Abstract
Background and Purpose
The SAMMPRIS medical group had a much lower primary endpoint rate than predicted from the preceding WASID trial. This result has been attributed to the aggressive medical therapy used in SAMMPRIS, but an alternative hypothesis is that SAMMPRIS patients were lower risk. We undertook analyses to evaluate these competing hypotheses.
Methods
Using proportional hazards regression, we compared the SAMMPRIS primary endpoint between SAMMPRIS medical patients and WASID patients meeting the same qualifying criteria adjusted for confounding baseline characteristics.
Results
The unadjusted comparison of the SAMMPRIS primary endpoint showed a significantly higher risk for WASID patients (p=0.009, logrank test) with 12 month Kaplan-Meier estimates of 21.9% in WASID and 12.6% in SAMMPRIS and hazard ratio (HR) 1.9 (95%CI=1.2–3.0). The analyses identified the following confounding factors that varied between the studies and that conferred a higher risk: lack of statin use at enrollment (HR=1.8, 95%CI=1.1–2.9, p=0.027) that was more prevalent among WASID patients (39% vs 14%, p< 0.0001) and prior infarcts in the territory of the symptomatic vessel (HR=1.8, 95%CI=1.1–2.9, p=0.023) that was more prevalent among SAMMPRIS patients (34% vs 22%, p=0.015). The HR for WASID vs SAMMPRIS adjusted for these two characteristics was 1.9 (95%CI=1.1–3.2).
Conclusion
After adjustment for confounding baseline characteristics, WASID patients had an almost two-fold higher risk of the SAMMPRIS primary endpoint, which supports the hypothesis that the lower rate of the primary endpoint in the medical arm of SAMMPRIS compared with WASID patients was due to the aggressive medical management used in SAMMPRIS.
Clinical Trials Registration
http://clinicaltrials.gov. Unique Identifier: NCT00576693
Keywords: Intracranial atherosclerosis, Clinical trials, Dyslipidemia, Atherosclerosis
The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial demonstrated superiority of aggressive medical management over stenting1. Additionally, the rate of the primary endpoint in the medical group in SAMMPRIS was much lower than predicted from the preceding Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial. 2
Although the much lower primary event rate among the SAMMPRIS patients has been attributed to the benefits of aggressive medical management3, an alternative explanation is that the patients in SAMMPRIS were lower risk or different in other meaningful ways compared with patients in WASID. We explored this hypothesis by performing a statistical analysis comparing the SAMMPRIS primary endpoint between patients in the two studies in which we evaluated the impact of baseline characteristics on the differences in outcome.
Methods
Qualifying Criteria and Outcome
The qualifying criteria for the two studies have been published in detail previously2, 4. Since WASID enrolled patients with a broader range of stenosis (50–99% vs. 70–99% in SAMMPRIS) and a longer qualifying period from the last symptomatic stroke or TIA to enrollment (90 days vs. 30 days in SAMMPRIS), the entire population of WASID was not comparable to that in SAMMPRIS. Therefore, for this analysis, we compared all 227 patients randomized to the medical arm of SAMMPRIS with the 143 patients in WASID who met the following primary SAMMPRIS qualifying criteria: 70–99% stenosis determined by the local investigator, < 81 years old, absence of tandem stenoses, and ≤ 30 days from qualifying event to randomization. The outcome for this analysis was the SAMMPRIS primary endpoint: any stroke or death within 30 days after enrollment or ischemic stroke in the territory of the qualifying artery beyond 30 days of enrollment.
Statistical Methods
An unadjusted comparison of the SAMMPRIS primary endpoint between the two studies, was made by estimating the cumulative probability of the outcome versus time for each study using the Kaplan-Meier method with the resulting curves compared between the studies using the log-rank test. Although we included in the analysis only the WASID patients who met the primary eligibility criteria that differed between WASID and SAMMPRIS, there could have been differences between the two selected study populations in terms of the baseline characteristics that were related to the outcome. We employed the following process to identify and adjust the comparison of the studies for such confounding factors. Baseline characteristics were compared between the two studies using Fisher’s exact test for percentages, t-test for means, and the Wilcoxon rank-sum test for medians. Also, the relationship of each of the baseline characteristics (other than the study participated in) to the outcome was assessed using bivariate and multivariable proportional hazards regression in the set of patients formed by combining the two studies. We identified the baseline characteristics that were both significantly different between the studies and also significantly associated with the outcome. A proportional hazards regression model was fit that included terms for the baseline characteristics identified and also a term for the study that was participated in. The hazard ratio comparing the two studies was estimated from that model. To further adjust for any potential confounding factors, the baseline characteristics that were statistically different between the studies and not already in the model were added one at a time to the model and were retained in the model if the hazard ratio comparing the studies changed by more than 10% from the hazard ratio estimated without the newly added characteristic. If a characteristic to be added had missing data, then the hazard ratios to be compared were estimated from the same set of patients. All analyses were done using SAS 9.3. All reported p-values are two-sided and those < 0.05 are considered statistically significant.
Results
Unadjusted Comparison of Outcome between Studies
The Kaplan-Meier curves for the risk of the SAMMPRIS primary endpoint were significantly different between WASID and SAMMPRIS (Figure 1, Table 1, p = 0.009). WASID patients had greater risk of the outcome at 1, 2, and 3 years after enrollment (21.9%, 23.7%, 28.9%, respectively) compared to SAMMPRIS patients (12.6%, 14.1%, 14.9%, respectively). The unadjusted hazard ratio (WASID relative to SAMMPRIS) was 1.9 (95% CI = 1.2 – 3.0).
Figure 1.
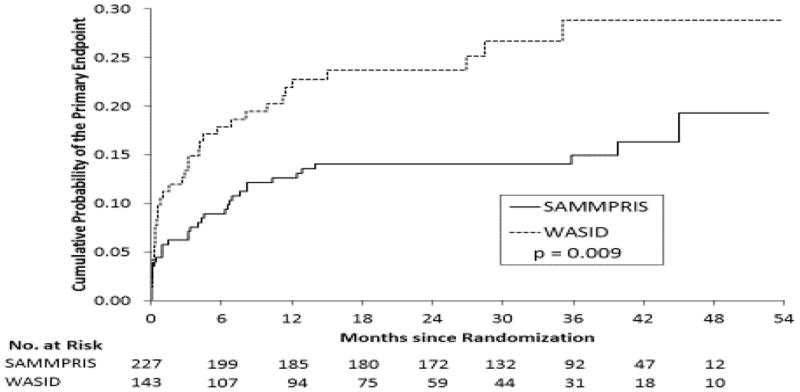
Kaplan-Meier estimates of the cumulative probability of the SAMMPRIS primary endpoint for SAMMPRIS medical patients and WASID patients meeting SAMMPRIS eligibility criteria.
Table 1.
Probability* of the SAMMPRIS Primary Endpoint For SAMMPRIS Medical Patients and SAMMPRIS-Eligible WASID Patients
| Months after Randomization | Study | |
|---|---|---|
| SAMMPRIS (n=227) Probability of Primary Endpoint (95% CI) |
WASID (n=143) Probability of Primary Endpoint (95% CI) |
|
| 1 | 5.8% (3.4% – 9.7%) | 10.5% (6.5% – 16.9%) |
| 6 | 8.9% (5.9% – 13.5%) | 17.9% (12.5% – 25.4%) |
| 12 | 12.6% (8.9% – 17.8%) | 21.9% (15.8% – 29.9%) |
| 24 | 14.1% (10.1% – 19.4%) | 23.7% (17.4% – 31.9%) |
| 36 | 14.9% (10.7% – 20.6%) | 28.9% (21.1% – 38.8%) |
| p-value† | 0.009 | |
Kaplan-Meier estimates of the cumulative probability of a primary endpoint at the specified months after randomization.
p-value for the log-rank test comparing the Kaplan-Meier curves of the two studies.
Comparison of Baseline Characteristics between Studies
Compared with WASID patients, SAMMPRIS patients were younger (mean age 59.5 years vs. 62.5 years; p=0.009) but had higher percentages of hypertension (89% vs. 76%; p=0.001), old infarcts in the territory of the symptomatic artery (34% vs 22%; p = 0.015), stenosis in the 80–99% range (55% vs. 35%; p=<0.0004), and anterior circulation stenosis (68% vs. 48%; p<0.0002) and a lower percentage of patients not taking a statin at enrollment (14% vs 39%; p < 0.0001).
SAMMPRIS patients had higher mean systolic blood pressure (SBP) (146.8 mmHg vs. 139.1; p= 0.0002), higher mean diastolic blood pressure (DBP) (82.3 vs 76.5; p < 0.0001), higher mean body mass index (30.7 kg/m2 vs. 29.4; p=0.048) but lower mean LDL (98 mg/dl vs. 125; p<0.0001) and HDL (39 mg/dl vs 44; p = 0.0004). There were no significant differences in the frequency of female gender, black race, stroke as the qualifying event, diabetes, smoking, physical activity, history of previous stroke, time from qualifying event to study entry, history of coronary artery disease, stroke scale scores or HgbA1c among patients with a history of diabetes (Table 2).
Table 2.
Baseline Characteristics for the SAMMPRIS Medical Group vs SAMMPRIS-Eligible WASID Patients*
| Characteristic | SAMMPRIS Medical Group (n=227) | WASID (n=143) | p-value† |
|---|---|---|---|
|
| |||
| Age (years) | 59.5 ± 11.8 | 62.5 ± 10.0 | 0.009 |
|
| |||
| Female Gender | 82 (36%) | 55 (38%) | 0.66 |
|
| |||
| Black Race | 49 (22%) | 37 (26%) | 0.38 |
|
| |||
| History of Hypertension | 203 (89%) | 109 (76%) | 0.001 |
|
| |||
| History of Diabetes | 97 (43%) | 62 (43%) | 0.91 |
|
| |||
| Previous or Current Smoker | 149 (66%) | 101 (71%) | 0.36 |
|
| |||
| History of Coronary Artery Disease | 59 (26%) | 41 (29%) | 0.63 |
|
| |||
| History of Ischemic Stroke (not the qualifying event) | 58 (26%) | 34 (24%) | 0.71 |
|
| |||
| Not Using Statin at Enrollment | 31 (14%) | 56 (39%) | < 0.0001 |
|
| |||
| Modified Rankin Grade ≥ 1 | 159 (70%) | 95 (66%) | 0.49 |
|
| |||
| NIH Stroke Scale > 1 | 88 (39%) | 50 (35%) | 0.51 |
|
| |||
| Physical Activity Out of Target‡ | 161 (71%) | 105 (73%) | 0.64 |
|
| |||
| Old Infarcts in the Territory of the Symptomatic Artery | 75/222 (34%) | 28/130 (22%) | 0.015 |
|
| |||
| Qualifying Event was a Stroke | 152 (67%) | 90 (63%) | 0.43 |
|
| |||
| On Antithrombotic Therapy at Qualifying Event | 140 (62%) | 75 (52%) | 0.084 |
|
| |||
| Time from Qualifying Event to Randomization (days) | 7 (4 – 19) | 8 (5 – 14) | 0.87 |
|
| |||
| Symptomatic Artery | < 0.0001 | ||
| Internal Carotid Artery | 49 (22%) | 26 (18%) | |
| Middle Cerebral Artery | 105 (46%) | 43 (30%) | |
| Vertebral Artery | 22 (10%) | 24 (17%) | |
| Basilar Artery | 51 (22%) | 38 (27%) | |
| Combination | 0 | 12 (8%) | |
|
| |||
| Symptomatic Artery in Posterior Circulation | 73 (32%) | 74 (52%) | 0.0002 |
|
| |||
| Percent Stenosis of Symptomatic Artery § | 81 ± 7 | 78 ± 7 | 0.0006 |
|
| |||
| Categories of Percent Stenosis of Symptomatic Artery § | 0.0004 | ||
| 70–79% | 102 (45%) | 94 (66%) | |
| 80–89% | 97 (43%) | 40 (28%) | |
| 90–99% | 28 (12%) | 9 (7%) | |
|
| |||
| Systolic BP (mmHg) | 146.8 ± 21.8 | 139.1 ± 17.8 | 0.0002 |
|
| |||
| Diastolic BP (mmHg) | 82.3 ± 12.0 | 76.5 ± 9.5 | < 0.0001 |
|
| |||
| BMI (kg/m2) | 30.7 ± 6.3 | (n=140) 29.4 ± 5.7 | 0.048 |
|
| |||
| LDL (mg/dl) | (n=226) 97.7 ± 36.6 | (n=120) 125.4 ± 39.7 | < 0.0001 |
|
| |||
| HDL (mg/dl) | (n=226) 38.8 ± 10.1 | (n=124) 43.8 ± 13.4 | 0.0004 |
|
| |||
| HgbA1c (%) (For patients with a history of diabetes) | (n=91) 7.6 (6.9 – 10.2) | (n=42) 8.0 (6.5 – 10.3) | 0.89 |
Values are mean ± standard deviation or number (%) or median (inter-quartile range).
Comparisons of the baseline characteristics of the two studies were made using either an independent groups t-test (for means), Fisher’s exact test (for percentages) or the Wilcoxon rank sum test (for medians).
Physical activity was measured in the two studies as follows: SAMMPRIS used the Physician-based Assessment and Counseling for Exercise (PACE) Current Physical Activity Status Score with a score of 1–3 considered in-target. WASID used four categories (Sedentary, Minimal, Moderate, Vigorous) with Moderate or Vigorous considered in-target. (see on-line supplement for details).
Percent stenosis according to the reading of the angiogram by the study physician at the patient’s clinical site.
Association of Baseline Characteristics and Outcome
In bivariate analyses, the following baseline characteristics were found to be significantly related to outcome in the combined set of WASID and SAMMPRIS patients (Table 3): female gender, history of diabetes, not using statin at enrollment, modified Rankin score ≥ 1, NIH stroke scale score ≥ 1, old infarcts in the territory of the symptomatic artery, and stroke as the qualifying event all conferred a significantly higher risk of the SAMMPRIS primary endpoint.
Table 3.
Bivariate Analyses of Baseline Characteristics vs the SAMMPRIS Primary Endpoint for the SAMMPRIS Medical Group and for SAMMPRIS-Eligible WASID Patients Combined
| Characteristic | # Patients | HR (95% CI) * | p-value* |
|---|---|---|---|
|
| |||
| Age† (years) | 370 | 1.0 (0.8 – 1.2) | 0.96 |
|
| |||
| Female Gender | 370 | 2.0 (1.2 – 3.2) | 0.004 |
|
| |||
| Black Race | 370 | 1.4 (0.8 – 2.3) | 0.21 |
|
| |||
| History of Hypertension | 370 | 1.0 (0.5 – 1.9) | 0.95 |
|
| |||
| History of Diabetes | 370 | 2.0 (1.2 – 3.2) | 0.0055 |
|
| |||
| Previous or Current Smoker | 370 | 0.8 (0.5 – 1.4) | 0.46 |
|
| |||
| History of Coronary Artery Disease | 370 | 0.9 (0.5 – 1.5) | 0.71 |
|
| |||
| History of Ischemic Stroke (not the qualifying event) | 370 | 1.3 (0.8 – 2.2) | 0.34 |
|
| |||
| Not Using Statin at Enrollment | 370 | 1.8 (1.1 – 2.9) | 0.027 |
|
| |||
| Modified Rankin Grade ≥ 1 | 370 | 1.8 (1.0 – 3.3) | 0.037 |
|
| |||
| NIH Stroke Scale > 1 | 370 | 1.9 (1.2 – 3.1) | 0.0067 |
|
| |||
| Physical Activity Out of Target‡ | 370 | 1.3 (0.7 – 2.2) | 0.42 |
|
| |||
| Old Infarcts in the Territory of the Symptomatic Artery | 352 | 1.8 (1.1 – 2.9) | 0.023 |
|
| |||
| Qualifying Event was a Stroke | 370 | 2.0 (1.2 – 3.6) | 0.014 |
|
| |||
| On Antithrombotic Therapy at Qualifying Event | 370 | 0.9 (0.6 – 1.4) | 0.65 |
|
| |||
| Time from Qualifying Event to Randomization† (days) | 370 | 0.9 (0.6 – 1.2) | 0.31 |
|
| |||
| Symptomatic Artery | 370 | 0.18 | |
| Internal Carotid Artery | Reference | ||
| Middle Cerebral Artery | 0.5 (0.3 – 0.9) | ||
| Vertebral Artery | 0.5 (0.2 – 1.2) | ||
| Basilar Artery | 0.6 (0.3 – 1.1) | ||
| Combination | 0.6 (0.1 – 2.4) | ||
|
| |||
| Symptomatic Artery in Posterior Circulation | 370 | 0.8 (0.5 – 1.3) | 0.41 |
|
| |||
| Percent Stenosis of Symptomatic Artery † | 370 | 1.1 (0.8 – 1.6) | 0.49 |
|
| |||
| Categories of Percent Stenosis of Symptomatic Artery | 370 | 0.98 | |
| 70–79% | Reference | ||
| 80–89% | 1.0 (0.6 – 1.7) | ||
| 90–99% | 1.1 (0.5 – 2.4) | ||
|
| |||
| Systolic BP† (mmHg) | 370 | 1.0 (0.9 – 1.1) | 0.53 |
|
| |||
| Diastolic BP† (mmHg) | 370 | 0.9 (0.8 – 1.2) | 0.60 |
|
| |||
| BMI† (kg/m2) | 367 | 1.0 (0.7 – 1.4) | 0.84 |
|
| |||
| LDL† (mg/dl) | 346 | 1.04 (0.98 – 1.11) | 0.19 |
|
| |||
| HDL† (mg/dl) | 350 | 1.06 (0.9 – 1.3) | 0.63 |
The hazard ratio and p-value from a proportional hazards regression model relating the individual characteristic to the time to a primary endpoint.
The hazard ratio is calculated for a 10 unit increase in the characteristic.
The baseline characteristics that were both different between the studies and related to outcome and thus potential confounders were no statin use at enrollment (more prevalent among WASID patients) and old infarcts in the territory of the symptomatic artery (more prevalent among SAMMPRIS patients). In a multivariable analysis, both characteristics were found to be related to the outcome with the following hazard ratios - no statin use at enrollment: 2.0 (95% CI = 1.1 – 3.3, p = 0.019), old infarcts in the territory of the symptomatic artery 1.9 (95% CI = 1.1 – 2.9, p = 0.019).
Adjusted Comparison of Outcome between Studies
When adjusted for no statin use at enrollment and old infarcts in the territory of the symptomatic artery, the estimated hazard ratio for WASID vs SAMMPRIS was 1.9 (95% CI = 1.1 – 3.2, p = 0.016) demonstrating a higher risk of the outcome among WASID patients (Table 4). To determine if there were additional confounding factors, the baseline characteristics other than no statin use at enrollment and old infarcts in the territory of the symptomatic artery that were significantly different between the studies (Table 2): age, history of hypertension, symptomatic artery, percent stenosis, systolic and diastolic blood pressure, BMI, LDL, and HDL were individually added to the model and the effect on the hazard ratio was noted. None of those factors changed the hazard ratio by more than 10%. The percent change ranged from 0.8% to 7.8%.
Table 4.
Adjusted Comparison of WASID and SAMMPRIS for the SAMMPRIS Primary Endpoint among SAMMPRIS Medical Patients and SAMMPRIS-Eligible WASID Patients*
| Characteristic | Parameter Estimate | Standard Error | p-value | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| WASID vs SAMMPRIS | 0.64745 | 0.26861 | 0.016 | 1.9 (1.1 – 3.2) |
| Not Using Statin at Enrollment | 0.31519 | 0.28168 | 0.26 | 1.4 (0.8 – 2.4) |
| Old Infarcts in the Territory | 0.66134 | 0.25311 | 0.009 | 1.9 (1.2 – 3.2) |
The estimates are based on a proportional hazards regression model with 352 patients.
Discussion
The 1, 2 and 3-year rates of the primary endpoint were 42%, 41% and 48% lower respectively in SAMMPRIS medically-treated patients compared with WASID patients who met the SAMMPRIS entrance criteria. Our analysis sought to explore whether SAMMPRIS patients had a lower rate of major vascular events due to a lower burden of vascular risk factors compared with WASID patients.
SAMMPRIS patients were younger, had lower LDL at baseline (because of higher statin use), and had a higher frequency of anterior circulation stenosis. Younger age and lower cholesterol have been associated with a lower risk of stroke in patients with intracranial stenosis5, 6, however, WASID showed no increased risk of stroke with posterior circulation stenosis in medically treated patients7. On the other hand, SAMMPRIS patients had higher frequencies of hypertension, mean SBP, mean body mass index, more severe stenosis (80–99%) and old infarct in the territory of the symptomatic artery. Raised SBP and severe stenosis were strongly associated with an increased risk of stroke in patients with intracranial stenosis in WASID6–8.
Among the baseline characteristics evaluated, we identified lack of statin use at baseline and old infarcts in the territory of the stenotic artery as confounding factors in that they were significantly different in frequency between WASID and SAMMPRIS patients and were associated with a worse outcome. After adjusting for these factors, WASID patients were still at a two-fold higher risk of the primary endpoint. This result support the hypothesis that the lower risk of the primary endpoint in SAMMPRIS patients is due to the differences in medical management between the two studies.
In addition to differences in risk factor control, changes in antithrombotic therapy between the two studies may have played a role. The SAMMPRIS regimen utilized dual antiplatelet therapy with aspirin and clopidogrel for 90 days compared to either aspirin monotherapy or warfarin in WASID. Dual antiplatelet therapy has been shown to reduce stroke in patients with recent minor stroke or transient ischemic attack in the Clopidogrel in High-Risk patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial9. In addition, a recent meta-analysis found that when treatment was initiated within three days of the index TIA or stroke, dual antiplatelet therapy reduced the risk of stroke by 31% compared to antiplatelet monotherapy10.
This study has some limitations. The increased frequency of hypertension in SAMMPRIS might reflect changes in the JNC definition of hypertension after the start of WASID11. However, this is an unlikely explanation given that SAMMPRIS patients had significantly higher SBP at baseline compared with WASID patients. In addition, the studies were done in different time periods and other variations in secular treatment patterns during the course of these two trials may have existed, which could explain some of the differences in event rates in these two trials12.
In conclusion, compared with WASID patients who met the SAMMPRIS qualifying criteria, SAMMPRIS patients were slightly younger but had a higher burden of other poor prognostic features. Analyses comparing the SAMMPRIS primary endpoint between the two studies adjusted for confounding factors demonstrated a higher risk of the outcome among WASID patients. These data suggest that the lower rate of stroke in the medical arm of SAMMPRIS compared with WASID patients with the same qualifying criteria is likely related to the aggressive medical management used in SAMMPRIS.
Acknowledgments
Funding sources
The SAMMPRIS trial was funded by a research grant (U01 NS058728) from the US Public Health Service National Institute of Neurological Disorders and Stroke (NINDS). In addition, the following Clinical and Translational Science Awards, funded by the National Institutes of Health, provided local support for the evaluation of patients in the trial: Medical University of South Carolina (UL1RR029882), University of Florida (UL1RR029889), University of Cincinnati (UL1RR029890), and University of California, San Francisco (UL1RR024131). Stryker Neurovascular (formerly Boston Scientific Neurovascular) provided study devices and supplemental funding for third party device distribution, site monitoring and study auditing. This research is also supported by the Investigator-Sponsored Study Program of AstraZeneca that donates rosuvastatin (Crestor) to study patients.
Footnotes
Disclosures
All the authors besides the first author serve on the Executive Committee of the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial which is funded by the National Institute of Neurological Disorders and Stroke (grant number: U01 NS058728). All but one (SJ) receive salary support from the SAMMPRIS grant. Additional support is listed below.
Seemant Chaturvedi MD has nothing to disclose.
Tanya N. Turan, MD is a past recipient of funding from the American Academy of Neurology (AAN) Foundation Clinical Research Training Fellowship and is the current recipient of a K23 grant from NIH/NINDS (1 K23 NS069668-01A1). She has also served as an expert witness in medical legal cases.
Michael J. Lynn, MS receives grant support from the National Eye Institute. He is the principal investigator of the Coordinating Center for Infant Aphakia Treatment Study (EY013287) and a co-investigator on the Core Grant for Vision Research (EY006360).
Colin Derdeyn MD receives other grant support from the NINDS (P50 55977; R01 NS051631). He is also on the Scientific Advisory Board for W.L Gore and Associates and is the Chair of the Scientific Advisory Board for Pulse Therapeutics.
David Fiorella MD, PhD has received institutional research support from Seimens Medical Imaging and Microvention, consulting fees from Codman/Johnson and Johnson, NFocus, W.L. Gore and Associates, and EV3/Covidien, and royalties from Codman/Johnson and Johnson. He has received honoraria from Scientia and has ownership interest in CVSL and Vascular Simulations.
Scott Janis PhD is a program director at the National Institute of Neurological Disorders and Stroke
Marc Chimowitz, MBChB is the grant recipient for the NINDS funded SAMMPRIS trial (U01 NS058728) and the WASID trial (1 R01 NS36643) discussed in this paper. The WASID and SAMMPRIS trials also received industry support as described in the funding sources. He has also received research grants from NINDS to fund other research on intracranial stenosis (1 K24 NS050307 and 1 R01 NS051688).
References
- 1.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New England Journal of Medicine. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New England Journal of Medicine. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 3.Turan TN, Lynn MJ, Nizam A, Lane B, Egan BM, Le NA, et al. Rationale, design, and implementation of aggressive risk factor management in the stenting and aggressive medical management for prevention of recurrent stroke in intracranial stenosis (sammpris) trial. Circulation. Cardiovascular quality and outcomes. 2012;5:e51–60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2011;20:357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong KS, Li H. Long-term mortality and recurrent stroke risk among chinese stroke patients with predominant intracranial atherosclerosis. Stroke. 2003;34:2361–2366. doi: 10.1161/01.STR.0000089017.90037.7A. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 7.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 8.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M for the WASID Investigators. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–2975. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New England Journal of Medicine. 2013;369:11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 10.Wong KSL, Wang Y, Leng X, Mao C, Tang J, Bath PMW, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: An updated systematic review and meta-analysis. Circulation. 2013;128:1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Egan BM, Zhao Y, Axon R. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]


