aINS), and the right posterior insula (pINS) compared to healthy controls (HCs). (B) For the orienting condition, patients showed greater suppression (cool voxels) in the left inferior frontal junction (IFJ) and supplementary motor area (SMA), whereas for the (C) executive control portion of the ANT, patients relative to HCs showed greater activation (warm voxels) and suppression (cool voxels) in the dorsal medial prefrontal cortex (dmPFC) and posterior thalamus, respectively.
Relationship between brain activity and cognitive measures
Table 4 displays the results for the exploratory correlational analyses conducted for regions that showed group differences in brain activity during the ANT task. Across both groups, GI-specific anxiety as measured by the VSI showed large effect size correlations with brain activity in all regions showing group differences across tasks. Pain catastrophizing showed medium correlations across most regional activity. Fear of uncertainty from the harm avoidance subscale of the TCI-R showed medium effect size associations with aINS activity during alerting, and thalamic and dmPFC activity during executive functioning. Within the IBS group, activity in the aMCC during alerting was negatively correlated with duration of GI-symptoms, [r(14) = −0.69, p = 0.007], and showed a moderate effect size correlation with overall symptom severity [r(15) = 0.45, p = 0.09].
Table 4.
Correlation between cognitive measures and activity in brain regions showing group differences during alerting, orienting, and executive control.
| Alerting | Orienting | Executive Control | ||||||
|---|---|---|---|---|---|---|---|---|
| aMCC | L aINS | R aINS | pINS | IFJ | SMA | THAL | dmPFC | |
| r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | |
| Fear of uncertaintya |
.39(.40) | −.40(.030) | .39(.036) | |||||
| VSI | .62(<.001) | .53(.003) | .63(<.001) | .50(.006) | −.54(.002) | −.41(.02) | −.53(.003) | .58(.001) |
| PCS | .32(.1) | .47(.009) | −.41(.028) | −.40(.02) | −.42(.020) | .55(.002) | ||
Fear of uncertainty from the Revised Temperament Characteristics Inventory (TCI-R).
Abbreviations: L = left; R = right; aMCC = anterior midcingulate; aINS = anterior insula; pINS = posterior insula; IFJ = inferior frontal junction; SMA = supplementary motor area; THAL = thalamus; dmPFC = dorsal medial prefrontal cortex; VSI = visceral sensitivity index; PCS = pain catastrophizing scale.
Conjunction analysis
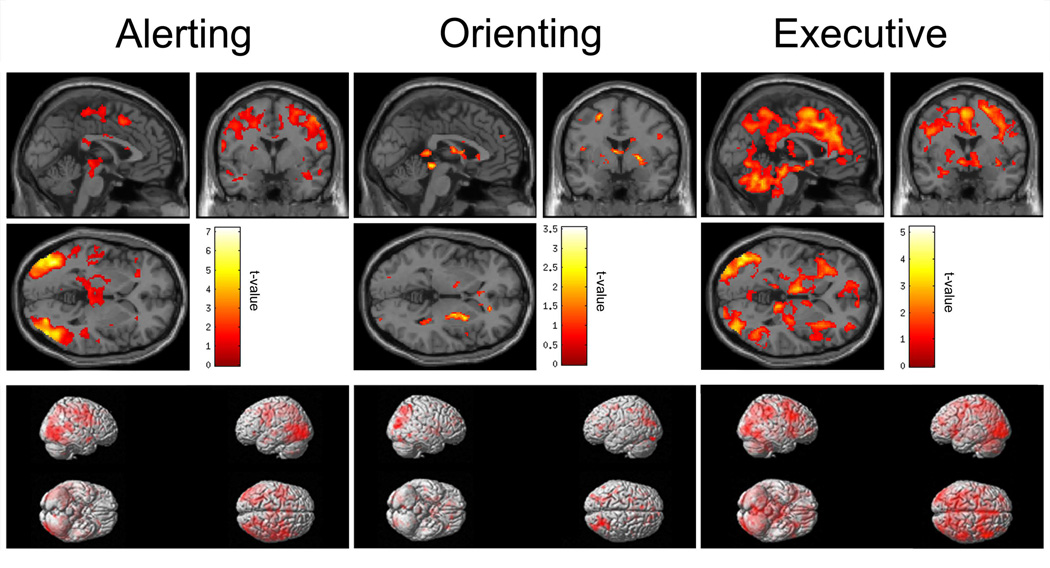
Results from the conjunction analysis for the alerting, orienting and executive control networks are displayed in Figure 3. Findings from our conjunction analysis for the alerting network are largely consistent with results reported in the literature16,58. Most notably, we found activations in the bilateral fusiform and inferior parietal gyri, as well as in the premotor cortex and SMA. However, unlike previous findings reporting left hemispheric lateralization using this task, activations for the majority of identified areas encompassed both hemispheres. Moreover, activation in the thalamus, although not to the extent previously reported by Fan and colleagues16, was also observed. For the orienting network, conjunction analysis showed that IBS and HCs combined had activations in the inferior parietal cortex. For the executive control network, patients and HCs showed activations in the aMCC and thalamus, also consistent with findings from Fan et al., 2005. In addition, widespread activity throughout the posterior parietal and inferior temporal cortices was observed, as well as in the cerebellum.
Figure 3.
Whole-brain conjunction maps displaying activations in IBS patients and healthy controls combined for the alerting (left panel), orienting (middle panel), and executive control (right panel) conditions of the Attention Network Test rendered onto MNI single-subject T1 brain template (top panels) and inflated brain (bottom panels) in SPM8.
Discussion
The aim of this study was to test the hypothesis that the core attentional networks (alerting, orienting, and executive control) were dysregulated in IBS patients compared to HCs. Behaviorally, IBS patients showed enhanced performance for both alerting and orienting, and this appeared to be reflected by differences in activity in known cognitive control and pain-related areas. Enhanced performance on the alerting task was related to symptom severity measures, suggesting hypervigilance to behaviorally relevant stimuli might be related to pain amplification in these patients. In contrast, we saw small effect size differences during the executive control portion of the ANT. Thus, while IBS patients appear to differ in alerting and orienting aspects of attention, from a behavioral standpoint, executive control functions appear to be less affected, at least as measured by the ANT.
Group differences in brain activation during alerting
We found that IBS patients, relative to HCs, showed greater activations in L aMCC, bilateral aINS, and R pINS during the alerting condition, which was positively associated with task performance. Alerting is defined as the process of achieving and maintaining a state of high sensitivity to incoming stimuli that are behaviorally relevant to an organism17. The alerting network is comprised of regions within the frontal and posterior parietal cortices, and subcortical structures such as the thalamus. The aMCC and aINS are thought to play an important role in processing the affective components of pain, as well as attentional and cognitive control functions, including monitoring and resolving conflict and the detection of salient stimuli59–61. In addition, the aMCC along with the aINS have been proposed to belong to the salience network62, and together these areas have been shown to be activated during cognitively demanding tasks involving inhibitory control and the ability to attend, quickly disengage, and re-engage in response to salient, novel stimuli63–64. Our findings of greater activity within the aINS and aMCC during the alerting task in IBS patients, and associated increased behavioral performance, may indicate heightened engagement of the salience network, leading to greater task efficiency in this group. This supposition is further supported by our behavioral results which demonstrated that increased task performance in IBS patients was positively associated with abdominal pain over the last week, usual symptom severity, and the VSI, which measures hypervigilance to IBS symptoms and the contexts in which they occur. Across patients and HCs, our exploratory analysis revealed medium to large effect size correlations between increased activity in aMCC and aINS regions, and pain catastrophizing and GI-related symptom measures in patients. Fear of uncertainty was also related to activation of the R aINS. These findings are intriguing and seem to suggest that pain amplification may be related to a general hyperresponsivity in IBS. Previous studies have reported enhanced pre-attentive processing in IBS patients65–66. Whether this enhancement in alerting functions observed here results from up-regulation of the arousal system or via diminished modulatory control mechanisms, and/or is a cause or consequence of IBS symptom chronification, remains an area for future study.
Another important finding was that IBS patients showed greater activation in the R pINS during alerting compared to HCs. The pINS is involved in homeostatic and sensory-discriminative components of pain processing via its reciprocal connections to the secondary somatosensory cortex and afferent projections from the ventroposterior lateral thalamus67. Our finding of greater pINS activity during alerting is in agreement with previous results from functional and morphometric imaging studies demonstrating increased pINS activity during rectal distension and increased gray matter in this region in female IBS patients compared to HCs68–69. Moreover, exploratory analysis showed a positive correlation between pINS activity and the visceral sensitivity measure, indicating that pINS activation may be related to an enhanced vigilance toward disease-related bodily sensations in these patients.
Group differences in brain activation during orienting
Another novel finding was that IBS patients showed reduced activity within areas involved in error processing and response inhibition, such as the L SMA and the L IFJ, compared to HCs during the orienting task57,70. Both areas are considered part of the ventral frontoparietal attention network57,71–72 which serves to detect, engage, and direct attention toward behaviorally relevant sensory stimuli while simultaneously ignoring irrelevant, competing stimuli. In addition, IBS patients also showed greater efficiency (RT) during the orienting task relative to HCs. These findings are, in part, in line with previous imaging studies demonstrating suppression of activity within the ventral attention network as cognitive demands of a task increase, perhaps due to sensory gating or filtering of irrelevant cues to prevent inappropriate behavioral responses73–74. Furthermore, our findings of decreased activity in the SMA during orienting and increased activation of the pINS during alerting in patients parallel findings reported by Aizawa et al. 2012 showing reductions in effective connectivity between the SMA and dorsolateral prefrontal cortex and increased pINS activation in IBS patients during set-shifting in response to error feedback76.
Group differences in brain activation during conflict monitoring
While we expected IBS patients to be behaviorally less efficient at the executive control task than HCs, this was not the case. However, we did observe group differences in brain function, with IBS patients showing greater deactivation in the R posterior thalamus along with greater R dmPFC activity. The spatial location of the thalamic cluster is consistent with the pulvinar, which has repeatedly been linked to the control of attention and pre-attentive visual processing16,77–78. The dmPFC (i.e., pre-SMA), like the aMCC, has also been implicated in the regulation of attentional control and conflict resolution79,80. Taken together, our finding of decreased activation in the thalamus and increased activation in the dmPFC in patients may reflect disruptions in executive network functioning related to enhanced attentional focus toward ongoing pain and IBS related symptoms. Although we saw no group differences in behavioral performance during the executive control task, we did observe a trend for a negative association between executive control efficiency scores and usual symptom severity in patients; greater efficiency was related to decreases in GI symptom severity. Additionally, we also observed medium to large effect size associations between thalamic and dmPFC activity and GI symptom severity scores with IBS, as well as cognitive and affective measures across groups, including pain catastrophizing and fear of uncertainty. The mPFC has been linked to fear of uncertainty81, the belief that uncertainty is negative and should be avoided82. More studies are needed to parse out the contribution of pain and related symptoms on attention in IBS patients, and the extent to which these factors may impact the functioning of the executive control network.
Limitations
Given the nature of the small to moderate effect size differences observed for reaction times during alerting and orienting tasks, these behavioral abnormalities need to be validated in a larger sample with greater power to detect differences. The stimuli used in the present study were not specific to IBS symptomology and therefore the findings point to alterations in global attention-related functioning. These findings have clear implications for understanding IBS, however, further work with more IBS relevant stimuli would be important to expand these results. Furthermore, we cannot rule out the possibility that higher levels of anxiety in IBS patients did not influence our findings. Lastly, the present study was also cross-sectional and therefore unable to directly address whether the findings observed here represent a vulnerability factor for development of IBS symptoms or a response to chronic pain and discomfort. Longitudinal studies will be necessary to address these questions.
Conclusion
When viewed together with previous behavioral reports on altered attentional processes and attentional network functioning in IBS patients14,83, the current results suggests that IBS patients may have specific abnormalities in attentional processing, perhaps due to lack of suppression via top-down inhibitory control mechanisms and/or up-regulation in brainstem arousal systems, such as those involving the locus coeruleus. These deficits may underlie the higher levels of hypervigilance (i.e., symptom specific anxiety) and pain catastrophizing seen in IBS, resulting in enhanced alerting and orienting network functioning. Interestingly, the brain regions demonstrating functional differences during this global attention task overlap with those showing altered functioning in IBS compared to HCs during supraliminal rectal distention15. These results provide further evidence that alterations in central cognitive control processes are an important component to chronic visceral pain. The clinical correlates of these altered attentional processes include fear of uncertainty, pain catastrophizing, and persistent worry about GI symptoms. Given the brain’s known plasticity, heightened attentional system functioning in IBS may be reversed by therapeutic interventions such as cognitive behavioral therapy, or at the very least, lead to improvements in cognitive and visceral pain-related symptoms frequently reported in these patients.
Key Messages.
We examined the behavioral and functional brain correlates of attentional network functioning in IBS patients.
We used the attention network test (ANT) during functional MRI (fMRI) in 15 IBS patients and 14 healthy controls to determine if core attentional networks were altered in patients.
Females with IBS showed increased efficiency in the orienting and alerting functions of attention, which was associated with differences in brain activity in known cognitive control areas.
Clinical correlates of attention included fear of uncertainty, pain catastrophizing, and worry about GI symptoms.
Our findings demonstrate that compared to controls, female IBS patients show altered attentional network functioning which was associated with measures of symptom-related anxiety, hypervigilance, and visceral sensitivity.
Acknowledgments
The authors would like to thank the Staglin IMHRO Center for Cognitive Neuroscience for their expert technical assistance.
Funding
This research was supported by grants from the National Institutes of Health including K08 DK071616 (JSL), R03 DK 084169, R01 DK48351 (EAM), P50 DK064539 CORE-Neuroimaging & Psychophysiology (EAM), CORE- Administrative (EAM), R24 AT002681(EAM), P30 DK041301 CORE-Administrative Core & Enrichment, R01 AT007137 (KT), K23 DK073451(KT), and T32 DK0718034 (CSH).
Footnotes
Author Contribution
- Funding (JSL, EAM, CSH)
- Study Conceptualization and Design (JSL, EAM, KT, CSH)
- Data Acquisition (ZJ, BE, BS, SS, NH, CSH)
- Data Analysis and Interpretation (JSL, CSH, EAM, KT, BN)
- Manuscript preparation and Critical Revisions (CSH, JSL, EAM, BN, KT)
Disclosures
Competing Interests: the authors have no competing interests.
Reference List
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 3.Kellow JE, Eckersley CM, Jones MP. Enhanced perception of physiological intestinal motility in the irritable bowel syndrome. Gastroenterology. 1991;101:1621–1627. doi: 10.1016/0016-5085(91)90400-f. [DOI] [PubMed] [Google Scholar]
- 4.Naliboff BD, Chang L, Munakata J, Mayer EA. Towards an integrative model of irritable bowel syndrome. Prog Brain Res. 2000;122:413–423. doi: 10.1016/s0079-6123(08)62154-8. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263–1271. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am J Med. 1999;107:41S–50S. doi: 10.1016/s0002-9343(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 7.van Tilburg MA, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74:486–492. doi: 10.1016/j.jpsychores.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzal M, Potokar JP, Probert CS, Munafo MR. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med. 2006;68:758–761. doi: 10.1097/01.psy.0000232270.78071.28. [DOI] [PubMed] [Google Scholar]
- 9.Chapman S, Martin M. Attention to pain words in irritable bowel syndrome: increased orienting and speeded engagement. Br J Health Psychol. 2011;16:47–60. doi: 10.1348/135910710X505887. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs-Gallagher N, Palsson OS, Levy RL, Meyer K, Drossman DA, Whitehead WE. Selective recall of gastrointestinal-sensation words: evidence for a cognitive-behavioral contribution to irritable bowel syndrome. Am J Gastroenterol. 2001;96:1133–1138. doi: 10.1111/j.1572-0241.2001.03759.x. [DOI] [PubMed] [Google Scholar]
- 11.Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: Evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res. 2014;77:13–19. doi: 10.1016/j.jpsychores.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Posserud I, Svedlund J, Wallin J, Simren M. Hypervigilance in irritable bowel syndrome compared with organic gastrointestinal disease. J Psychosom Res. 2009;66:399–405. doi: 10.1016/j.jpsychores.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. 2014 doi: 10.1016/j.clinre.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 18.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. J Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudsen E. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- 21.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 22.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 24.Mackie MA, Van Dam NT, Fan J. Cognitive control and attentional functions. Brain Cogn. 2013;82:301–12. doi: 10.1016/j.bandc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 26.Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122:1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 27.Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain. Pain. 1989;39:345–352. doi: 10.1016/0304-3959(89)90048-1. [DOI] [PubMed] [Google Scholar]
- 28.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 29.Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Locke GR, III, Weaver AL, Melton LJ, III, Talley NJ. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350–357. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller AR, North CS, Clouse RE, Wetzel RD, Spitznagel EL, Alpers DH. The association of irritable bowel syndrome and somatization disorder. Ann Clin Psychiatry. 2001;13:25–30. doi: 10.1023/a:1009060731057. [DOI] [PubMed] [Google Scholar]
- 32.Tillisch K, Mayer EA. Pain perception in irritable bowel syndrome. CNS Spectr. 2005;10:877–882. doi: 10.1017/s1092852900019830. [DOI] [PubMed] [Google Scholar]
- 33.Miró E, Lupiáñez J, Hita E, Martínez MP, Sanchez AI, Buela-Casal G. Attentional deficits in fibromyalgia and its relationships with pain, emotional distress and sleep dysfunction complaints. Psycholo Health. 2011;26:765–80. doi: 10.1080/08870446.2010.493611. [DOI] [PubMed] [Google Scholar]
- 34.Togo F, Lange G, Natelson BH, Quigley KS. Attention network test: Assessment of cognitive function in chronic fatigue syndrome. J Neuropsychol. 2015;9:1–9. doi: 10.1111/jnp.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21:275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- 36.Adolfsdottir S, Sorensen L, Lundervold AJ. The attention network test: a characteristic pattern of deficits in children with ADHD. Behav Brain Funct. 2008;4:9. doi: 10.1186/1744-9081-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan J, Bernardi S, Van Dam NT, Anagnostou E, Gu X, Martin L, Park Y, Liu X, Kolevzon A, Soorya L, Grodberg D, Hollander E, Hof PR. Functional deficits of the attentional networks in autism. Brain Behav. 2012;2:647–660. doi: 10.1002/brb3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rhematoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47:639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 39.Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995;33:391–405. doi: 10.1016/0005-7967(94)00057-q. [DOI] [PubMed] [Google Scholar]
- 40.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 41.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 42.Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 43.Faul F, Erdfelder E, Buchner A, Lang A. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 44.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 45.Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Luzi C, Andreoli A. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19–32. doi: 10.1016/0022-3999(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 46.Cloninger CR. The Temperament and Character Inventory--Revised. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1999. [Google Scholar]
- 47.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Spielberger CD, Gorsuch RL, Lushene RE. STAI: Manual for the State–Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 49.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothman KJ. Curbing type I and type II errors. Eur J Epidemiol. 2010;25:223–4. doi: 10.1007/s10654-010-9437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang JM, Rothman KJ, Cann CI. That confounded P-value. Epidemiol. 1998;9:7–8. doi: 10.1097/00001648-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Stang A, Poole C, Kuss O. Th ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–30. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 53.Cohen J. Things I have learned (so far) American Psychologist. 45:1304–1312. [Google Scholar]
- 54.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. (6 ed.) 1981:107–128. [Google Scholar]
- 55.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 2014. [Google Scholar]
- 56.Cumming G, Finch S. Inference by eye: confidence intervals and how to read pictures of data. Am Psychol. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- 57.Brass M, Derrfuss J, Forstmann B, Von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Coull JT, Nobre AC, Frith CD. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- 59.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 60.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 61.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menon V, Uddin L. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ham T, Leff A, de BX, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013;33:7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berman SM, Naliboff BD, Chang L, Fitzgerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97:2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- 66.Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, Naliboff BD. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biol Psychol. 2010;84:272–278. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin. 2012;42:299–313. doi: 10.1016/j.neucli.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, Pearlson G, Kiehl KA. A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013;256:529–536. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 72.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- 75.Vossel S, Weidner R, Fink GR. Dynamic coding of events within the inferior frontal gyrus in a probabilistic selective attention task. J Cogn Neurosci. 2011;23:414–424. doi: 10.1162/jocn.2010.21441. [DOI] [PubMed] [Google Scholar]
- 76.Aizawa E, Sat Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, Kanazawa M, Shima K, Mushiake H, Hongo M, Fukudo S. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on fMRI and dynamic causal modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 77.Arend I, Machado L, Ward R, McGrath M, Ro T, Rafal RD. The role of the human pulvinar in visual attention and action: evidence from temporal-order judgment, saccade decision, and antisaccade tasks. Prog Brain Res. 2008;171:475–83. doi: 10.1016/S0079-6123(08)00669-9. [DOI] [PubMed] [Google Scholar]
- 78.Marzinik F, Wahl M, Gerd-Helge Schneider, Kupsch A, Curio G, Klostermann F. The human thalamus is crucially involved in executive control operations. J Cog Neurosci. 2008;20:1903–1914. doi: 10.1162/jocn.2008.20124. [DOI] [PubMed] [Google Scholar]
- 79.Aarts E, Roelofs A, van TM. Attentional control of task and response in lateral and medial frontal cortex: brain activity and reaction time distributions. Neuropsychologia. 2009;47:2089–2099. doi: 10.1016/j.neuropsychologia.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 80.Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- 81.Buhr K, Dugas MJ. The Intolerance of Uncertainty Scale: psychometric properties of the English version. Behav Res Ther. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 82.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]