Abstract
Background
Poor brain reserve in alcoholic cirrhosis could worsen insight regarding disease severity and increase the patients’ vulnerability towards further deterioration.
Aim
To analyze brain reserve in abstinent alcoholic (Alc) compared to non-alcoholic (Nalc) cirrhosis patients in the context of hepatic encephalopathy (HE) and evaluate relative change in brain reserve between groups over time and before/after elective TIPS placement.
Methods
Cross-sectional study
46 Alc and 102 Nalc outpatients with or without HE. Cognitive tests followed by magnetic resonance (MR) imaging including 1-H MR Spectroscopy (MRS), Diffusion tensor (DTI) and T1-weighted imaging.
Prospective study
MRS on subset of 10 patients before/after TIPS placement. Another subset of 26 patients underwent MRS at least one year apart.
Results
Cross-sectional study
Alc patients were worse on cognitive tests than Nalc. MR results suggest a greater effect of hyperammonemia, brain edema and significantly higher cortical damage in Alc as compared to Nalc patients. Effect of HE status on cognitive tests and brain reserve was more marked in Nalc than in Alc group.
TIPS study
Nalc patients showed a greater adverse relative change after TIPS compared to Alc group.
1-year follow-up
Both groups remained stable between the two visits. However, Alc patients continued to show poor brain reserve than Nalc over time.
Conclusions
Patients with alcoholic cirrhosis, despite abstinence, have a poor brain reserve while, non-alcoholic cirrhosis patients have a greater potential for brain reserve deterioration after HE and TIPS. Information regarding the brain reserve in cirrhosis could assist medical teams to refine their communication and monitoring strategies for different etiologies.
Keywords: hepatic encephalopathy, cognitive reserve, alcohol, diffusion tensor imaging, voxel-based morphometry
Introduction
Global brain reserve, which consists of structural changes (brain reserve) and the ability of the individual to tolerate changes (cognitive reserve), can affect the ultimate impact of brain diseases (1). Changes in global reserve have been shown to impact the progression of dementia and multiple sclerosis (2, 3). In cirrhotic patients with hepatic encephalopathy (HE), cognitive reserve has been shown to modulate the impact of the disease (4), however a detailed analysis of structural brain reserve is unclear. Unlike cognitive reserve, which is a dynamic adaptation based on socio-economic and educational factors, brain reserve is less adaptable and is dependent on disease severity, etiology and progression of disease (2). Specifically, the impact of alcohol, which has a multi-pronged injury on the brain, liver and gut individually and together on brain reserve, in cirrhosis is critical (5). Poor brain reserve in alcoholic cirrhosis could worsen insight regarding disease severity and increase the patients’ vulnerability towards further deterioration, which can impact their transplant suitability. There is evidence in non-cirrhotic studies that brain reserve may not recover despite abstinence (6). If that were indeed the case, then patients with alcoholic cirrhosis would require closer monitoring to detect and prevent cirrhosis progression, simpler communications and greater involvement of caregivers compared to non-alcoholic cirrhotic patients.
We hypothesized that there is worsened brain reserve in alcoholic cirrhotic patients [higher consequences of hyperammonemia on white and gray matter on MR spectroscopy (MRS), increased brain white matter edema on diffusion tensor imaging (DTI) and reduced gray matter density on voxel-based morphometry(VBM)] despite abstinence compared to non-alcoholic cirrhotic patients, which could modulate the impact of hepatic encephalopathy (HE) and the disease course.
Our aim in this study was to (1) analyze brain reserve in the context of HE on multi-modal brain MR imaging in abstinent alcoholic compared to non-alcoholic cirrhotic patients cross-sectionally and (2) evaluate relative change in brain reserve between the groups over time and before/after transjugular intrahepatic portosystemic shunt (TIPS) placement.
Patients and Methods
Outpatients with cirrhosis between 21 and 65 years of age who were able to give informed consent were enrolled from two sites (VAMC and VCU). Cirrhosis was diagnosed using liver biopsy, evidence or history of decompensation (prior HE, ascites, history of variceal bleeding) or endoscopic evidence of varices in patients with chronic liver disease. We excluded patients who were unable to give informed consent, had contra-indications for MRI, and were using alcohol/illicit drugs within 6 months. Patients with alcoholic cirrhosis (based on history, clinical impression and ruling out concomitant diseases) were included if they were abstinent for ≥6 months (based on interviews of patients/family members, random alcohol screening and detailed questioning) were considered alcohol-related (Alc) while the remaining patients were considered non-alcohol related cirrhotics (Nalc). Patients with prior HE were only included if they were controlled on therapy and had a mini-mental status examination score >25 at the day of the study. These patients were sub-grouped as “HE” while others were considered “no-HE” and analyzed separately.
Cross-sectional study
All patients underwent a cognitive battery consisting of psychometric hepatic encephalopathy score (PHES) and inhibitory control tests (ICT) followed by multi-modal MRI scanning (7, 8). PHES and ICT were used to assess psychometric function. The MRI scanning consisted of MRS, DTI and VBM. MR Spectra from both sites were included for the analysis since the analysis method for MRS intrinsically accounts for inter-site variations if the acquisition protocol is the same. DTI and structural scan analysis, which do not have this capability, were performed in the site with the highest number of patients (site 2: VCU).
Prospective study
A subset of patients underwent MRS analysis at least one year apart; during this time disease course was stable without change in medications or development of complications. Another set were studied before and after 30 days of an elective TIPS placement. We then compared the MRS values before/after TIPS and the relative change in these profiles over time. The protocol was approved by the VCU and VA Medical Center IRB.
MRI Methods Outline (details in supplementary information)
MRS can detect and quantify brain metabolites in localized brain regions. Studies have shown a decrease in myo-inositol (mI) and increase in Glutamine+Glutamate (Glx) in cirrhosis, which is widely hypothesized as an astrocytic reaction to hyperammonemia (9).
DTI (10) provides information about the amount and directionality of the motion of water molecules in the brain via three diffusion based metrics. Fractional anisotropy (FA) is a measure of microstructural integrity of the white that ranges from 0 to 1 with higher values representing less random motion of water molecules, which is associated with greater white matter structural integrity. Mean diffusivity (MD) measures the degree of water mobility across cell membranes and is higher with the increase in water movement. Spherical Isotropy (CS) measures isotropic diffusion i.e. the degree to which the water molecule can move freely in the three-dimensional space. Increase in CS indicates higher amounts of extracellular water. VBM(11) is an automated tool to detect localized structural changes in gray matter density due to an underlying disease process.
Statistical and MRI analysis
Statistical analysis
We analyzed clinical and cognitive parameters between Alc and Nalc patients as well as those with and without prior HE. Specific MRI measures (noted below) were compared within the groups with and without HE. Appropriate parametric and non-parametric tests were used with statistical adjustment for multiple comparisons. The data are presented as mean±SEM and p<0.05 was considered significant.
MRS
Choline (Cho), creatine (Cr), myo-inositol (mI) and glutamate+glutamine (Glx) complex peak areas were computed using a quantitative assessment of the metabolite concentration by means of LCModel software and their ratios with creatine were analyzed (12). Alc vs. Nalc groups were compared cross-sectionally, before/after TIPS and after 1-year follow-up.
DTI
Whole brain voxel-wise maps of FA, MD and CS were constructed using FSL tools (13). Thirteen bilateral a priori regions of interest were created using the DTI-based probabilistic white matter atlases (14) using a probability threshold of 40%: frontal and posterior white matter, anterior/posterior internal and external capsule, corpus callosum (genu, body, splenium), cingulum, inferior/superior longitudinal and uncinate fasciculi.
VBM
Structural data acquired at MRI Site 2 was analyzed with FSL-VBM(15) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM).
RESULTS
Patients
174 patients were screened; 26 were excluded (12 current drinkers, 14 could not complete MRI). Ultimately, 46 Alc and 102 Nalc patients were included. Of the 102 Nalc patients, 54 had HCV, 29 had NASH (non-alcoholic steatohepatitis) and 19 had other etiologies. The median duration of alcohol use for Alc patients was 31 years (IQR 16–52 years) and the median abstinence duration was 19 months (IQR 7–49 months). Alcoholic patients had a higher proportion of prior HE and worse cognition (Table 1). Overall HE patients performed worse in both groups (Table 2), however the effect of HE was much more pronounced on Nalc patients. NAlc HE patients had a worse performance on 6 out of 9 tests compared to Nalc no-HE. However, Alc HE patients had worse performance on only 3 tests compared to the Alc No-HE.
Table 1.
Demographic and cognitive testing within the cirrhosis group
| Alcoholic Cirrhosis (n=46) | Non-Alcoholic Cirrhosis (n=102) | |
|---|---|---|
| Age | 53±6 | 56±8 |
| Years of Education | 13±5 | 13±6 |
| Gender (men/women) | 26/20 | 65/37 |
| Race (Caucasian/African-American/Hispanic) | 31/10/5 | 78/21/3 |
| MELD score | 15.2 ± 0.9 | 13.5 ± 0.6 |
| Number (%) with prior OHE | 27 (58%) | 44 (43%)* |
| Serum Sodium (mEq/L) | 135.5 ± 0.8 | 136.8 ± 0.6 |
| Total bilirubin (mg/dL) | 3.0 ± 0.6 | 2.6 ± 0.3 |
| INR | 1.4 ± 0.06 | 1.3 ± 0.03 |
| Serum Creatinine (mg/dL) | 1.06 ±0.06 | 1.01 ± 0.07 |
| Venous Ammonia | 49.9 ± 3.5 | 50.0 ± 2.9 |
| Cognitive testing | ||
| Number connection-A (sec) | 46.4 ± 3.2 | 42.5 ± 2.0 |
| Number connection-B (sec) | 135.9 ± 16.0 | 122.5 ± 9.25 |
| Inhibitory Control Lures (number) | 13.7 ± 1.4 | 11.5 ± 0.9 |
| Digit Symbol (score) | 45.5 ± 2.6 | 52.1 ± 1.7 * |
| Block design (score) | 21.3 ± 1.8 | 26.2 ± 1.3 * |
| Line tracing time (sec) | 122.2 ± 8.6* | 118.3 ± 6.3 |
| Line tracing errors (number) | 41.9 ± 4.9 | 31.57 ± 3.2 |
| Serial Dotting (sec) | 90.1 ± 6.9* | 71.29 ± 3.1 |
| Inhibitory Control Targets (%) | 89.8 ± 2.1 | 95.01 ± 0.61* |
p<0.05 Alc vs. Nalc, data is presented as Mean±SEM; a high score on block design, digit symbol and inhibitory control targets and a low score on the rest indicate poor cognition.
Alc: abstinent alcoholic cirrhosis, Nalc: non-alcoholic cirrhosis.
Table 2.
Comparison of cognitive testing between patients with and without HE within each group. Patients with HE performed worse than those without HE in both Alc and Nalc groups.
| Alcoholic cirrhosis | Non-alcoholic cirrhosis | |||
|---|---|---|---|---|
| No-HE (n=19) | HE (n=27) | No-HE (n=48) | HE (n=44) | |
| Number connection-A (sec) | 41 ± 4.4 | 48.9 ± 4.3 | 37 ± 2.1 | 48.0 ± 3.5** |
| Number connection-B (sec) | 103.8 ± 12.8 | 153.9 ± 22.8 | 89.7 ± 5.2 | 156.23 ± 17.4** |
| Inhibitory Control Lures (number) | 12.9 ± 2.5 | 14.3 ± 1.7 | 11.1 ± 1.3 | 12.6 ± 1.2 |
| Digit Symbol (score) | 53.7 ± 4.5** | 40.4 ± 2.7 | 56.6 ± 2.1** | 47.5 ± 2.8 |
| Block design (score) | 24.5 ± 3.3 | 19.2 ± 2.2 | 27.5 ±1.9 | 24.4 ± 2.0 |
| Line tracing time (sec) | 120.5 ± 15.6 | 120.7 ± 10.6 | 112.5 ± 8.0 | 124.9 ± 10.4 |
| Line tracing errors (number) | 33.1 ± 7.1 | 47.8 ± 6.5 | 28.7 ± 4.3 | 36.3 ± 5.0 |
| Serial Dotting (sec) | 71.5 ± 5.3 | 100.1 ± 9.7* | 65.8 ± 4.3 | 77.4 ± 4.7* |
| Inhibitory Control Targets (%) | 95.5 ± 2.2* | 85.8 ± 3.0 | 97.1 ± 0.6** | 92.6 ± 1.1 |
p<0.05
p<0.005 no-HE vs. HE (within each group); data is presented as Mean±SEM; a high score on block design, digit symbol and inhibitory control targets and a low score on the rest indicate poor cognition.
HE: prior overt hepatic encephalopathy, No-HE: no history of overt hepatic encephalopathy
All 148 patients underwent MRS cross-sectionally. 77 VCU patients underwent DTI and structural scans. Ten patients were studied pre/post TIPS and 26 patients over one year follow-up.
Brain MRI results
Cross-sectional analysis
MRS
Compared to Nalc, Alc group had higher Glx (p=0.017) and lower mI (p=0.008) in parietal white matter, lower mI (p=0.001) in posterior gray matter and higher Glx (p=0.014) and lower mI (p=0.039) in the anterior cingulate (Table 3). Since HE was seen in significantly higher Alc patients, we compared metabolite ratios separately in those with/without HE. In no-HE patients, we found similar significant differences as listed above i.e. in Glx and mI between Alc and Nalc groups. However, in the HE patients these differences disappeared. Furthermore, to test whether HE influenced the metabolite ratios differently in Alc and Nalc groups, we compared the no-HE vs. HE in each group separately. We found that the Nalc group’s brain metabolites were significantly affected by development of HE, unlike the Alc group, which mirrors the cognitive test results.
Table 3.
Cross-sectional MRS
| Right Parietal White Matter | Posterior Gray Matter | Anterior Cingulate Cortex | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cho | Glx | mI | Cho | Glx | mI | Cho | Glx | mI | |
| All | |||||||||
| Alc (n=46) | 0.256 | 2.788* | 0.302 | 0.192 | 2.784 | 0.348 | 0.245 | 3.314* | 0.425 |
| Nalc (n=102) | 0.266 | 2.439 | 0.465* | 0.186 | 2.617 | 0.493* | 0.253 | 2.925 | 0.526* |
| No-HE | |||||||||
| Alc (n=13) | 0.261 | 2.587* | 0.513 | 0.180 | 2.760* | 0.432 | 0.249 | 3.128* | 0.522 |
| Nalc (n=46) | 0.287 | 2.040 | 0.644‡‡ | 0.190 | 2.268 | 0.621*‡‡ | 0.262 | 2.525 | 0.635‡‡ |
| HE | |||||||||
| Alc (n=27) | 0.254 | 2.911‡‡ | 0.229 | 0.196 | 2.773 | 0.336 | 0.241 | 3.299 | 0.386 |
| Nalc (n=44) | 0.240‡‡ | 2.791‡‡ | 0.296 | 0.186 | 2.953‡‡ | 0.389 | 0.245 | 3.355‡‡ | 0.417 |
p<0.05 Alc vs. Nalc ;
p<0.005 no-HE vs. HE (for Alc & Nalc separately).
Alcoholic cirrhosis group (Alc) had higher Glx and lower mI than non-alcoholic cirrhosis group (Nalc). This difference disappeared with the presence of HE. Furthermore, results show that HE influence is predominant in Nalc but not in Alc.
DTI metrics
We found that in all regions studied; the Alc group had lower FA, higher MD and higher CS as compared to Nalc group with differences reaching statistical significance in many brain regions such as external capsule, inferior longitudinal fasciculus, corpus callosum, cingulum and the uncinate fasciulus. Similar to MRS findings, we found that HE status predominantly affects FA and CS but not MD in the Nalc patients as compared to Alc group (Table 4).
Table 4.
Diffusion metrics
| Fractional Anisotropy | Left External capsule | Right External capsule | Left Posterior Internal capsule | Right Posterior Internal capsule | Left Uncinate fasciculus | |
|---|---|---|---|---|---|---|
| All | Alc (n=23) | 0.404 | 0.404 | 0.664 | 0.663 | 0.491 |
| Nalc (n=54) | 0.423** | 0.422** | 0.674 | 0.676* | 0.530* | |
| No-HE | Alc (n=7) | 0.399 | 0.404 | 0.664 | 0.663 | 0.452 |
| Nalc (n=24) | 0.432** | 0.430* | 0.684* | 0.686* | 0.542** | |
| HE | Alc (n=16) | 0.406 | 0.404 | 0.664 | 0.664 | 0.508‡ |
| Nalc (n=26) | 0.419*‡ | 0.418*‡ | 0.665*‡ | 0.666‡‡ | 0.528 |
| Mean Diffusivity | Left External capsule | Right External capsule | Left Longitudinal fasciculus | Right Longitudinal fasciculus | Left Uncinate fasciculus | |
|---|---|---|---|---|---|---|
| All | Alc (n=23) | 0.813* | 0.805* | 0.823 | 0.822 | 0.803* |
| Nalc (n=54) | 0.790 | 0.785 | 0.791 | 0.804 | 0.781 | |
| No-HE | Alc (n=7) | 0.802 | 0.800 | 0.821 | 0.819 | 0.810** |
| Nalc (n=24) | 0.788 | 0.783 | 0.794 | 0.804 | 0.773 | |
| HE | Alc (n=16) | 0.818** | 0.808* | 0.825** | 0.823* | 0.800 |
| Nalc (n=26) | 0.789 | 0.785 | 0.786 | 0.801 | 0.782 |
| Spherical Isotropy | Left External capsule | Right External capsule | Left Longitudinal fasciculus | Right Longitudinal fasciculus | Left Uncinate fasciculus | |
|---|---|---|---|---|---|---|
| All | Alc (n=23) | 0.628** | 0.621* | 0.527* | 0.515 | 0.538** |
| Nalc (n=54) | 0.610 | 0.606 | 0.498 | 0.493 | 0.502 | |
| No-HE | Alc (n=7) | 0.636** | 0.622 | 0.523 | 0.504 | 0.571** |
| Nalc (n=24) | 0.604 | 0.599 | 0.495 | 0.489 | 0.495 | |
| HE | Alc (n=16) | 0.624 | 0.620* | 0.529* | 0.520 | 0.523 |
| Nalc (n=26) | 0.615‡ | 0.610‡ | 0.499 | 0.495 | 0.503 |
p<0.05
p<0.005 Alc vs. Nalc;
p<0.05
p<0.005 no-HE vs. HE (for Alc & Nalc separately).
Alc group had lower fractional anisotropy (FA), higher mean diffusivity (MD) and spherical isotropy (CS) in major white matter regions indicating higher interstitial edema compared to Nalc. As with MRS findings, HE status was a predominant factor in the Nalc group alone but not in Alc. EC external capsule; PIC posterior internal capsule; UF uncinate fasciculus; L/R left/right; MD expressed in × 10−3 mm2/s
VBM results
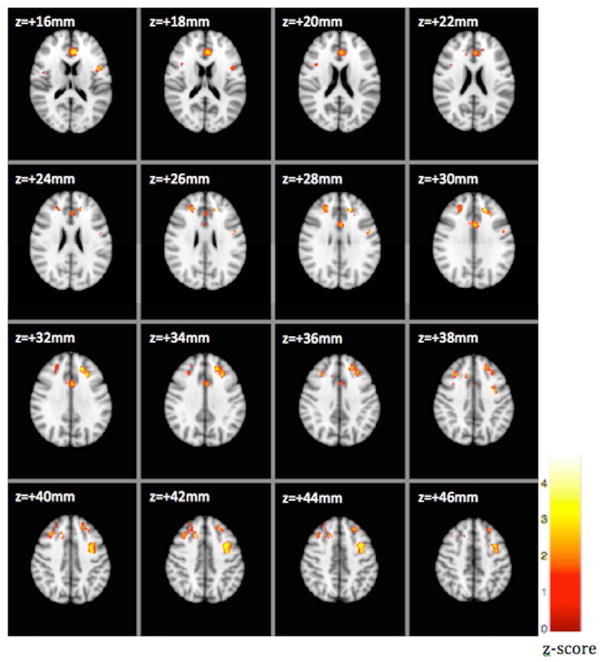
We analyzed structural images from 21 Alc and 21 Nalc patients. The number of patients chosen in each group was the same because VBM is sensitive to the potential bias in gray matter density if groups are unequal. We found that the gray matter density in the Alc was significantly reduced when compared with the Nalc group in many gray matter regions such as inferior, superior and middle frontal gyri, anterior cingulate gyrus, precentral gyrus, central opercular cortex and postcentral gyrus. Significant clusters corrected for multiple comparisons, locations and peak z-scores are shown in Figure 1 and Supplementary Table 1, respectively. Influence of HE on each group could not be tested because of the limited degrees of freedom available for VBM analysis.
Figure 1.
FSLVBM showed reduced gray matter density in the alcoholic cirrhosis group as compared to the non-alcoholic cirrhosis group. Voxelwise GLM was applied using permutation-based non-parametric testing, correcting for multiple comparisons across space. Threshold p-value of 3.1 was then applied to the resultant map to form clusters for reporting purposes. FSLVBM, voxel-based morphometry as implemented in FMRIB’s Software Library
Pre-Post TIPS
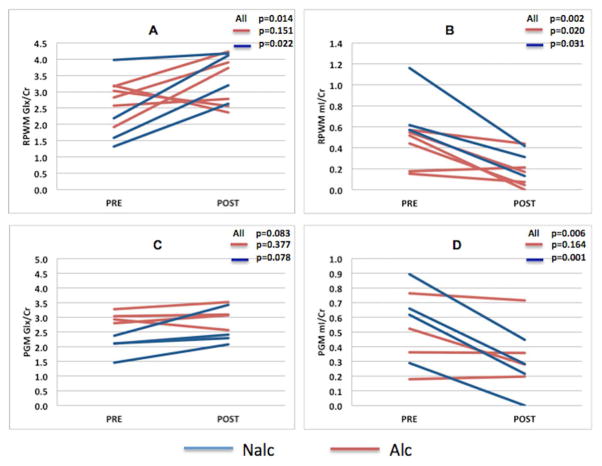
10 patients were scanned pre and post TIPS procedure (mean 34±7 days before and 25±14 days after TIPS placement). Six patients had alcoholic cirrhosis and 4 had non-alcohol related cirrhosis. The reason for TIPS placement was refractory ascites in 8 and hepatic hydrothorax in 2 patients. The change in hepatic portal venous pressure gradient was 7±3 mmHg (Alc 8±5 mmHg, Nalc 7±5 mmHg, p=0.62). In the right parietal white matter (RPWM) a brain region associated with attention and working memory ability, after TIPS there was a significant overall (Alc+Nalc combined) increase in Glx (pre: 2.776 ± 0.77, post: 3.257 ± 0.80, p=0.014) and decrease in mI (pre: 0.431 ± 0.29, post: 0.173 ± 0.16, p=0.002, whereas in posterior gray matter (PGM) there was a decrease in mI alone (pre: 0.402 ± 0.24, post: 0.312 ± 0.21, p=0.006). These results suggest a neuro-metabolic profile conducive to the development of HE, after TIPS. When the effect of TIPS on brain metabolites was compared separately in Alc and Nalc subgroups, we found that in parietal white matter, only the Nalc group showed significant change in Glx (pre: 2.402 ± 1.08, post: 3.253 ± 0.90, p=0.043) whereas mI decreased significantly both in Alc (pre: 0.352 ± 0.17, post: 0.157 ± 0.16, p=0.020) and Nalc (pre: 0.609 ± 0.44, post: 0.193 ± 0.17, p=0.031) groups. In the posterior gray matter, only Nalc group showed a significant decrease in mI (pre: 0.491 ± 0.28, post: 0.236 ± 0.19, p=0.001). This could be explained by the observation that before TIPS, the Glx and mI levels in Alc were already much higher and lower respectively, relative to Nalc group (Supplementary Table 2). Seven patients (3 in each Alc/Nalc group) developed an HE episode within 3 months of TIPS placement, however due to the sample size relative MRI changes were not significantly different.
Prospective study after 1-year follow-up
MR Spectroscopy data from 26 patients (11 Alc 15 Nalc) was considered for analysis. These patients completed both visits with a median of 11 months (IQR 10–13 months) apart. No significant changes were found in Cho, Glx and mI in any region between the two visits when considering Alc and Nalc groups separately or combined. However Glx was consistently higher and mI was consistently lower in Alc group compared to Nalc in all three spectroscopic volumes of interest, which was similar to the baseline results (Alc vs. Nalc; baseline visit: PGM Glx p=0.012; PGM mI p=0.041; visit 2 : RPWM Glx p=0.006; RPWM mI p=0.050; PGM Glx p=0.041; PGM mI p=0.007; ACC Glx p=0.02, ACC mI p=0.04) (supplementary Table 3).
Discussion
The current results demonstrate that despite abstinence, brain reserve as shown using the MRI neuro-metabolic and neuro-structural profile is significantly impaired in abstinent alcoholic patients compared to non-alcoholic cirrhosis patients. Further, these differences remain stable over time. Prior HE status or development of new HE after TIPS results in brain reserve deterioration to a larger extent in Nalc patients.
The concept of global reserve, defined as the combination of brain and cognitive reserve is clinically relevant in cirrhosis and especially in HE because it could determine the disease course and impact the patient’s ability to comprehend and follow treatment strategies. In our particular population, we found changes in both cognitive and brain reserve that translated into impaired cognitive performance in Alc patients. This indicates a double insult in Alc patients. Brain reserve was impaired on several different aspects of the multi-modal MR imaging and remained stable over time. On the other hand, we found that since brain reserve was already impaired in Alc subjects, the differential change with HE in that population was not as marked compared to Nalc patients. Therefore a nuanced approach to treating Nalc patients who have developed HE, and would consequently suffer greater brain reserve changes compared to Alc patients, is needed. Ultimately, in those who have a poor brain reserve, or those who are expected to worsen, the clinical team may require closer monitoring to detect and prevent cirrhosis progression, simplified communication and greater caregiver involvement in the treatment plan. This may also prevent non-adherence to medications and clinic visits that ordinarily would lead to inactivation or delay in liver transplant listing.
The study of alcohol in cirrhosis is complicated by the ability of alcohol to affect the brain directly or indirectly through involvement of the liver and the gut. These effects are manifested as gray matter atrophy and microstructural damage in the white matter. Brain volume recovers after short-term abstinence in non-cirrhotics (6-weeks) but does not result in brain volumes equivalent to controls (6). It has been reported that even with continued abstinence some neuronal loss may be irreversible (16). Based on DTI studies, white matter reduction is seen in alcoholics who relapse (17); and continued abstinence is associated with increase in white matter regions like corpus callosum and subcortical white matter (18). This suggests that white matter regions maybe more susceptible to alcohol effects than gray matter, and more likely to recover after abstinence. The impact of alcohol and cirrhosis together is more nuanced given that there may be evidence of direct alcohol-related brain injury as shown in the studies above and also indirect brain injury related to cirrhosis caused by the alcohol (5). This would explain why there was continued impairment in the neuro-metabolic profile despite longer abstinence over time in Alc patients as compared to 6-weeks abstinence reported previously.
The pathogenesis of brain functional abnormalities in cirrhosis is hypothesized to be due to a combination of hyperammonemia (usually detected with MRS), systemic inflammation leading to neuro-inflammation (white matter DTI changes) along with structural changes (morphometric analysis)(19). The current results demonstrate that the poor brain reserve is related to structure, edema and neuro-metabolic profile changes. The cross-sectional analysis in patients without prior HE clearly showed worse consequences of ammonia in the brain demonstrated by a higher Glx and compensatory mI decrease in all regions tested compared to Nalc patients. These consequences of hyperammonemia were further exacerbated by the DTI-associated impairment in the white matter in Alc patients. Alc patients showed higher MD and higher CS compared to Nalc patients. This indicates reduced intracellular water and increased extracellular water respectively, which is suggestive of interstitial edema. We also observe a reduction in FA in the Alc patients possibly due to dilution effect of extracellular water (20) or chronic alcohol induced disruption of cell cytoskeleton (21). Previous diffusion studies have also found similar elevation in MD and reduction in FA in alcoholic cirrhosis patients with or without HE compared to controls (22, 23) in similar regions found in this study. DTI findings in uncomplicated alcoholism too have found reduced FA in tracts of the corpus callosum, internal and external capsules, fornix, longitudinal fasciculi (10) and fronto-limbic fibers (24). These results suggest that the direct effect of alcohol (microstructural damage) on the brain may preexist and combine with cirrhosis-related effects at least in certain white matter regions to a degree that they are prevalent even after accounting for abstinence. We found similar results in gray matter since Alc patients had lower gray matter density in key reward-related and attentional areas of the frontal brain. Morphometry-based studies have implicated these regions in alcohol dependent and alcohol-abstinent non-cirrhotics (17). Our VBM based results extended a prior study showing similar gray matter areas being affected in a larger population (11). Future studies evaluating cirrhotic patients before and after abstinence are needed to evaluate the potential reversibility of these effects. Strengths of the present study include a large sample of Alc and Nalc patients with and without HE, and use of acquired MRS and DTI data in addition to VBM to facilitate insight concerning the underlying etiologic differences. It is discouraging that abstinence from alcohol in the setting of cirrhosis has limited improvement, with regard to impact on brain function. The patients who develop alcoholic cirrhosis typically have sustained a pattern of drinking over several decades. Relatively greater improvement in brain reserve with abstinence in non-cirrhotic binge drinkers may reflect the brain’s ability to recover with temporal gaps in toxin exposure (25).
A striking finding was that super-added HE or insertion of a TIPS that mimic HE, did not significantly worsen the MR imaging parameters or brain reserve in Alc patients, in contrast to Nalc. Overall results suggest that changes in Alc patients before HE potentially predispose them to have a significantly lower brain reserve compared to Nalc patients. Interestingly, similar changes are found in most HE patients regardless of the etiology of cirrhosis. Indeed in our population, Alc and Nalc subjects did not differ from each other with regard to MRS values once they reached HE. This indicates that the brain-associated consequences of hyperammonemia and neuro-inflammation starts earlier in the Alc patients. It is interesting that this difference in Alc and Nalc patients occurred similar venous ammonia levels, which underlines the difficulty in extrapolating systemic ammonia levels to changes in the brain milieu (26). The onset of HE in our population extinguished differences in etiologies on brain MRS findings but not on predominant white-matter changes. The supremacy of HE in the determination of the ultimate brain metabolic profile is in agreement with prior cognitive studies showing that once patients reach HE, there is impaired learning that is not etiology-dependent (27, 28). Therefore, this should alert the clinical teams to closely monitor and potentially initiate therapy for HE prevention in those with alcoholic cirrhosis compared to non-alcoholics. Our 1-year follow-up MRS study provided further evidence in support of this. We found that in the absence of progression of underlying liver disease, medication and change in HE status, the MRS profiles did not change significantly. However, metabolite profiles in Alc were worse than Nalc to begin with and continued to be so after a year. Interpreting these findings of altered neuro-metabolic profile, decreased gray matter density, and increased white matter edema, points to several layers of brain dysfunction that conspire with a poor cognitive reserve in making these patients more vulnerable to further deterioration.
Despite a few reports showing that Alc patients are relatively protected from HE development after TIPS, the potential mechanism was unclear (29). Our results show that the relative deterioration in the neuro-metabolic profile in Alc patients’ post-TIPS is insignificant compared to Nalc patients. This is probably due to the fact that these patients have already been exposed to the “maximal” Glx in their astrocytes even before the TIPS. In contrast, Nalc patients have lower pre-TIPS Glx. Therefore, these subjects will be vulnerable to experience a higher scope of change after TIPS and subsequent HE development. Therefore the potential for brain reserve change in Nalc patients is greater and they should be counseled regarding a significantly higher potential loss of functionality after TIPS.
Our study is limited by the exclusion of currently drinking cirrhotics; however it would have been difficult to interpret the relative impact of alcohol compared to cirrhosis in that setting. Our sample size did not allow us to focus on individual non-alcoholic etiologies, which were all grouped as one. However, the overall impact of alcoholic etiology overrode these potential discrepancies within the non-alcoholic group. The lack of patients who developed HE on 1 year follow-up and those pre/post-TIPS limited the ability of the brain MR profile to predict these events, which would require future studies.
We conclude that cirrhotic patients with an alcohol based etiology suffer a specific impact on brain reserve, which is modulated by HE despite abstinence. The relative changes after HE development vary between alcoholic and non-alcoholic cirrhotic patients. The knowledge of the structural and functional basis of cognitive and brain reserve in cirrhotic patients of different etiologies could guide medical teams to tailor their communications and monitoring of the affected patients and caregivers.
Supplementary Material
Figure 2.
Changes in brain metabolites after TIPS. Overall, Glx increases and mI decreases post-TIPS in the right parietal white matter (RPWM) (A,B) and posterior grey matter (PGM) (C,D). Relative change in metabolites before and after TIPS was higher in the non-alcoholic cirrhosis (Nalc) group as compared to alcoholic cirrhosis patients(Alc).
Acknowledgments
Grant and Financial Support: This was partly supported by VA Merit Review CX001076, RO1AA020203 from the National Institute on Alcohol Abuse and Alcoholism, by grant RO1DK087913 from the National Institute of Diabetes and Digestive and Kidney Diseases, and by the McGuire Research Institute.
Abbreviations
- no-HE
no prior overt hepatic encephalopathy
- HE
prior overt hepatic encephalopathy
- MRS
MR spectroscopy
- DTI
diffusion tensor imaging
- VBM
voxel-based morphometry
- Alc
abstinent alcoholic cirrhotic patients
- Nalc
non-alcoholic cirrhotic patients
- TIPS
transjugular intra-hepatic porto-systemic shunt
- mI
myoinositol
- Glx
glutamate+glutamine
- PHES
psychometric hepatic encephalopathy score
- ICT
inhibitory control test
- CS
spherical isotropy
- FA
fractional anisotropy
- MD
mean diffusivity
- RPWM
right parietal white
- PGM
posterior gray
- ACC
anterior cingulate cortex
Footnotes
Conflicts of interest: none for any author
References
- 1.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 2.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 3.Sumowski JF, Chiaravalloti N, Krch D, Paxton J, Deluca J. Education attenuates the negative impact of traumatic brain injury on cognitive status. Arch Phys Med Rehabil. 2013;94:2562–2564. doi: 10.1016/j.apmr.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Patel AV, Wade JB, Thacker LR, Sterling RK, Siddiqui MS, Stravitz RT, Sanyal AJ, et al. Cognitive Reserve Is a Determinant of Health-related Quality of Life in Patients With Cirrhosis, Independent of Covert Hepatic Encephalopathy and Model for End-Stage Liver Disease Score. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 7.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. e1591. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Sarma MK, Huda A, Nagarajan R, Hinkin CH, Wilson N, Gupta RK, Frias-Martinez E, et al. Multi-dimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. Metab Brain Dis. 2011;26:173–184. doi: 10.1007/s11011-011-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guevara M, Baccaro ME, Gomez-Anson B, Frisoni G, Testa C, Torre A, Molinuevo JL, et al. Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol. 2011;55:564–573. doi: 10.1016/j.jhep.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 14.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tractspecific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, et al. Anatomically related grey and white matter abnormalities in adolescentonset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 16.Harper C. The neurotoxicity of alcohol. Hum Exp Toxicol. 2007;26:251–257. doi: 10.1177/0960327107070499. [DOI] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 18.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rovira A, Alonso J, Cordoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008;29:1612–1621. doi: 10.3174/ajnr.A1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath K, Saraswat VA, Krishna YR, Thomas MA, Rathore RK, Pandey CM, Gupta RK. Quantification of cerebral edema on diffusion tensor imaging in acute-onchronic liver failure. NMR Biomed. 2008;21:713–722. doi: 10.1002/nbm.1249. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 22.Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Gupta RK, Elderkin-Thompson V, Huda A, Sayre J, Kirsch C, Guze B, et al. Voxel-based diffusion tensor magnetic resonance imaging evaluation of lowgrade hepatic encephalopathy. J Magn Reson Imaging. 2008;27:1061–1068. doi: 10.1002/jmri.21342. [DOI] [PubMed] [Google Scholar]
- 24.Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, Orduna J, et al. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry. 2010;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188–193. doi: 10.1016/s0002-9343(02)01477-8. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riggio O, Ridola L, Pasquale C, Nardelli S, Pentassuglio I, Moscucci F, Merli M. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2010;9:181–183. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Somberg KA, Riegler JL, LaBerge JM, Doherty-Simor MM, Bachetti P, Roberts JP, Lake JR. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995;90:549–555. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.