Abstract
Background and Purpose
Acute infarct volume, often proposed as a biomarker for evaluating novel interventions for acute ischemic stroke (AIS), correlates only moderately with traditional clinical endpoints such as the modified Rankin Scale (mRS). We hypothesized that the topography of acute stroke lesions on diffusion-weighted MRI (DWI) may provide further information with regard to presenting stroke severity and long-term functional outcomes.
Methods
Data from a prospective stroke repository were limited to AIS subjects with MRI completed within 48 hours from last known well, admission NIH Stroke Scale (NIHSS), and 3-to-6 months mRS scores. Using voxel-based lesion symptom mapping techniques including age, sex and DWI lesion volume as covariates, statistical maps were calculated to determine the significance of lesion location for clinical outcome and admission stroke severity.
Results
490 subjects were analyzed. Acute stroke lesions in the left hemisphere were associated with more severe NIHSS at admission and poor mRS at 3 to 6 months. Specifically, injury to white matter (corona radiata, internal and external capsules, superior longitudinal fasciculus, and uncinate fasciculus), post-central gyrus, putamen, and operculum were implicated in poor mRS. More severe NIHSS involved these regions as well as the amygdala, caudate, pallidum, inferior frontal gyrus, insula, and pre-central gyrus.
Conclusions
Acute lesion topography provides important insights into anatomical correlates of admission stroke severity and post-stroke outcomes. Future models that account for infarct location in addition to DWI volume may improve stroke outcome prediction and identify patients likely to benefit from aggressive acute intervention and personalized rehabilitation strategies.
Keywords: Acute stroke, magnetic resonance imaging, statistical model, VLSM, topography
Introduction
Human stroke pathophysiology heterogeneity has been implicated in the limited success of therapeutic interventions for the treatment of acute ischemic stroke (AIS).1 Neuroimaging biomarkers such as infarct volume have been proposed as potential surrogates for clinical outcome in the evaluation of novel AIS therapies. To date, studies2 have found that lesion volumes are only moderately correlated with long-term clinical outcome measures. Small pilot studies have demonstrated that integration of lesion location and size can estimate stroke severity better than volume alone.3 Here, we propose to build upon these early studies by investigating the relationship between topography of acute diffusion-weighted MRI (DWI) lesions and measures of AIS severity and long-term functional outcomes, using voxel-based lesion symptom mapping (VLSM) techniques.
VLSM compares neurobehavioral scores between patients with and without lesions on a voxel-wise basis.4 VLSM methods have been used to examine motor recovery,5 spatial neglect6 and aphasia4, 7 in chronic stroke patients, and one-month modified Rankin Scale (mRS) score in sub-acute stroke (2–3 days)8 patients. These studies have provided insight into clinical deficits linked to lesions in particular brain regions, but did not take into consideration important factors such as age, sex, and lesion volume that are known to be associated with long-term functional outcome after stroke. Furthermore, these studies used MRI scans acquired relatively late (greater than 48 hours) in the clinical course of stroke, i.e., completed outside of the time window when clinical decision-making occurs. To improve clinical relevance, we investigated the role of stroke lesion topography of acute DWI acquired in AIS patients within 48 hours from last known well (LKW) on initial stroke severity (admission NIH Stroke Scale [NIHSS]) and long-term disability (3–6 month mRS), accounting for age, sex, and lesion volumes.
Methods
Participants
Ischemic stroke patients prospectively enrolled in our Institutional Review Board-approved Genes Associated with Stroke Risk and Outcomes Study (GASROS) between 2007–2011 with an MRI performed within 48 hours of LKW were retrospectively analyzed. GASROS is a cross-sectional, hospital based cohort of consecutive adults admitted to our neurology service with diagnosis of ischemic stroke confirmed by neuroimaging (CT or MRI). Exclusion criteria include inability to obtain informed consent from the subject or proxy, or a verified diagnosis of secondary cerebral ischemia (e.g. vasculitis, subacute bacterial endocarditis, vasospasm, or tumor). All patients were evaluated emergently by a neurologist at the time of admission, and clinical and laboratory data from this encounter (e.g. admission NIHSS score) were abstracted from corresponding medical records. The long-term functional outcomes were assessed using the mRS score collected either in person or via a telephone interview at 3–6 months post-stroke. If the subject could not be reached, the mRS was reconstructed from the subject’s medical record. Only the subset of stroke patients with evidence of infarction on acute DWI MRI and available admission NIHSS and follow-up mRS was included in this analysis.
Magnetic Resonance Imaging
DWI was performed for the majority of studies on 1.5T General Electric Signa scanners with a few cases performed on 1.5T or 3T Siemens scanners (N=5) with the following acquisition parameters: repetition time of 5000 ms, minimum echo time of 62 ms to 117 ms, 220 mm field-of-view, 128×128 acquisition matrix upsampled to 256×256, 5-mm slice thickness with a 1-mm gap, and 0 s/mm2 (b-zero) and 1000 s/mm2 b-values. DWI datasets were corrected for motion and eddy current distortions.9 Apparent diffusion coefficient maps were calculated from the slope of the linear regression fit of the log of the DWI and b-zero images. Lesion volumes were outlined on the acute DWI using a semi-automated algorithm10 by a reader blinded to the admission NIHSS and follow-up mRS scores. All DWI datasets were co-registered to one another using non-linear co-registration techniques (MNI Autoreg11, 12) and to the MNI 152 1 mm atlas.13
VLSM was performed on the co-registered datasets using VLSM version 2.55 (http://neuroling.arizona.edu/resources.html) with age, and sex as covariates against admission NIHSS or follow-up mRS.14 Analyses were repeated with DWI lesion volume as a covariate. Subset analyses were performed in survivors (i.e. follow-up mRS<6) in order to remove potential confounds from death being unrelated to the stroke. For all analyses, a voxel was only tested if at least 10 subjects exhibited a lesion at its location. Resulting T-scores maps were thresholded voxelwise (P < 0.001), then corrected for multiple comparisons based on cluster size permutation method (1000 permutations; P<0.05) in which mRS or NIHSS were randomly reassigned.15 The Harvard-Oxford Cortical and Sub-cortical Structural Atlases16 and the JHU ICBM-DTI-81 white matter atlas17 distributed as part of FSL (FMRIB’s Software Library)18 were combined for region of interest (ROI) analysis. Results were displayed using MRIcroGL (http://www.mricro.com) in radiologic convention, with MNI coordinates provided in mm.
Statistical Analysis
Pearson’s Chi-squared test was used to evaluate differences between categorical variables. Continuous variables were compared using two-tailed Wilcoxon rank-sum tests. Differences in patient demographics with respect to lesion laterality were compared using one-way analysis of variance (ANOVA) with post-hoc Tukey-Kramer Honest Significant Different testing. Correlation between acute DWI volumes and NIHSS and mRS scores were performed using Spearman’s test. Backward step-wise regression was performed to investigate the relationship between age, sex, lesion volume, admission NIHSS and IV thrombolysis (tPA), time-to-MRI and/or endovascular therapy (EVT) on follow-up mRS. Multicollinearity was assessed by calculating variance inflation factors (VIF) for the final model parameters for which VIF > 10 was considered an indication of multicollinearity.19 Statistical analyses were performed using JMP Pro 11.0 (SAS Institute Inc) with significance at P<0.05 unless otherwise noted
Results
490 subjects met inclusion and exclusion criteria. Patient demographics are shown in Table 1. 19% of the subjects were treated with tPA and/or EVT and had significantly worse mRS than those not receiving revascularization treatment (2 [1–4] vs. 1 [0–2]; P=0.006), most likely due to more severe admission NIHSS in these patients (11 [5–16] vs. 3 [1–5]; P<0.0001) and larger acute DWI lesion volumes (12.1 [3.6–44.2] vs. 2.2 [0.8–14.8]; P<0.0001). MRI was performed prior to the revascularization therapy in 9 patients (−1 [−0.5 – 3.5] h), post-intervention in 77 (3.2 [1.5–9.6] h) with the precise time of treatment not documented for 7. Acute DWI lesion volume significantly correlated with both admission NIHSS score (ρ=0.51, P<0.0001) and follow-up mRS (ρ=0.32, P<0.0001). Subjects who died before follow-up (mRS=6) presented with significantly more severe stroke symptoms and larger lesion volumes.
Table 1.
Demographic Characteristics.
| All (N=490) | mRS<6 (N=439) | mRS=6 (N=51) | |
|---|---|---|---|
|
| |||
| Age (y)* | 65.0±14.9 | 63.8±14.8 | 75.2±12.1 |
| Male (%)* | 303 (62%) | 284 (65%) | 19 (37%) |
| Admission NIHSS* | 3 [1–8] | 3 [1–6] | 12 [5–20] |
| Time-to-MRI (h)† | 15.9 [6.7–26.5] | 16.2 [6.9–27.1] | 11.5 [4.8–20.0] |
| DWI lesion volume (cm3)* | 3.7 [1–21.2] | 3.0 [0.9–17.3] | 12.9 [2.8–52.3] |
| 3–6 month mRS* | 1 [0–3] | 1 [0–2] | 6 |
| Treated with tPA/EVT (%)* | 93 (19%) | 73 (17%) | 20 (39%) |
| Stroke Location* | |||
| Left | 232 (47%) | 209 (48%) | 23 (45%) |
| Right | 212 (43%) | 197 (45%) | 15 (29%) |
| Bilateral | 46 (9.4%) | 33 (7.5%) | 13 (25%) |
Values are Mean±SD or Median [IQR].
P<=0.0001,
P=0.03 mRS<6 versus mRS=6.
Multivariable regression analysis found that age (P<0.0001), sex (P=0.0002), acute DWI lesion volume (P=0.004) and admission NIHSS (P<0.0001) were significant factors for follow-up mRS. This relationship between mRS and age (P<0.0001), sex (P=0.001), acute DWI lesion volume (P<0.0001) and NIHSS (P<0.0001) held for the survivors subset. Age, sex, and DWI volume VIF were 1.04, 1.05, 1.01 respectively, indicating no multicollinearity issues. Age and sex were, therefore, used as covariates in subsequent VLSM analysis. In a separate analysis, lesion size was also included. Because admission NIHSS was likely a reflection of extent and location of brain injury, NIHSS was not included as a covariate in the mRS analysis.
Figure 1 shows the incidence map of lesions for all 490 subjects, along with the statistical power for alpha=0.001. The distribution of stroke lesions was comparable for both left and right hemispheres, with slightly greater incidence/higher power in the right hemisphere (maximum incidence of 64, 99.8%) than the left hemisphere (maximum incidence 39, 94.8%). Table 2 shows the differences between patients with left, right or bilateral (including brainstem) strokes. Patients with bilateral/brainstem strokes had more severe acute stroke symptoms and worse follow-up outcome.
Figure 1.
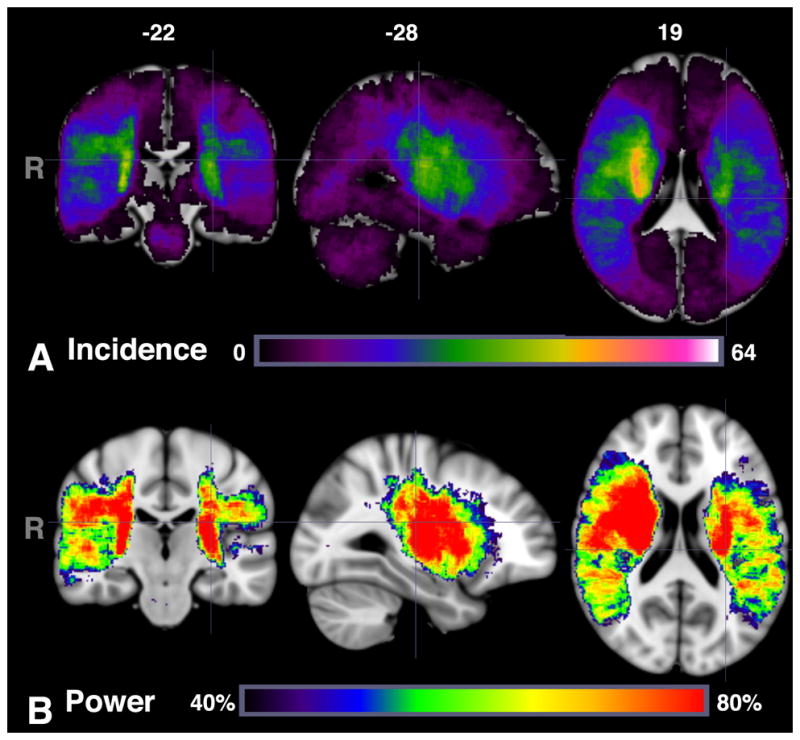
Number of patients with lesions within each voxel (A) using range 0 to 64 (maximum incidence) and (B) power map between 40 and 80% for alpha=0.001 for VLSM analysis for follow-up mRS.
Table 2.
Demographic differences between patients with left versus right hemispheric versus bilateral (including brain stem) strokes.
| Left (N=232) | Right (N=212) | Bilateral (N=46) | P-value | |
|---|---|---|---|---|
| Age (y) | 65.9±14.2 | 64.2±15.8 | 64.3±14.5 | 0.45 |
| Male (%) | 146 (63%) | 126 (59%) | 31 (67%) | 0.54 |
| Admission NIHSS | 3 [1–7] | 4 [1–9] | 3 [1–14]* | 0.01 |
| Time-to-MRI (h) | 15.8 [6.5–26.0] | 15.9 [6.7–27.8] | 16.8 [7–29.8] | 0.88 |
| DWI volume (cm3) | 2.7 [0.8–17.8] | 5.4 [1.0–28.9] | 2.8 [1.1–10.5] | 0.59 |
| 3–6 month mRS | 1 [0–3] | 1 [0–2] | 2 [1–6]† | 0.0002 |
| Treated with tPA/EVT (%) | 44 (19%) | 39 (18%) | 10 (22%) | 0.87 |
P=0.01,
P<0.001 bilateral vs. left or right hemisphere.
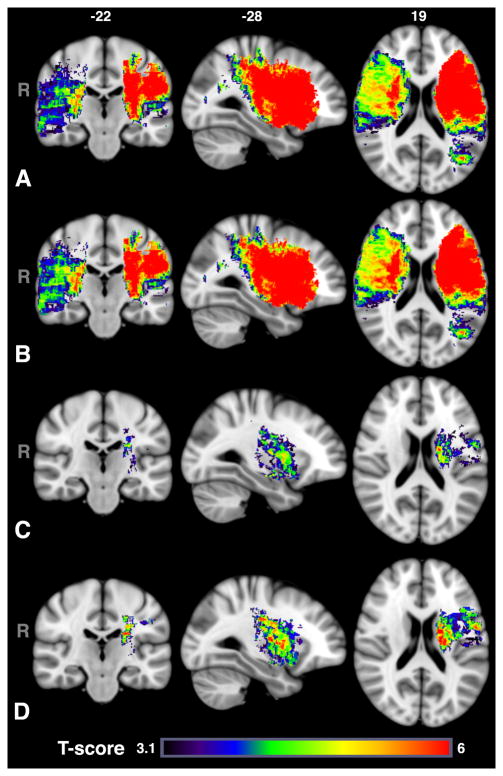
Figure 2 shows the VLSM results for admission NIHSS overlayed on the MNI 152 1mm atlas using a voxel-wise threshold of P<0.001, corrected for multiple comparison. The differences between T-score maps including no covariates and those including age and sex was minimal for both the left (187 cm3 vs. 185 cm3) and right (184 cm3 vs. 192 cm3) hemispheres. Poor admission NIHSS scores were associated with injury in both left and right hemispheres, principally in middle cerebral artery (MCA) vascular territories. Table 3 shows the percentage of each ROI that overlap with the T-map clusters. Only regions that were found to be significant for all T-maps for either NIHSS or mRS are shown. Online Supplement Tables I and II in http://stroke.ahajournals.org provide a detailed breakdown by ROI for both left and right hemispheres. The T-scores were higher in the left hemisphere than right, despite lower power in the left hemisphere, for VLSM results without covariates (5.9 [4.7–7.0] vs. 4.4 [3.8 – 4.9]) as well as with age and sex (5.8 [4.6–6.8]) vs. 4.5 [3.9–5.1]) included. Furthermore, with acute DWI lesion size included as a covariate, the volume of tissue locations associated with more severe NIHSS was reduced to 24.8 cm3 and limited to the left hemisphere – in particular to white matter (56% of total cluster), pre- and postcentral gyri (10%), putamen (11%), insula (10%), operculum (5%), inferior frontal gyrus (IFG, 2%), pallidum (2%), caudate (1%), and amygdala (1%). The thalamus, frontal orbital cortex, hippocampus and unclassified cerebral cortex each made up less than 1% of the cluster. Subset analysis in survivors produced similar distributions in a slighter larger cluster (36.6 cm3) involving more of the IFG, insula, operculum, and white matter (Table 3).
Figure 2.
T-maps with voxel-wise threshold of P<0.001, thresholded based on cluster size (P<0.05) and permutation method for admission NIHSS scores using (A) no covariates, (B) sex and age covariates or (C) sex, age and lesion volume. The differences between (B) and (C) demonstrate the importance of lesion volume for severity of stroke symptoms at admission. However, even after taking into account lesion size, injury to the left hemisphere, in particular motor pathways and white matter tracts, insula and putamen was associated with more severe symptoms. (D) Subset analysis for patients, who were alive at 6 months, demonstrates similar findings, but more involvement of the insula and operculum.
Table 3.
Distribution of the cluster in the left hemisphere as a percentage of the region of interest.
| NIH (N=490) | NIH age+sex (N=490) | NIH age+sex +volume* (N=490) | NIH age+sex +volume* (Alive N=439) | mRS (N=490) | mRS +age+sex (N=490) | mRS age+sex +volume* (N=490) | mRS+ age+sex +volume* (Alive N=439) | |
|---|---|---|---|---|---|---|---|---|
| Amygdala | 30 | 30 | 10 | 4 | 28 | 28 | 1 | 0 |
| Caudate | 34 | 35 | 10 | 11 | 20 | 30 | 0 | 1 |
| Corona Radiata | 65 | 65 | 17 | 24 | 63 | 58 | 5 | 9 |
| External Capsule | 98 | 98 | 70 | 70 | 98 | 96 | 8 | 16 |
| Fornix | 31 | 32 | 3 | 0 | 15 | 21 | 0 | 0 |
| Frontal Orbital Cortex | 30 | 30 | 1 | 0 | 27 | 17 | 0 | 0 |
| Inferior Frontal Gyrus pars opercularis | 80 | 80 | 8 | 31 | 47 | 25 | 0 | 0 |
| Insula | 76 | 76 | 23 | 34 | 65 | 67 | 0 | 1 |
| Internal Capsule | 81 | 81 | 33 | 35 | 68 | 68 | 5 | 12 |
| Operculum | 93 | 93 | 8 | 18 | 72 | 68 | 1 | 0 |
| Pallidum | 71 | 71 | 29 | 31 | 64 | 68 | 0 | 19 |
| Postcentral Gyrus | 33 | 33 | 2 | 3 | 32 | 32 | 1 | 0 |
| Precentral Gyrus | 29 | 29 | 5 | 9 | 19 | 14 | 0 | 0 |
| Putamen | 91 | 91 | 52 | 61 | 86 | 88 | 3 | 17 |
| Superior longitudinal fasciculus | 92 | 91 | 6 | 21 | 80 | 78 | 12 | 4 |
| Thalamus | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| Uncinate fasciculus | 87 | 87 | 18 | 5 | 86 | 87 | 7 | 6 |
Volume = acute DWI lesion volume.
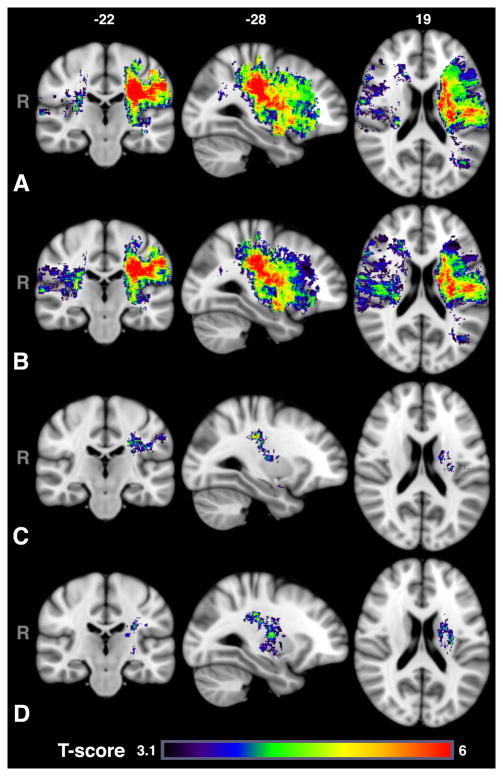
For the mRS results (Figure 3), despite comparable incidence of acute left and right lesions, lesions primarily in the left MCA territory were associated with poor mRS. When age and sex were included as covariates, larger regions of tissue in the right hemisphere were implicated with worse mRS (46.9 cm3 vs. 81.8 cm3) while the opposite was true for the left hemisphere (138.9 cm3 vs. 116.9 cm3). When including lesion volume as a covariate, only injury to the left hemisphere (4.1 cm3) was associated with poor mRS. The distribution of the total cluster by ROI were predominantly white matter (84%), with some involvement of the post-central gyrus (9%), putamen (4%), and operculum (2%). The anterior division of the supramarginal gyrus, amygdala, caudate, insula and unclassified cerebral cortex made up the remainder of the cluster (less than 1% each). Subset analysis in survivors resulted in a slightly larger cluster (6.0 cm3) that encompassed more of the internal and external capsules, and involved more of the pallidum and putamen, but not the postcentral gyrus, likely due to reduced power in this region (see Online Supplement Figure I for power maps among survivors).
Figure 3.
T-maps with voxel-wise threshold of P<0.001, thresholded based on cluster size (P<0.05) and permutation method for follow-up mRS scores (A) without covariates and using (B) sex and age or (C) sex, age and lesion volume as covariates. The differences between (B) and (C) demonstrate the association between lesion volume and severity of stroke symptoms at admission. In comparison with admission NIHSS, injury to the right hemisphere appears less important for long-term outcome than acute stroke symptoms. Injury to primarily white matter tracts in the left hemisphere was associated with worse long-term outcome with lesion volume included as a covariate. (D) Subset analysis for patients who were alive at 6 months demonstrates similar findings, but more involvement of the putamen.
Discussion
VLSM techniques have been developed to understand which regions of the brain are critical for brain functions.14 We used these methods to investigate how ischemic injury to particular brain regions is associated with acute stroke severity and long-term functional outcome. We found that despite greater incidence of right-hemispheric lesions in this cohort, injury to the left hemisphere – and in particular, to the motor pathway (i.e., posterior limb of the internal capsule, corona radiata) and white matter tracts were associated with greater severity of acute stroke symptoms and poor long-term outcome. The association between poor mRS and injury to the motor pathway may partly be explained by the mRS score being heavily weighted by the degree of motor disability from the dominant hemisphere. In contrast, the NIHSS score reflects various symptomatology, including aphasia, dysarthria, ataxia, and neglect that are captured in a limited way by the mRS score; hence, more severe NIHSS scores were associated with greater expanses of tissue injury in both hemispheres. However, even for NIHSS, asymmetry between the left and right hemisphere T-scores was observed, with more severe NIHSS associated with the left-sided infarcts. This suggests that study designs, which “flip” right-sided lesions into left-sided ones in order to increase statistical power, may lead to inaccurate conclusions and should be avoided, if possible.
Our findings suggest that if one does not take into consideration age, sex or lesion volume, locations of the lesion are associated with degree of stroke severity and long-term disability – and more so with lesions in the left hemisphere. Interestingly, including age and sex into our model for mRS increased the number of voxels in the right hemisphere. This suggests that for a given age and sex, the risk that a patient will have greater admission stroke severity and long-term disability is increased by where the stroke is located. Patients with strokes in certain regions of the right hemisphere - in particular the insula, operculum or putamen - are more likely to have more severe long-term disability. This effect is less pronounced on the admission NIHSS, for which lesion location appears to be an important factor independent of age and sex.
Once volume is included in the models, lesion location in the right hemisphere is no longer significant for either NIHSS or mRS. That is for a given acute DWI lesion volume for a patient with specific age and sex, if the lesion is located in certain regions (in particular, left-hemispheric white matter and subcortical gray matter), the likelihood of greater severity on admission and long-term disability is increased. We speculate that the reason individual voxels in the right hemisphere are no longer significant once volume is taken into consideration is that the size of the lesion in right hemispheric strokes determine the degree of admission stroke severity and outcome, independent of where the large lesion is located in the right hemisphere. A major determinant of poor outcome in right hemispheric stroke is unilateral neglect,20 which is typically associated with large strokes.
Our findings are consistent with previously published results in a subacute stroke patient population reporting that injury to the corticospinal tract in the left hemisphere was associated with poor outcomes.21 Furthermore, the right angular, left middle, and superior temporal gyri were also implicated in that analysis that did not account for a lesion volume, and was also seen in our intermediate models. However, after including lesion volume as a covariate in our study, only injury to the left hemisphere further explained high NIHSS or poor mRS. These findings highlight the importance of accounting for the acute infarct volume in prediction models of post-stroke outcomes. Significant correlation between the acute DWI lesion volume and long-term mRS score observed in our data and in the large Virtual International Stroke Trials Archive (VISTA) collaborative show that initial lesion volumes were an independent predictor of mRS scores at day 90.22 Thus, studies of brain topology and outcomes that do not account for lesion size may mistakenly attribute disproportionate significance of injury to regions that tend to be associated with large lesion volumes (e.g. the insular cortex21); however, these regions may not be independently associated with poor outcome. On the other hand, despite being highly significant, the correlation of acute DWI lesion volume with mRS was only moderate, suggesting that other factors, such as lesion location, are necessary for building better stroke outcome prediction models, which our results support.
Our findings show the promise and importance of incorporating lesion location and lesion volume into models that predict stroke outcome. In addition, we can use a similar framework to combine multimodal imaging information with clinical information to predict disability and stroke severity. The output of these models will be a voxel-based map of predicted disability scores, by which one weighs actual DWI lesion location. These models will need to be validated in an independent cohort of patients and compared against actual outcome to assess for accuracy.
There are a few limitations to our study. Our findings are primarily limited to MCA territory strokes as reflected in our power map. It is likely that with greater number of patients, the brain stem would also be highlighted. Indeed, we found a cluster in the brainstem with T-scores > 3.1 for both NIHSS and mRS (see Online Supplement Figure II), however after correction for multiple comparisons, that cluster did not meet statistical significance. In addition, the importance of the asymmetry between left compared to the right hemisphere may not generalize to investigations that involve thousands of patients for which a difference of 4% between incidences of left versus right hemispheric strokes may become significant. Another limitation is our study’s retrospective nature in that MRI was not performed at pre-specified time points. The acute DWI lesion may not fully encompass dysfunctional tissue at presentation, which perhaps would be better captured using MR perfusion-weighted imaging. 110 of our subjects were imaged before 6 hours, a time frame in which lesion evolution is highly dynamic.23 Furthermore, for the IV tPA/EVT subset, MRI was not uniformly acquired prior to intervention. Subject enrollment into GASROS required informed consent, potentially skewing out patient population toward milder strokes. Another potential limitation is the use of the MNI 152 1mm atlas, which was derived from young healthy adults, as a reference template. Co-registration errors when transforming the clinical low-resolution DWI scans to the high-resolution atlas may have led to inaccuracies, when assessing regional involvement of small structures; therefore, caution is needed in the interpretation of their results. An alternate approach is to perform manual segmentation and to co-register all images to one another; however, manual segmentation is also subject to measurement errors. We also did not record the cause of death for patients. It may be that patients either had care withdrawn as a result of large DWI lesion volumes, thereby leading to a self-fulfilling prophecy, or died due to a cause unrelated to the stroke. We attempted to control for this by performing subset analysis in only survivors and found similar results, albeit at the cost of power. Furthermore, both NIHSS and mRS do not measure unilateral neglect, 24 which is a major predictor of poor outcome in right hemispheric strokes. In addition, mRS25 is a measure of global disability that is relatively insensitive to cognitive dysfunction. We chose the mRS score as our outcome measurement because it is the most widely used validated disability scale, and is the traditional endpoint for many clinical trials. Future prospective studies should utilize outcome instruments that are sensitive to deficits across a variety of cognitive domains. There is increased realization that, for stroke trials targeting specific brain regions, outcome scales sensitive to recovery of brain function rather than only physical disability will be needed.26 With such instruments, VLSM may be useful for identifying potential responders to novel treatments as has been recently done in a study of brain stimulation treatment for aphasia.27
Conclusions
Our results confirm the hypothesis that the location of AIS lesions is an important determinant of presenting stroke severity and long-term functional outcomes. Outcome prediction models that account both for acute infarct topography and volume, as well as clinical characteristics, highlight the complex mechanisms that contribute to the severity of the neurological syndrome and its long-term recovery. Therefore, integration of VLSM into clinical assessment of AIS patients may facilitate early identification of patients at risk for poor long-term functional outcomes and enhance significantly our current strategies for selection of patients for aggressive acute intervention and focused post-stroke rehabilitation programs.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported in part by the National Institutes of Health (R01NS59775, P50NS051343, R01NS063925, K23NS064052, R01NS082285 and NIBIB P41EB015896), American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N), and Deane Institute for Integrative Study of Atrial Fibrillation and Stroke.
Footnotes
Disclosures: None.
References
- 1.Muir KW. Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke. 2002;33:1545–1550. doi: 10.1161/01.str.0000018684.86293.ab. [DOI] [PubMed] [Google Scholar]
- 2.Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, et al. Is the association of national institutes of health stroke scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 3.Menezes NM, Ay H, Wang Zhu M, Lopez CJ, Singhal AB, Karonen JO, et al. The real estate factor: Quantifying the impact of infarct location on stroke severity. Stroke. 2007;38:194–197. doi: 10.1161/01.STR.0000251792.76080.45. [DOI] [PubMed] [Google Scholar]
- 4.Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 5.Lo R, Gitelman D, Levy R, Hulvershorn J, Parrish T. Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage. 2010;49:9–18. doi: 10.1016/j.neuroimage.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain. 2011;134:903–912. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnusdottir S, Fillmore P, den Ouden DB, Hjaltason H, Rorden C, Kjartansson O, et al. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study. Hum Brain Mapp. 2013;34:2715–2723. doi: 10.1002/hbm.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng B, Forkert ND, Zavaglia M, Hilgetag CC, Golsari A, Siemonsen S, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke. 2014;45:1695–1702. doi: 10.1161/STROKEAHA.114.005152. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: Detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 10.Mocking S, Garg P, Chutinet A, Copen WA, Sorensen AG, Wu O. Accuracy and execution speed of automatic voxel-based algorithms for segmenting stroke lesions in clinical DWI imaging (abstract). ISMRM 19th Scientific Meeting; 2011. [Google Scholar]
- 11.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3d intersubject registration of MR volumetric data in standardized talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 12.Laboratory of Neuro Imaging UCLA. ICBM 452 T1 atlas. 2008
- 13.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: An application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 14.Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia research. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. Boston; New York: McGraw-Hill/Irwin; 2004. [Google Scholar]
- 20.Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: Predictor of stroke severity and vascular lesion. Arch Neurol. 2005;62:1081–1085. doi: 10.1001/archneur.62.7.1081. [DOI] [PubMed] [Google Scholar]
- 22.Vogt G, Laage R, Shuaib A, Schneider A, Collaboration V. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: An analysis of the virtual international stroke trials archive (VISTA) database. Stroke. 2012;43:1266–1272. doi: 10.1161/STROKEAHA.111.646570. [DOI] [PubMed] [Google Scholar]
- 23.Schellinger PD, Fiebach JB, Jansen O, Ringleb PA, Mohr A, Steiner T, et al. Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol. 2001;49:460–469. [PubMed] [Google Scholar]
- 24.Hillis AE, Wityk RJ, Barker PB, Ulatowski JA, Jacobs MA. Change in perfusion in acute nondominant hemisphere stroke may be better estimated by tests of hemispatial neglect than by the national institutes of health stroke scale. Stroke. 2003;34:2392–2396. doi: 10.1161/01.STR.0000089681.84041.69. [DOI] [PubMed] [Google Scholar]
- 25.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 26.Krakauer JW, Hillis AE. The future of stroke treatment: Bringing evaluation of behavior back to stroke neurology. JAMA Neurol. 2014;71:1473–1474. doi: 10.1001/jamaneurol.2014.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campana S, Caltagirone C, Marangolo P. Combining voxel-based lesion-symptom mapping (VLSM) with a-tDCS language treatment: Predicting outcome of recovery in nonfluent chronic aphasia. [published online ahead of print Jan 30, 2015] [Accessed May 12, 2015];Brain Stimul. 2015 doi: 10.1016/j.brs.2015.01.413. http://www.ncbi.nlm.nih.gov/pubmed/25732786. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.