Abstract
Hepatitis B virus (HBV) infections are a global health problem afflicting approximately 360 million patients. Of these individuals, 15–20 million are co-infected with hepatitis delta virus (HDV). Progress towards curative therapies has been impeded by the highly restricted host tropism of HBV, which is limited to productive infections in humans and chimpanzees. Here, we will discuss different approaches that have been taken to study HBV and HDV infections in vivo. The development of transgenic and humanized mice has lead to deeper insights into HBV pathogenesis. An improved understanding of the determinants governing HBV and HDV species tropism will aid the construction of a small animal model with inheritable susceptible to HBV/HDV.
Keywords: hepatitis B virus, hepatitis delta virus, animal models, host tropism
Introduction
Hepatitis B virus (HBV) is a global health problem affecting an estimated 360 million chronic carriers. Persistent infection frequently results in liver disease, including fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Liver pathogenesis is accelerated and exacerbated in the approximately 15–20 million individuals co-infected with HBV and hepatitis delta virus (HDV).
A prophylactic vaccine is available and anti-HBV drugs can efficiently suppress the virus. However, these drug regimens rarely eradicate the HBV to completely cure the patient. Therefore, chronically infected patients with an active disease are usually treated for life with anti-viral agents, resulting in significant morbidity and costs, not to mention the risk of emerging mutants and viral re-activation. Currently, there are no treatments that directly act on HDV. Rather, the use of IFN is the standard of care. However, since HDV requires HBV for producing infectious virions, it is thought that eliminating HBV would also cure HDV [1,2].
Our understanding of the intricate interplay between HBV/HDV and the human host as well as the development of new curative therapeutics have been impeded by the lack of a small animal model that is genetically tractable, immunocompetent, mimics the disease phenotypes caused by HBV and HDV, and is conducive to high-throughput use. Here, we discuss existing models that have been used to study HBV and HDV in vivo. Furthermore, we highlight blocks in the HBV and HDV lifecycles in non-permissive species, such as rodents, that potentially need to be overcome in order to create models with inheritable susceptibility to infection.
The host range of HBV and HDV
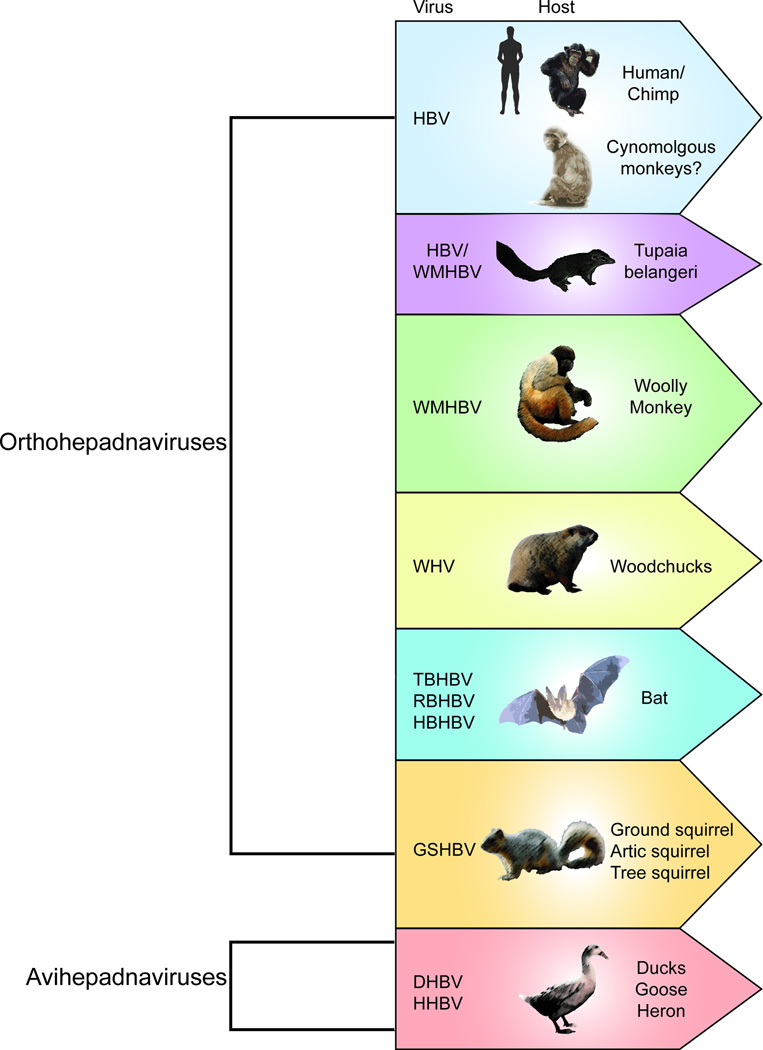
HBV belongs to the Hepadnaviridae family, which is divided into two genera, orthohepadnaviruses and avihepadnaviruses, infecting mammals and birds, respectively. HBV appears to robustly infect only higher primates, specifically humans and chimpanzees. Undoubtedly, the chimpanzee model has played a critical role in the characterization of HBV, defining the natural course of infection, including the host immune response and consequences of persistent infection, such as liver inflammation [3]. HDV infection in chimpanzees has been studied by using the envelope proteins from HBV. Chronic infection occurs with liver inflammation, cirrhosis, fibrosis, and HCC development [4–6]. However, studies in chimpanzees are hampered by their limited availability, high costs, and ethical concerns, which have led to a ban on the use of these animals for biomedical research in most countries, making the development of suitable alternatives critical. Due to these limitations and the lack of adequate cell culture systems, viruses genetically related to HBV have been widely used as alternatives (Figure 1).
Figure 1. Host range of Hepatitis B virus (HBV) and related surrogate hepadnaviruses.
Woodchuck hepatitis virus (WHV) was first identified in a colony of woodchucks at the Penrose Zoo in Philadelphia, where animals presented with liver disease including cirrhosis, fibrosis, and HCC, which is reminiscent of the disease progression observed with HBV in humans [7]. HDV virions pseudotyped with WHV envelope proteins can infect woodchuck hepatocytes, causing liver disease and HCC [8]. Additionally, woodchucks have been used for testing the ability of nucleoside analogs (NA) to suppress viremia and to investigate how mutations in the reverse transcriptase (RT) domain of the viral polymerase can lead to NA resistance [9,10]. However, woodchucks are limited in their use as a model organism because of their genetic diversity as an outbred species, and the scarcity of reagents to monitor their immune response to infection.
Duck hepatitis B virus (DHBV), another related hepadnavirus, has been instrumental in deciphering the mechanism of HBV replication and the formation of covalently closed circular DNA (cccDNA), the stable template for all HBV transcripts. In a series of seminal papers, the study of DHBV has provided the basis of understanding: (i) the synthesis of both the (+) and (−) strands of relaxed circular DNA (rcDNA); (ii) the RT activity of the viral polymerase and its covalent attachment to the (−) strand of rcDNA in the cytosol up until the viral genome separates from the polymerase and moves into the host nucleus; (iii) the mechanism by which the pool of cccDNA increases in the host nucleus [11–16]. However, a major drawback with DHBV is that the pathology is not the same as that observed with HBV in humans.
Several other hepadnaviruses have been identified, such as ground squirrel hepatitis B virus (GSHBV), heron hepatitis B virus (HHBV) and woolly monkey hepatitis B virus (WMHBV). Each has a limited tropism and has not been extensively used as a surrogate model [17].
Viruses resembling orthohepadnaviruses in sequence and genome structure were recently identified in bats [18]. While these viruses cannot infect primary human hepatocytes (PHH) or Tupaia belangeri primary hepatocytes (TPH), HDV particles pseudotyped with tent bat HBV (TBHBV) can. Further investigation of these viruses’ tropism and pathogenicity is required to determine if bat HBVs can cause chronic infection.
A few studies suggest that HBV may have other zoonotic reservoirs. It was previously reported that HBV could be transmitted to Macaca mulatta, resulting in viremia, and that HBV could be passaged into naïve monkeys [19]. However, these results could not be reproduced. HBV infection had not been observed in any small Old World monkeys until the recent isolation of a hepadnavirus from the livers of M. fascicularis from Mauritius Island [20]. The isolated M. fascicularis HBV was most similar to HBV genotype D ayw3. Chronic HBV infections exist naturally in the M. fascicularis population on the Mauritius Island and have also developed in a related species, M. sylvanus, when experimentally challenged. However, further studies need to be conducted in order to understand the tropism and pathogenesis of these newly found hepadnavirus [21].
The study of related hepadnaviruses has provided great insight into hepadnavirus life cycle and pathogenesis. However, there are significant sequence differences between human HBV and related hepadnaviruses. For example, WHV has 70% nucleotide identity with HBV, while DHBV has only 40% identity [17]. This makes the potential usage of these surrogates for drug testing problematic as therapeutics are often highly virus-specific.
Host adaptation: xenotransplantation models
Human liver chimeric mice
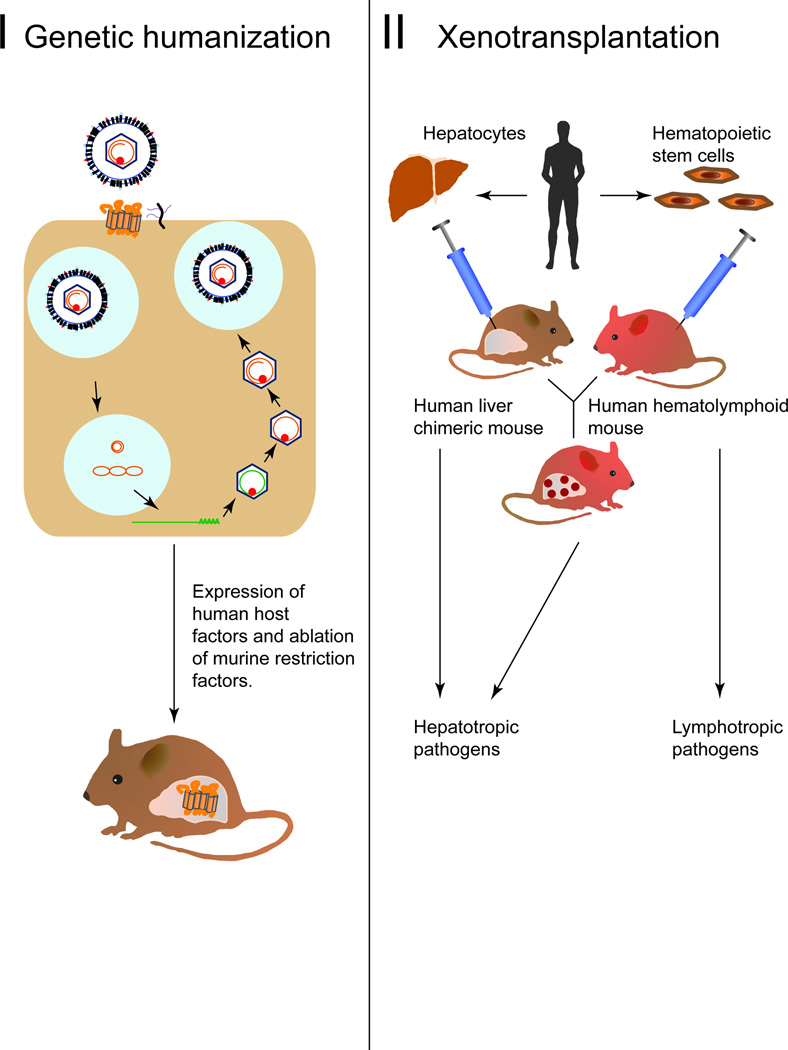
As an alternative to using surrogate viruses, progress has been made in adapting the murine environment to support the replication of human HBV (Figure 2). Chimeric mice harboring human tissues permissive to HBV and HDV infection can be generated by engrafting human hepatocytes into suitable murine xenorecipients strains. The most commonly utilized and best characterized xenotransplantation models for HBV and HDV are human liver chimeric mice. To facilitate engraftment of human hepatocytes, xenorecipient strains must be immunodeficient to avoid graft rejection, and liver injury must be inducible by selective ablation of mouse hepatocytes in order for the promotion of human hepatocyte proliferation. Robust engraftment of human hepatocytes has been shown in a number of immunodeficient liver injury models. These include fumaryl acetoacetate hydrolase (FAH) deficient mice [22], mice harboring transgenes that are directly hepatotoxic, specifically the urokinase type plasminogen activator driven by an albumin (Alb-uPA) [23] or major urinary protein promoter (MUP-uPA) [24], or transgenes whose hepatotoxic effect can be induced, such as an FK506 binding protein caspase 8 fusion protein (AFC8) [25] or herpes simplex thymidine kinase (HSK-TK) [26].
Figure 2. Host adaptation approaches to render mice permissive to HBV and HDV infections.
I. Genetic humanization of the mouse through knowledge of what host factors are necessary for supporting the HBV life-cycle. II. Creation of a human immune system engrafted, human liver chimeric and dual engrafted mice for the investigation of lymphotropic and hepatotropic pathogens respectively.
The resultant human liver chimeric mice are currently the only small animal model that supports the entire viral life cycle, as they are susceptible to HBV and HBV/HDV co-infection [22,23]. HBV cccDNA and all viral intermediates have been detected in infected hepatocytes [23]. These models have also provided a platform to test the effect of anti-HBV therapies [27]. HDV was found to infect and persist in engrafted human hepatocytes for up to six weeks, with HDV viremia only occurring upon super-infection with HBV [28]. In addition, the above mouse models have been used to investigate the metabolic and toxicological response of the donor hepatocytes to drug compounds (reviewed in [29]).
While robust human hepatic chimerism can routinely be achieved with adult hepatocytes, this limits the analysis of host responses to often randomly selected donor lots. To minimize inter-experimental variability in infection due to differences in hepatocyte donor genotypes and to create a renewable source of human donor hepatocytes, stem cell-derived hepatocyte-like cells (HLCs) have been pursued as a possible solution [30,31]. HLCs do not engraft efficiently in most xenorecipient models possibly due to their incomplete differentiation phenotype. However, recently it was shown that transplantation of HLCs into immunodeficient MUP-uPA mice yielded a sufficiently high hepatic chimerism to support hepatitis C virus infection [32]. While these results require independent confirmation, this system holds promise for systematically analyzing the impact of host polymorphisms on HBV and HDV infections in mice engrafted with patient-specific hepatocytes.
Dual engraftment of human hepatocytes and a human immune system (HIS) in mice
The usually highly immunocompromised status of human liver chimeric mice currently precludes mechanistic analysis of interactions of HBV and/or HDV with the human immune system. To overcome this, protocols have been established to co-engraft human liver cells and components of a human immune system in a single murine xenorecipient. While donor matching would be desirable, this can currently only be achieved logistically with hematopoietic stem cells (HSCs) and hepatoblasts derived from the same fetal donor. Injection of HSCs gives rise to multilineage engraftment with human immune cells. However, similar to HLCs, fetal hepatoblasts engraft poorly in the commonly used Alb-uPA mice [33]. Engraftment efficiency appears to be strain-dependent as some studies report more robust engraftment with these fetal progenitor cells in AFC8 mice [25]. However, similar to liver transplantation in humans, close donor matching may not be imperative as it was recently shown that extensive humanization of both the liver and the immune system can been achieved through the use of allogeneic adult hepatocytes and HSCs without any overt rejection [34,35]. Dually engrafted mice mounted virus-specific immune responses following HBV infection, resulting in human-specific liver fibrosis [36]. While this first report established an important proof-of-concept for the approach, independent validations and further refinements of xenorecipient and humanization protocols are necessary. Importantly, since human immune responses are generally weak in HSC-transplanted mice, further modifications will be needed to improve both the cellular complexity and functionality of engrafted HIS mice (reviewed in [37]). Such dually engrafted humanized mice may also be suitable for dissecting the mechanisms of exacerbated viral hepatitis during HBV and HDV co-infections.
The HBV life-cycle is blocked at multiple steps in rodent cells
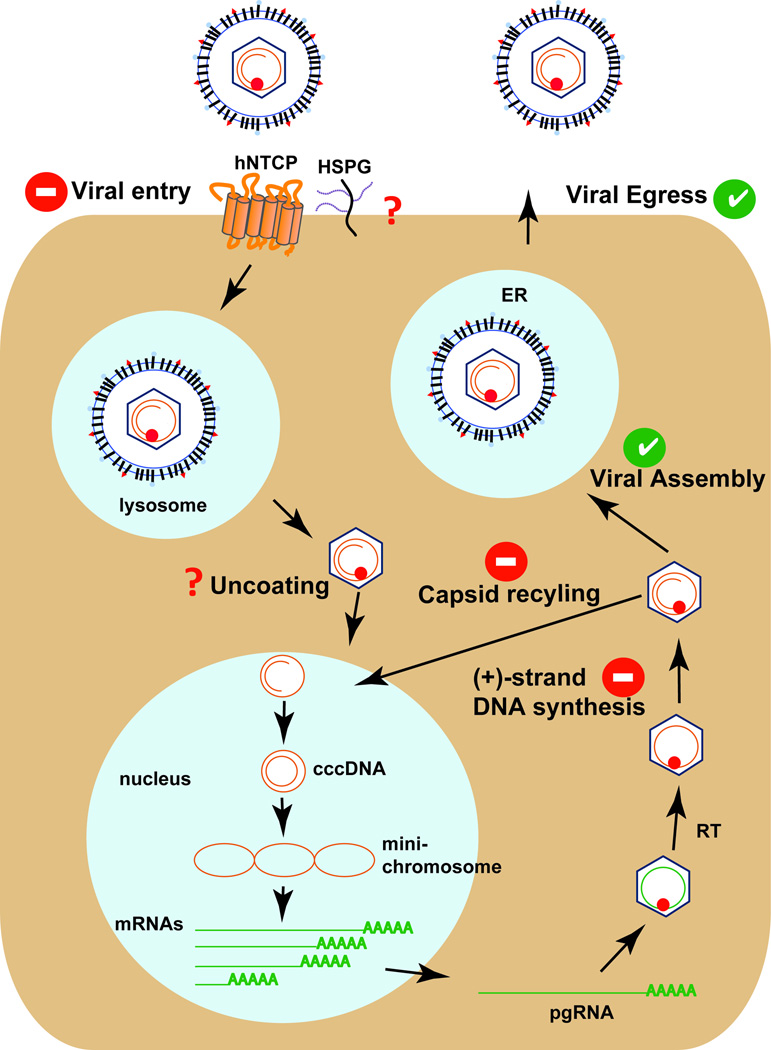
An animal model with inheritable susceptibility to HBV would overcome the technical and logistical difficulties of xenotransplantation models. The challenge is to systematically identify and overcome restrictions to HBV infection in non-permissive species. Because of the vast number of experimental tools and ease with which genetic manipulations can be performed, mice may be a preferred target species. However, mice are not naturally susceptible to HBV infection and interspecies transmission is presumably blocked at multiple steps (Figure 3). The mechanism by which HBV enters hepatocytes is incompletely understood. Radiolabelled preS1 peptides, the part of the viral envelope that is critical for HBV uptake, but not control peptides are retained specifically in the livers of mice [38]. This demonstrates that attachment of HBV and HDV to hepatocytes, presumably via interaction with heparan sulfate proteoglycans, is support in mice. While HBV binding to hepatocytes in non-permissive species appears to occur, subsequent steps including binding of specific receptors, virion internalization, and membrane fusion, may all be blocked in mouse hepatocytes. Recently, human sodium taurocholate co-transporting polypeptide (NTCP or SLC10A1) was identified as a receptor for HBV and HDV [39,40]. Importantly, while expression of human NTCP in human hepatoma cell lines facilitates uptake of HBV and HDV, the murine NTCP orthologue does not. Residues critical for HBV and HDV uptake have been mapped and are not conserved in the murine NTCP orthologue [41]. This may partially explain the differences in the susceptibility of mice and humans. Furthermore, expression of human NTCP in murine cells only enables infection with HDV but is not sufficient for HBV uptake [41,42]. This argues for the existence of additional entry factors or post-entry blocks. Conceivably, additional human-specific host factors are needed or dominant negative murine factors need to be eliminated in order to establish HBV infection in murine hepatocytes.
Figure 3. The HBV life-cycle is blocked at several steps in murine cells.
Green checks represent those steps that are also supported in murine hepatocytes. Red question marks and red/white dashes indicate those steps that are most likely or are known not to be supported in murine hepatocytes.
Following productive entry into human cells, the HBV genome is uncoated and the relaxed circular genome (rcDNA) is inserted into the nucleus. Here, cccDNA, which serves as the transcriptional template for all four viral gene products, is formed through poorly defined mechanisms. There is little experimental evidence that these steps are supported in murine cells. In mice stably expressing the HBV genome, pre-genomic HBV RNAs are transcribed off the transgenic integrant, but cccDNA does not form [43,44]. Conceivably, human-specific factors may be missing or rodent-specific factors may limit formation and/or maintenance of cccDNA. However, the block does not seem to be absolute as it was shown that in HBV transgenic mice lacking hepatocyte nuclear factor (HNF) 1α, cccDNA becomes detectable [45]. The latter data are peculiar but require independent validation. Notably, later steps of the HBV life-cycle, including virion assembly and egress, are supported in mouse cells as experimental inoculation of serum from HBV transgenic mice causes persistent HBV infection in chimpanzees [46].
Short of having a mouse model with inheritable susceptibility to HBV, mice expressing a larger than genome size HBV transgene have contributed substantially to our understanding of HBV viral replication but have also provided insight into immunobiology and pathogenesis, specifically the role of cytotoxic T lymphocytes in viral pathogenesis [47]. In addition, HBV transgenic mice expressing subgenomic fragments have provided great insights into the contribution of HBV proteins to viral life-cycle and pathogenesis. For example, the role of the large (L) HBV envelope protein in the secretion of the small (S) envelope protein was ascertained by overexpressing L in mice, resulting in the retention of S in the ER and reduced secretion from hepatocytes [48,49]. Similarly, constitutive expression of the HBx protein in a CD1 transgenic mouse was shown to promote the development of HCC [50].
HDV genome propagation is closely tied to productive HBV replication, as HDV requires the HBsAg for packaging of infectious virions. Little is known about whether the HDV life cycle is fully supported in mice post-entry. What is known is that when HDV cDNA and RNA are injected hydrodynamically, a single round of replication and HDAg production are observed in murine hepatocytes [51]. HDV virions pseudotyped with WHV envelope proteins have also been used to infect CB17 and CB17/SCID mice [52]. It was observed that these infections led to an increase in HDV genomic RNA detection post-infection. Detection of the viral antigenomic RNA was also observed, indicating that the viral replication cycle is at least partially supported in murine cells. Additionally, HDAg was detected in hepatocytes throughout the murine liver. No viral spread was detected, and viral clearance occurred 10–20 days post-infection, indicating that helper virus super-infection was needed for viral persistence and spread. This is further corroborated by recent studies showing that delta antigen accumulates in human NTCP-expressing murine cell lines following HDV infection [41,42]. Transgenic mouse models for HDV RNA replication have also been created. HDAg has been expressed in several tissues, including skeletal muscle and hepatocytes, but no pathology was observed. This lends support to HDV being non-cytopathic [53].
Conclusions
To deepen the understanding of HBV and HDV biology and address the lack of a curative treatment for chronic hepatitis B, animal models are needed. This issue is particularly pressing in light of the highly restricted access to the chimpanzee model, which is the only non-human species readily permissive to HBV and HDV infections. To create more tractable animal models, a number of distinct but putatively complementary approaches likely need to be pursued, including a search for better surrogates as well as viral and host adaption approaches. Such a multipronged effort will likely produce a plethora of complementary models each with their own unique strengths and weaknesses. To ensure the relevance of emerging models, any development efforts need to demonstrate that new and refined models accurately reflect important hallmarks of HBV and HDV infection as observed in patients.
Highlights.
HBV and HDV have a narrow host range limited to humans and chimpanzees.
Human immune system and liver chimeric mice are powerful tools for studying the host response to HBV and HDV.
The HBV life-cycle is blocked at multiple steps in murine hepatocytes.
The discovery of human NTCP as a receptor may aid in the development of an inbred animal model with inheritable susceptibility for HBV and HDV.
Acknowledgements
We would like to thank Markus von Schaewen and Jenna Gaska for their helpful discussion and comments on drafts of this paper. Work in the laboratory is in part supported by grants from the National Institutes of Health (2 R01 AI079031-05A1, 1 R01 AI107301-01, 1 R56 AI106005-01), the Grand Challenge Program and Innovation Award of Princeton University. B.Y.W. is supported by co-funding from NIAID on iNRSA 5T32GM007388. We apologize to all colleagues whose work could not be cited due to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 3. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. Showcased the importance of the T cell response in viral clearence for HBV acute infections.
- 4.Rizzetto M, Canese MG, Gerin JL, London WT, Sly DL, Purcell RH. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J. Infect. Dis. 1980;141:590–602. doi: 10.1093/infdis/141.5.590. [DOI] [PubMed] [Google Scholar]
- 5.Fields HA, Govindarajan S, Margolis HS, Schable CD, Ebert JW, Maynard JE. Experimental transmission of the delta virus to a hepatitis B chronic carrier chimpanzee with the development of persistent delta carriage. Am J Pathol. 1986;122:308–314. [PMC free article] [PubMed] [Google Scholar]
- 6.Govindarajan S, Fields HA, Humphrey CD, Margolis HS. Pathologic and ultrastructural changes of acute and chronic delta hepatitis in an experimentally infected chimpanzee. Am J Pathol. 1986;122:315–322. [PMC free article] [PubMed] [Google Scholar]
- 7.Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponzetto A, Cote PJ, Popper H, Hoyer BH, London WT, Ford EC, Bonino F, Purcell RH, Gerin JL. Transmission of the hepatitis B virus-associated delta agent to the eastern woodchuck. Proc Natl Acad Sci U S A. 1984;81:2208–2212. doi: 10.1073/pnas.81.7.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob JR, Korba BE, Cote PJ, Toshkov I, Delaney WEt, Gerin JL, Tennant BC. Suppression of lamivudine-resistant B-domain mutants by adefovir dipivoxil in the woodchuck hepatitis virus model. Antiviral Res. 2004;63:115–121. doi: 10.1016/j.antiviral.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Korba BE, Cote P, Hornbuckle W, Tennant BC, Gerin JL. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology. 2000;31:1165–1175. doi: 10.1053/he.2000.5982. [DOI] [PubMed] [Google Scholar]
- 11.Molnar-Kimber KL, Summers J, Taylor JM, Mason WS. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983;45:165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molnar-Kimber KL, Summers JW, Mason WS. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984;51:181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers J, Mason WS. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 14.Summers J, Smith PM, Horwich AL. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 16.Wu TT, Coates L, Aldrich CE, Summers J, Mason WS. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 17.Kidd-Ljunggren K, Miyakawa Y, Kidd AH. Genetic variability in hepatitis B viruses. J Gen Virol. 2002;83:1267–1280. doi: 10.1099/0022-1317-83-6-1267. [DOI] [PubMed] [Google Scholar]
- 18. Drexler JF, Geipel A, Konig A, Corman VM, van Riel D, Leijten LM, Bremer CM, Rasche A, Cottontail VM, Maganga GD, et al. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc Natl Acad Sci U S A. 2013;110:16151–16156. doi: 10.1073/pnas.1308049110. Identified several hepadnaviruses in bats.
- 19.London WT, Alter HJ, Lander J, Purcell RH. Serial transmission in rhesus monkeys of an agent related to hepatitis-associated antigen. J Infect Dis. 1972;125:382–389. doi: 10.1093/infdis/125.4.382. [DOI] [PubMed] [Google Scholar]
- 20.Dupinay T, Gheit T, Roques P, Cova L, Chevallier-Queyron P, Tasahsu SI, Le Grand R, Simon F, Cordier G, Wakrim L, et al. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius Island. Hepatology. 2013;58:1610–1620. doi: 10.1002/hep.26428. [DOI] [PubMed] [Google Scholar]
- 21.Lucifora J, Vincent IE, Berthillon P, Dupinay T, Michelet M, Protzer U, Zoulim F, Durantel D, Trepo C, Chemin I. Hepatitis B virus replication in primary macaque hepatocytes: crossing the species barrier toward a new small primate model. Hepatology. 2010;51:1954–1960. doi: 10.1002/hep.23602. [DOI] [PubMed] [Google Scholar]
- 22.Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. First demonstration that human liver chimeric mice are susceptible to HBV.
- 24.Tesfaye A, Stift J, Maric D, Cui Q, Dienes HP, Feinstone SM. Chimeric mouse model for the infection of hepatitis B and C viruses. PLoS One. 2013;8:e77298. doi: 10.1371/journal.pone.0077298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosaka K, Hiraga N, Imamura M, Yoshimi S, Murakami E, Nakahara T, Honda Y, Ono A, Kawaoka T, Tsuge M, et al. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem Biophys Res Commun. 2013;441:230–235. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529–537. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giersch K, Helbig M, Volz T, Allweiss L, Mancke LV, Lohse AW, Polywka S, Pollok JM, Petersen J, Taylor J, et al. Persistent hepatitis D virus mono-infection in humanized mice is efficiently converted by hepatitis B virus to a productive co-infection. J Hepatol. 2014;60:538–544. doi: 10.1016/j.jhep.2013.11.010. First detailed characterization of HDV infection in human liver chimeric mice.
- 29.Strom SC, Davila J, Grompe M. Chimeric mice with humanized liver: tools for the study of drug metabolism, excretion, and toxicity. Methods Mol Biol. 2010;640:491–509. doi: 10.1007/978-1-60761-688-7_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 32.Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, Thorgeirsson SS, Feinstone SM, Liang TJ. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, Narain N, Bock M, Norder M, Legrand N, et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175:1483–1492. doi: 10.2353/ajpath.2009.090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson EM, Bial J, Tarlow B, Bial G, Jensen B, Greiner DL, Brehm MA, Grompe M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13:404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutti TL, Knibbe JS, Makarov E, Zhang J, Yannam GR, Gorantla S, Sun Y, Mercer DF, Suemizu H, Wisecarver JL, et al. Human hepatocytes and hematolymphoid dual reconstitution in treosulfan-conditioned uPA-NOG mice. Am J Pathol. 2014;184:101–109. doi: 10.1016/j.ajpath.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. doi: 10.1371/journal.ppat.1004032. First demonstration that mice dually engrafted with liver and components of a human immune system mount immune resonses to HBV infection
- 37.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schieck A, Schulze A, Gahler C, Muller T, Haberkorn U, Alexandrov A, Urban S, Mier W. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58:43–53. doi: 10.1002/hep.26211. Study shows that radiolabelled preS1 peptides are retained in mouse livers which suggests that binding of HBV and HDV is supported in murine hepatocytes.
- 39. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. Identified human NTCP as a receptor for HBV and HDV.
- 40. Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. Identified that human NTCP is the receptor that the HBV large evenlope protein binds to.
- 41. Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, Gao Z, Jing Z, Song M, Xu G, et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol. 2013;87:7977–7991. doi: 10.1128/JVI.03540-12. Showed that expression of human NTCP facilitates both HBV and HDV uptake when expressed in human cells but only HDV is taken up by murine cells expressing human NTCP.
- 42. Li H, Zhuang Q, Wang Y, Zhang T, Zhao J, Zhang Y, Zhang J, Lin Y, Yuan Q, Xia N, et al. HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell Mol Immunol. 2014;11:175–183. doi: 10.1038/cmi.2013.66. Expanded on the observation by Yan et al 2013 that murine cells expressing human NTCP were susceptible to HDV but not to HBV.
- 43.Araki K, Miyazaki J, Hino O, Tomita N, Chisaka O, Matsubara K, Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farza H, Hadchouel M, Scotto J, Tiollais P, Babinet C, Pourcel C. Replication and gene expression of hepatitis B virus in a transgenic mouse that contains the complete viral genome. J Virol. 1988;62:4144–4152. doi: 10.1128/jvi.62.11.4144-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raney AK, Eggers CM, Kline EF, Guidotti LG, Pontoglio M, Yaniv M, McLachlan A. Nuclear covalently closed circular viral genomic DNA in the liver of hepatocyte nuclear factor 1 alpha-null hepatitis B virus transgenic mice. J Virol. 2001;75:2900–2911. doi: 10.1128/JVI.75.6.2900-2911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117–1132. doi: 10.1016/s0002-9440(10)64980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chisari FV. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr. Top. Microbiol. Immunol. 1996;206:149–173. doi: 10.1007/978-3-642-85208-4_9. [DOI] [PubMed] [Google Scholar]
- 48.Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chisari FV, Filippi P, McLachlan A, Milich DR, Riggs M, Lee S, Palmiter RD, Pinkert CA, Brinster RL. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol. 1986;60:880–887. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 51.Chang J, Sigal LJ, Lerro A, Taylor J. Replication of the human hepatitis delta virus genome Is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J Virol. 2001;75:3469–3473. doi: 10.1128/JVI.75.7.3469-3473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Netter HJ, Kajino K, Taylor JM. Experimental transmission of human hepatitis delta virus to the laboratory mouse. J Virol. 1993;67:3357–3362. doi: 10.1128/jvi.67.6.3357-3362.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polo JM, Jeng KS, Lim B, Govindarajan S, Hofman F, Sangiorgi F, Lai MM. Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol. 1995;69:4880–4887. doi: 10.1128/jvi.69.8.4880-4887.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]