Abstract
Background context
Intervertebral discs (IVD) are attractive targets for local drug delivery because they are avascular structures with limited transport. Painful IVDs are in a chronic inflammatory state. While anti-inflammatories show poor performance in clinical trials their efficacy treating IVD cells suggests that sustained, local drug delivery directly to painful IVDs may be beneficial.
Purpose
To determine if genipin crosslinked fibrin (FibGen) with collagen type I hollow spheres (CHS) can serve as a drug delivery carrier for the anti-TNFα drug, infliximab. Infliximab was chosen as a model drug because of the known role of TNFα in increasing downstream production of several pro-inflammatory cytokines and pain mediators. FibGen was used as drug carrier because it is adhesive injectable, slowly degrading hydrogel with potential to seal annulus fibrosus (AF) defects. CHS allow simple and non-damaging drug loading and could act as a drug reservoir to improve sustained delivery.
Study Design/Setting
Biomaterials and human AF cell culture study to determine drug release kinetics and efficacy.
Methods
Infliximab was delivered at low and high concentrations using FibGen with and without CHS. Gels were analyzed for structure, drug release kinetics, and efficacy treating human AF cells following release. This work was funded by grants from the NIAMS/NIH (R01 AR057397), AO Foundation, and from the Icahn School of Medicine at Mount Sinai.
Results
Fibrin showed rapid infliximab drug release but degraded quickly. CHS alone showed a sustained release profile but the small spheres may not remain in a degenerated IVD with fissures. FibGen showed steady and low levels of infliximab release that was increased when loaded with higher drug concentrations. Infliximab was bound in CHS when delivered within FibGen and was only released following enzymatic degradation. The infliximab released over 20 days retained its bioactivity as confirmed by the sustained reduction of IL-1β, IL-6, IL-8, and TNFα concentrations produced by AF cells.
Conclusions
Direct mixing of infliximab into FibGen was the simplest drug loading protocol capable of sustained release. Results show feasibility of using drug-loaded FibGen for delivery of infliximab and, in the context with the literature, show potential to seal AF defects and partially restore IVD biomechanics. Future investigations are required to determine if drug-loaded FibGen can effectively deliver drugs, seal AF defects, and promote IVD repair or prevent further IVD degeneration in vivo.
Keywords: intervertebral disc, annulus fibrosus repair, biomaterials, anti-inflammatory drug, drug delivery, spinal injection
Introduction
Regenerative repair of degenerated intervertebral discs (IVDs) is a clinical challenge due to the large spinal loading that must be resisted immediately following repair and the chronic inflammatory environment of injured and degenerated IVDs [1–3]. IVDs have limited self-repair capabilities following traumatic injuries or degenerative damage, and un-repaired ruptures can lead to accelerated IVD degeneration which is implicated as a source of low back pain [1, 2, 4]. The patterns of IVD pathology are distinct between degenerative disc disease and IVD herniation [5] with few treatments available for either condition. Herniated IVDs are more amenable to IVD repair as they often involve annulus fibrosus (AF) injury in an otherwise ‘healthy’ IVD [1]. Tears and fissures of the AF can progress to herniations that interact mechanically and chemically with spinal nerves and tissues to induce painful conditions [1, 6–9]. Microdiscectomy surgery to remove the prolapsed tissue responsible for painful conditions is effective at resolving acute pain from herniation [10]. However, herniation with or without microdiscectomy likely leads to accelerated IVD degeneration [11], which may be due to increased inflammation and altered biomechanics associated with the unrepaired AF defects [2, 12, 13]. Repair of the AF is required to restore the IVD structure and normal function; yet effective IVD repair strategies remain an unmet clinical need [1, 2, 14, 15]. We believe that microdiscectomy procedures can be improved with the development of effective strategies that are capable of repairing the AF, sealing the IVD, and providing local delivery of therapeutic drugs directly to the IVD. Determining the repair capacity of injured human AF requires the development of novel biomaterials and effective repair strategies to be tested using animal models and clinical studies. This study focuses on biomaterial development for drug delivery.
Pro-inflammatory cytokines have an important role in all aspects of IVD disease including pain and are a relevant therapeutic target [3, 16]. TNFα and IL1-β increase with severity of disease [17, 18] and TNFα up-regulates pain-related proteins [19]. TNFα is prominently implicated in injured and diseased human IVDs since it increases downstream production of pro-inflammatory cytokines (IL-1β, IL6, IL-8) and propagates matrix degrading enzymes and pain mediators [19–22].
Anti-inflammatory cytokines exhibited strong potential to ameliorate structural damage and mitigate neuropathic pain in cell, organ culture, and animal models but have not provided consistent results in human clinical trials directed at alleviating the symptoms of back or radicular pain associated with IVD pathologies [3]. In human trials a TNFα-blocking agent was intravenously injected to treat disc herniation induced sciatica, yet there was no improvement in conditions compared to placebo treatment [23, 24]. In contrast, local delivery of the anti-TNFα drug for sciatica via epidural administration or sub-cutaneous injection was effective in reducing pain with no observed adverse side effects [25] and also reduced the long-term need for surgery [26]. In another study, epidural delivery of anti-TNFα was less effective than epidural steroidal treatments in improving pain in a multicenter trial, although no statistically significant differences were detected and the authors noted that it was impossible to conclude whether a TNFα inhibitor delivered for longer times or at higher doses could have been more effective [27]. In an in vivo experimental study in rats, lumbar disc puncture through the AF induced pain associated behavioral changes, which were decreased with local infliximab delivery to the IVD [28]. Systemic drug administration for back pain is often not effective, which is likely due to the poor transport environment inhibiting delivery of drugs into the IVDs or due to rapid absorption from the epidural space [4, 16, 29]. Therefore, local drug delivery to the IVD may offer more promise. We believe that the mixed results in clinical trials are related to the lack of sustained delivery of drugs at appropriate dose to the painful IVD, and may also be associated with assessments of pain alone without evaluation of IVD degeneration and the inherent causes of that pain.
Local delivery of anti-inflammatories to the IVD shows some promise, yet few technologies are available for such delivery. IL-1ra delivery to agarose-based engineered nucleus pulposus constructs in vitro were able to inhibit degradation from IL-1β, with decreased mRNA expression and activity of catabolic enzymes and pro-inflammatory cytokines, improved GAG content, and restored mechanical properties [30]. Local and sustained delivery of anti-inflammatory drugs into the IVD has the potential to target cells in the IVD and epidural space and may inhibit cell catabolism, reduce painful conditions, and promote IVD regeneration.
Herniation and IVD injuries more generally involve increased TNFα and can also lead to stress adjacent to fissures and defects in the tissue. TNFα can interact with mechanical stretch to amplify the downstream production of multiple pro-inflammatory cytokines (IL-1b, IL-6, IL-8) by AF cells [31]. As a result, therapies that can restore mechanical properties of damaged IVDs and inhibit the pro-inflammatory cascade may offer the most promise. FibGen is a fibrin-based adhesive hydrogel that has been crosslinked with genipin to tune its biomechanical behaviors to match native AF tissue and allow for cell proliferation [32, 33]. FibGen undergoes slow in vitro and in vivo degradation, can be easily mixed and injected to seal the AF, and was able to partially restore IVD biomechanical behaviors in organ culture [32, 34]. These properties make FibGen an attractive carrier for drug delivery for IVD repair.
The objective of this study was to functionalize FibGen for controlled and sustained release of an anti-TNFα drug, infliximab. FibGen was functionalized by directly incorporating the drug into FibGen or by embedding drug-loaded collagen type I hollow spheres (CHS) into FibGen. CHS are an extracellular matrix-based biomolecule delivery reservoir system that allow for simple drug loading through an electrostatic mechanism [35, 36]. The FibGen carrier system has greater stiffness and slower degradation rates than fibrin alone, suggesting it may serve as a stable sealant for AF repair. CHS were included to act as a drug reservoir and to extend sustained release. We hypothesized that FibGen can be used as a carrier of anti-TNFα either by direct incorporation or by embedding anti-TNFα loaded CHS, and that the incorporation of CHS will improve the sustained release of infliximab.
Methods
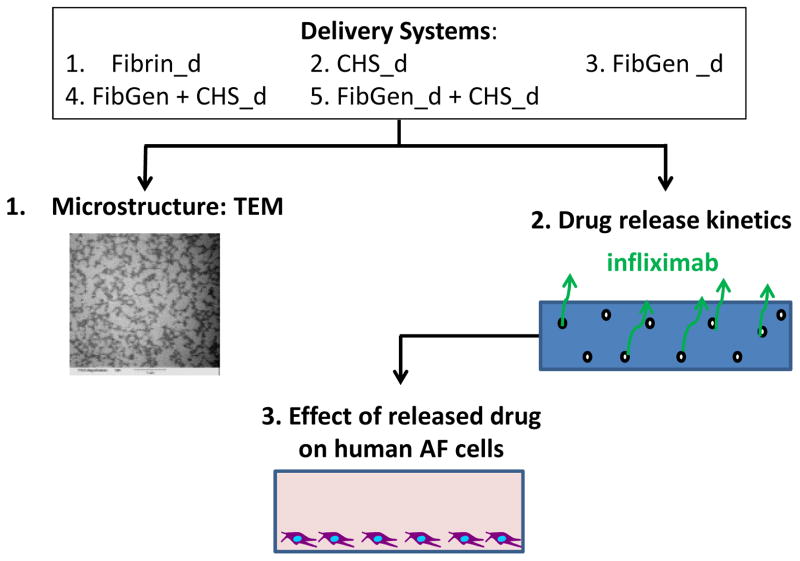
Experimental groups comprising different combinations of fibrin, FibGen and CHS and different doses of infliximab were evaluated with TEM and ELISA (Figure 1). The microstructure of un-loaded CHS were observed using TEM and compared to FibGen alone, FibGen+CHS, and Fibrin alone. Kinetics of drug release were assessed with an infliximab-specific enzyme-linked immunosorbent assay (ELISA), and feasibility of enhanced drug release in vivo was evaluated by additional culturing with plasmin, a broad spectrum serine proteinase capable of rapidly degrading fibrin. Efficacy of released infliximab was assessed with a bioactivity assay by treating TNFα-stimulated AF cells with the released drug and measuring their pro-inflammatory cytokine production using a multiplexed ELISA.
Figure 1. Study Design.
Five delivery systems were prepared using fibrin loaded with infliximab (Fibrin_d), collagen hollow spheres loaded with infliximab (CHS_d), FibGen loaded directly with infliximab (FibGen_d), FibGen with infliximab loaded collagen hollow spheres (FibGen+CHS_d), and Infliximab loaded FibGen with infliximab loaded collagen hollow spheres (FibGen_d+CHS_d), where ‘_d’ refers to the drug dose. Collagen hollow sphere morphology was examined via transmission electron microscopy. Drug release kinetics were measured over 20 days for all delivery systems. Pro-inflammatory cytokine concentration production from AF cells was measured over 20 days.
Collagen Hollow Sphere Fabrication and Characterization
CHS were fabricated using 1 μm diameter polystyrene beads which were sulfonated and coated in a type I collagen solution as previously described [35]. The coating was cross-linked with pentaerythritol poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-PEG), and the inner polystyrene bead was dissolved by washing in tetrahydrofuran (THF) to create a hollow sphere. CHS were characterized with transmission electron microscopy (TEM) to insure they retained their hollow configuration once embedded within the FibGen hydrogel and to characterize morphology of the sphere and gel.
Drug Delivery System Preparation
Five drug delivery systems for infliximab delivery were prepared: 1) CHS alone (1μm, 400μg) loaded with 320 μg infliximab, 2) FibGen alone (250 μl) directly mixed with 320 μg, 2.5 mg or 7.5 mg drug, 3) FibGen +CHS loaded with 320 μg drug, and 4) FibGen with 2.5 mg drug+ CHS with 320 μg drug and 5) Fibrin alone (250 μl) directly mixed with 394 μg infliximab.
Infliximab was obtained from the pharmacy at Mount Sinai Medical Center in a powder form and was reconstituted with PBS to create a stock solution. Doses were determined from pilot optimization studies. The 320 μg dose of infliximab in the CHS was the maximum amount we were able to accurately and repeatedly load. The 394 μg infliximab dose in fibrin was intended to be comparable to the dose in CHS but was slightly adjusted to allow easy mixing based on concentrations of the infliximab stock dissolved into the thrombin component of the fibrin solution. FibGen was loaded with 320 μg to be comparable to the dose in CHS and was also loaded with much larger doses, 2.5 mg or 7.5 mg drug in total gel volume, to assess feasibility of mixing high concentrations of these drugs. The reconstituted infliximab at a concentration of 10 mg/ml or 30 mg/ml was used to dissolve the fibrinogen component of FibGen, respectively. The 7.5mg dose started approaching limitation in mixing and handling due to increased viscosity of the dissolved fibrinogen with high drug stock concentration.
Drug Loading into Collagen Hollow spheres
To assess drug loading efficiency, 400 μg of collagen hollow spheres (average diameter of 1μm) and 320 μg Infliximab (in PBS) were added into a 1.5 ml low-binding tube, and incubated for 24 hours at room temperature on the Glas-Col Terre Haute rotator. Using the Infliximab assay, we measured the residual drug in the media to determine the amount of drug successfully loaded into the CHS.
Production of Infliximab Conditioned Media
Each gel-based delivery system was incubated on a shaker in 37°C and the microspheres suspended in media delivery systems were incubated on a rotating shaker in 37°C. We measured the released drug in media at 3 or 4 day intervals over the course of 35 days. The microspheres were centrifuged at 4000 revolutions per minute (rpm) for 10 minutes, supernatant was collected, and the microspheres were suspended in fresh experimental media. For the gel-based delivery systems, supernatant media was collected and fresh experimental media was added into these systems. The collected supernatant media were kept frozen at −80C until later application onto cells for bioactivity testing.
ELISA for Infliximab and Concentration Calculations
To determine the feasibility of using a carrier to deliver infliximab to the IVD space, the cumulative concentration of drug released from Fibrin alone, CHS alone, FibGen alone, and FibGen+CHS over 20 days was measured using an ELISA as described [37]. A 96-well plate was sensitized with 100 μl of 0.75 μg/ml TNFα in coating buffer (1 mol/L carbonate-bicarbonate buffer, pH 9.6) overnight at 4°C. The well-plate was washed four times with PBS + 0.05% Tween 20. Then we added 200 μl of blocking buffer (PBS + 1% BSA) to block the protein-binding sites for two hours at room temperature. The plate was again washed four times with PBS + 0.05% Tween 20. Duplicates of standard Infliximab concentrations of 0, 0.5, 5, 10, 25, 50, 100, 200 ng/ml in experimental media were added into the first two columns of the well-plate and the samples in appropriate dilutions in experimental media were added into the wells of the plate. The plate was placed on the C1 Platform Shaker (New Brunswick Scientific Classic Series) for two hours at 37C at the speed of 350 rpm. Followed by 4 washes, 100 μL of 1:30,000 HRP-anti IgG diluted in 1% PBS-BSA was added into each well for one hour in room temperature. After 4 additional washes, 100 μL of OPD peroxidase substrate (dissolve tablet in 20 ml H2O within one hour before use) at room temperature in the dark for 20 minutes. The color reaction was stopped by adding 50 μl of 2 mol/L sulfuric acid, and plates were read using Molecular Device’s SpectraMax M5 and SoftMax Pro 5 program at 492 and 620 nm.
A standard curve for Infliximab was generated for each time point during the 35-day experimental period. The concentration of the drug released from each delivery system was determined using dilutions of samples in each delivery system ranging from 1:1 to 1:10,000 so that the yielded absorbance would fall in the calibrated range of the standards. A four parameter curve fit of log (concentration) vs. absorbance was used to calculate concentrations based on a curve-fit absorbance = Bottom + (Top-Bottom)/(1+10^((LogEC50-concentration)*HillSlope)), where Top, Bottom, HillSlope and EC50 are the 4 parameters describing the shape of the curve.
Bioactivity Assay for Measuring Efficacy of Released Infliximab
Media from two selected delivery systems, CHS_320μg and FibGen_7.5mg, was collected at three time points to test bioactivity of released infliximab. This infliximab conditioned media was then applied to TNFα-treated (10 ng/ml) human AF cells that had previously been isolated from surgical samples (n=4). Human AF cells were cultured in a 96 well-plate at a density of 100,000 cells/mL in 125 μL of day 6, day 12, or day 20 infliximab conditioned media in culture conditions of 37°C, 5% O2, 5% CO2 for two days.
ELISA for Pro-Inflammatory Cytokines
ELISA was used to measure pro-inflammatory cytokine concentrations in AF cell supernatant from infliximab conditioned media treated cells and TNF-α treated cells. Culture medium was analyzed using a multiplex ELISA specific for human TNF-α, IL-1β, IL-6, and IL-8 (MSD N45025B-1, http://www.mesoscale.com) following manufacturer’s instructions.
Results
Collagen Hollow Sphere Morphology and Drug Loading Efficiency
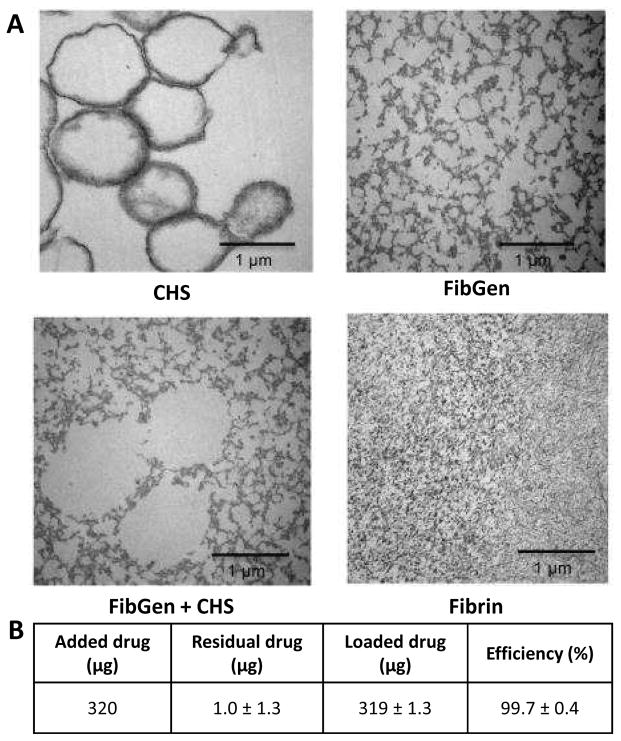
TEM demonstrated that fibrin had smaller pore size than FibGen, and that FibGen exhibited a more crosslinked structure than fibrin (Figure 2). CHS exhibited spherical shape when investigated under TEM. CHS loaded within FibGen also retained its hollow structure and spheres size indicating CHS could be successfully loaded within FibGen. To assess drug loading efficiency, 320 μg infliximab was initially loaded into CHS and the measured residual drug release (1.0±1.3 μg) indicated high loading efficiency (99.7±0.4 %, Figure 2B).
Figure 2. Characterization of collagen hollow sphere and FibGen morphology and drug release efficiency using transmission electron microscopy (TEM).
(A) Collagen hollow sphere morphology was observed using transmission electron microscopy. Sphere size and shape was maintained when CHS were mixed with FibGen. FibGen porosity was not altered with addition of CHS. Fibrin porosity was markedly smaller compared to FibGen. (B) Drug loading efficiency was assessed by loading initial 320 μg into CHS and measuring residual drug release (1.0±1.3μg) and loading efficiency was calculated (99.7%).
Infliximab Release Kinetics
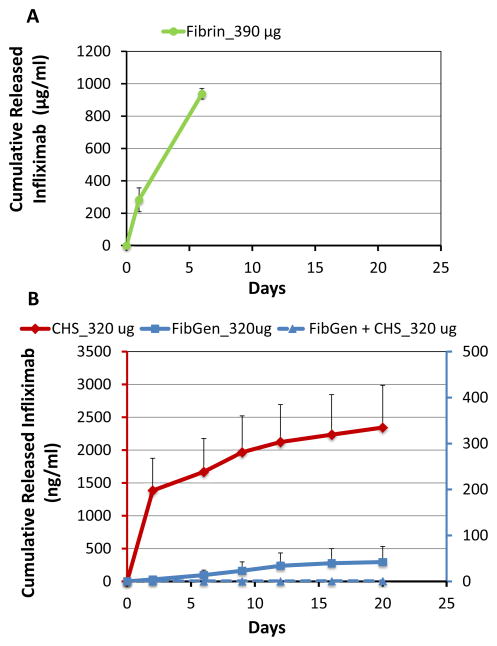
Fibrin alone exhibited an initial burst release of infliximab, but was not measured beyond 6 days due to its complete degradation so that the full amount of infliximab was released (Figure 3A). CHS alone loaded with 320 μg infliximab demonstrated steady release at low levels for 20 days (Figure 3). FibGen directly mixed with 320 μg infliximab showed steady drug release but at very low levels. Interestingly, CHS loaded with 320 μg infliximab did not exhibit any detectable drug release when it was mixed in FibGen (FibGen+CHS_320μg).
Figure 3. Collagen hollow spheres release the greatest amount of infliximab over time.
(A) Cumulative infliximab concentration in Fibrin alone showed large amounts of drug released but Fibrin degraded completely within 6 days indicating is is not capable of long-term release. (B) Cumulative infliximab concentration over 20 days showed increased drug release from CHS alone loaded with 320 μg infliximab, steady release at low levels from FibGen alone directly mixed with 320 μg infliximab, and impeded drug release in FibGen+CHS loaded with 320 μg infliximab.
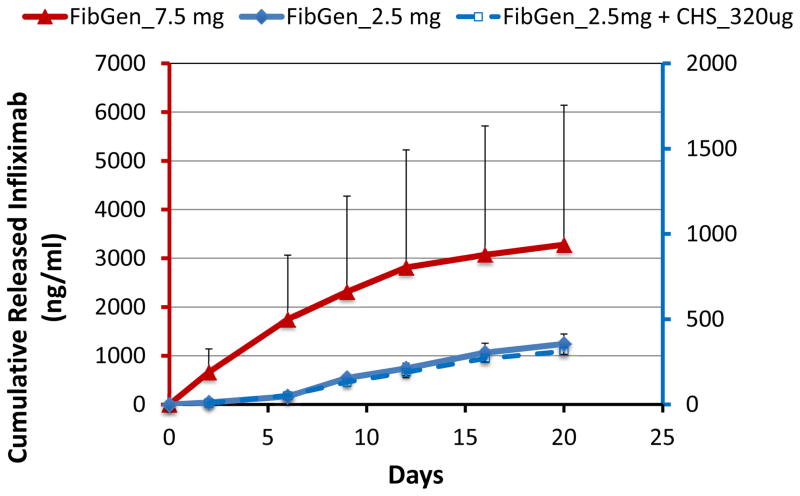
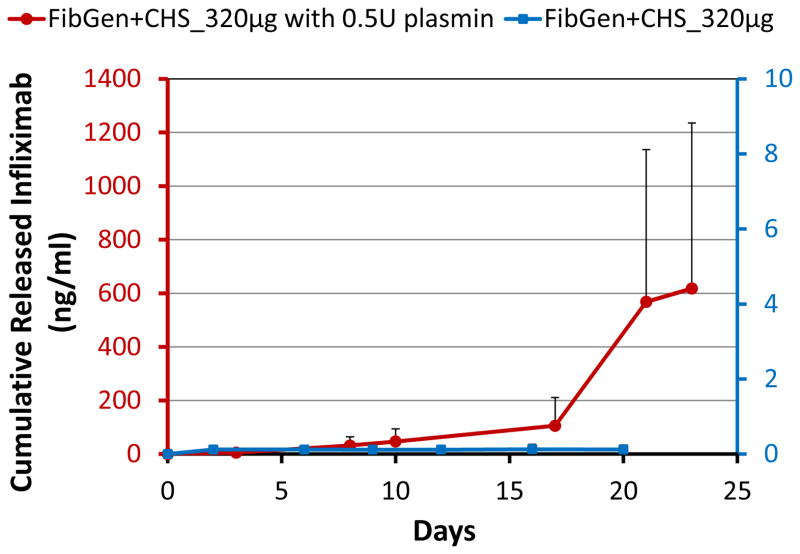
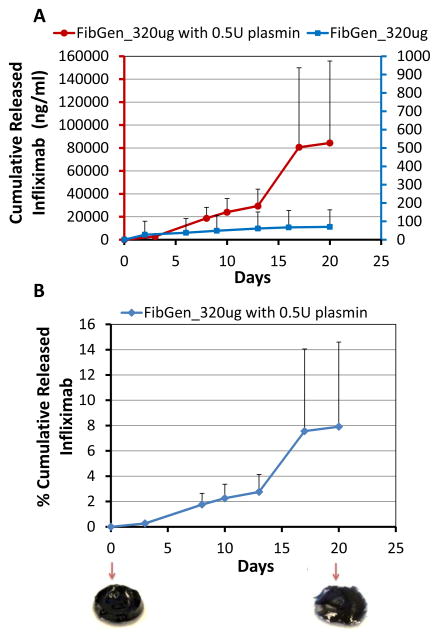
FibGen was loaded with higher concentrations of infliximab to assess if greater amounts of infliximab could be released, and if dosing could be controlled. Results confirmed that greater amounts of infliximab were released following loading with higher concentrations of infliximab (2.5 mg and 7.5 mg, Figure 4). Interestingly, the loading of 7.5 mg infliximab resulted in almost 6× greater drug release as compared to the 2.5 mg loaded FibGen, suggesting nonlinear interactions. Also, FibGen was amenable to loading with sufficient infliximab to allow greater amounts of drug release as compared to maximum drug released with fully loaded CHS alone. The addition of infliximab-loaded CHS into FibGen did not increase drug release (Figure 4). For FibGen+CHS_320μg, very little drug was released from day 0 to day 10, although drug release rate substantially increased in the presence of 0.5U plasmin, particularly from day 10 to day 23 as the gel degraded (Figure 5). Plasmin also substantially increased cumulative drug released from FibGen directly mixed with 320 μg infliximab as compared to the non-plasmin treated system (Figure 6A) and resulted in approximately 8% cumulative release over the 20 day culture period (Figure 6B).
Figure 4. Increasing infliximab concentration improves drug release in the FibGen delivery system but not FibGen+CHS.
Loading FibGen with increased amount of infliximab (2.5 mg and 7.5 mg) showed that the addition of 7.5 mg infliximab resulted in increased cumulative drug release comparable to cumulative drug release in CHS alone; however addition of infliximab-loaded CHS into FibGen did not increase drug release.
Figure 5. Infliximab is released from CHS within FibGen in the presence of plasmin.
Cumulative drug release from FibGen with 320 μg infliximab loaded CHS in the presence of plasmin, a broad spectrum serine proteinase. Infliximab was released from CHS within FibGen in the presence of plasmin, but the rate of release increased after some initial degradation after approximately day 10.
Figure 6. Plasmin degraded FibGen and increased cumulative infliximab release.
(A) Cumulative drug release from FibGen directly mixed with 320 μg infliximab in the presence of 0.5U plasmin, a broad spectrum serine proteinase, was increased over 20 days compared to that of the non-plasmin treated system and (B) resulted in approximately 8% cumulative drug release with observable degradation of the FibGen carrier.
Attenuation of Pro-inflammatory Cytokines
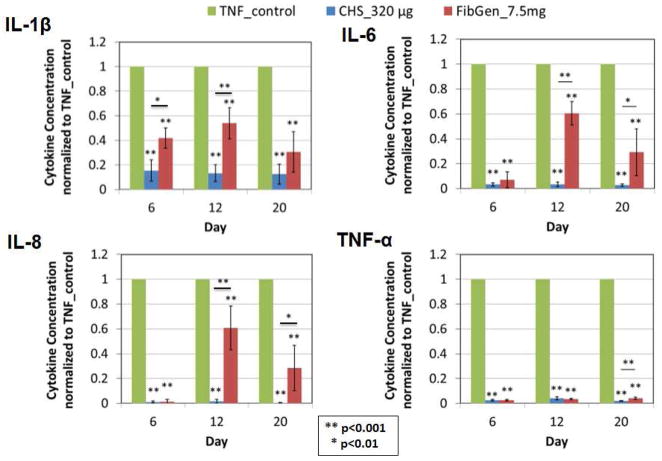
The pro-inflammatory cytokines IL1-β, IL-6, IL-8 and TNFα that were produced by AF cells were significantly reduced following treatment with infliximab conditioned media, confirming that infliximab remained bioactive following release from CHS loaded with 320 μg infliximab and FibGen directly mixed with 7.5 mg infliximab (p<0.001, Figure 7).
Figure 7. Pro-inflammatory cytokine production is reduced validating efficacy of released infliximab.
Pro-inflammatory cytokines, TNFα, IL1-β, IL-6, IL-8, produced by TNFα stimulated AF cells were significantly reduced with infliximab conditioned media confirming infliximab remained bioactive following release over 20 days.
Discussion
Small AF defects (e.g., from discography injections) and large AF defects (e.g., from herniation) are implicated as causes of low back pain due to accelerated IVD degeneration and increased pro-inflammatory cytokine production [1, 2, 38]. TNFα is implicated as an important pro-inflammatory cytokine in painful IVD degeneration. Mixed results using infliximab to treat spinal pathology in clinical trial may be related to a low dosage reaching the IVD and/or limited duration of treatments [3]. The overall strategy of our study was to develop an injectable treatment capable of sustained and local drug delivery to be used for treating painful IVD degeneration or for enhancing current microdiscectomy procedures. FibGen, as was previously proposed as an adhesive hydrogel with potential to seal IVD defects was functionalized in this study for controlled and sustained release of infliximab, a model anti-inflammatory drug. Fibrin, CHS, and FibGen with and without CHS were used as drug carriers, kinetics of infliximab drug release was characterized, and the efficacy of the released drug was validated. This optimization study was intended to create a material capable of promoting repair following IVD injury, as might be warranted during microdiscectomy procedures.
Fibrin is a commonly utilized material in many surgical and tissue engineering applications. Fibrin was able to serve as a drug delivery vehicle in this study. However, the full release of the drug and complete degradation of fibrin in vitro within 6 days indicated that it would not be useful for long-term sustained drug release. Therefore, results suggested fibrin would not be an effective drug delivery carrier for a long-term IVD repair strategy.
Because CHS exhibited infliximab drug loading efficiency of over 99% and were effective at releasing high and sustained doses of infliximab, they were an attractive drug carrier. However the small spherical structure of CHS might not be retained in an injured and degenerated human IVDs that contain fissures and defects. Therefore, an additional carrier would enhance retention of CHS within the IVD space.
The FibGen formulation in this study involved fibrin and genipin at concentrations previously optimized to match the AF shear modulus and to perform with greatly inhibited degradation rates as compared to fibrin [32–34]. FibGen was functionalized by direct mixing with infliximab in order to provide slow and sustained release of the drug. The drug delivery rate was modifiable by loading different amounts of the anti-inflammatory drug. Only a small percentage of the loaded drug was released during this 20 day in vitro study. However, the catabolic environment of IVDs would likely induce more degradation and release more drug in vivo. The in vivo degradation conditions were loosely simulated using plasmin, which is known to degrade fibrin. Plasmin greatly increased the amount of drug released and the release rate of infliximab from FibGen, providing a proof-of-concept that greater drug release rates for FibGen would be observed in vivo. Since FibGen was previously shown to remain present in subcutaneous rat studies for more than 16 weeks [34] we speculate that FibGen would be able to release infliximab for months during in vivo applications in animals and humans; however, further experiments are required to test this concept.
The infliximab released from FibGen remained active and was validated to be effective in inhibiting multiple pro-inflammatory cytokines produced by human AF cells. FibGen was able to be loaded with a high amount drug, and the 7.5 mg infliximab that was loaded within FibGen demonstrated sustained bioactivity over 20 days. The production of IL-1β, IL-6, IL-8, and TNFα from human AF cells were all decreased following treatment with the infliximab containing media released from all treatments, suggesting that infliximab remained effective during an extended period of time, and confirming the suitability of FibGen as a drug carrier for anti-inflammatory treatments.
Local delivery of infliximab within the IVD has the potential to target IVD cells, neural cells, and other cells in the spine region that could cause painful responses; however further testing would be required to understand targeted effects. An appropriate intradiscal dose of infliximab for human subjects is not clear from the literature. Infliximab has been used at a dose 5mg/kg epidurally in humans with limited effect [23, 24]. However, infliximab has also been used at a dose of 0.5 – 5 mg/kg systemically and 5 mg/kg intradiscally in rats with more promising results [28]. Scaling intradiscal dosing from rats to humans is not a linear relationship based on body weight due to differences in transport and cell density [39]. While 7.5 mg total delivery in our high dose gel was lower by weight than the intradiscal rat study by Nakame et al., we delivered more absolute amount of infliximab, and the released infliximab was effective in cell culture without any evidence for cytotoxicity. Taken together, we believe the 2.5–7.5 mg dose is a reasonable starting point. However, we highlight that in vivo animal and clinical dose response studies are necessary to determine the appropriate dose, and to test the efficacy of this repair strategy for painful spine conditions.
CHS were added to FibGen to enhance sustained delivery since it serves as a drug reservoir capable of releasing drugs with relatively simple fabrication and non-damaging drug loading procedures [35]. CHS retained their spherical shapes and sizes when incorporated into FibGen demonstrating their potential as a drug delivery carrier into the IVD space. The addition of drug loaded CHS into FibGen showed no detectable drug release, and it is therefore likely that genipin crosslinking inhibited the release infliximab from the CHS within FibGen. However, infliximab was released from FibGen+CHS_320μg following plasmin treatment at a later time points than when directly mixed in FibGen. Furthermore, this released infliximab remained active. The increased drug binding of CHS within FibGen may provide the ability for a multiple-stage drug release strategy with a more rapid release of drugs directly mixed in FibGen and a later release of drugs loaded within CHS within FibGen. As a result, a custom delivery strategy could be designed, implemented and eventually evaluated. Therefore, we speculate that CHS can be used to deliver infliximab or other drugs at longer time points, although this speculation requires validation and optimization.
In conclusion, FibGen was an effective delivery system for the sustained release of the anti-TNFα drug infliximab, and may be useful for delivery other anti-inflammatory drugs. We could control the release rate of infliximab in FibGen by adjusting the amount of drug loaded, and the released infliximab was validated to be effective at reducing the production of multiple pro-inflammatory cytokines by human AF cells. The drug loaded CHS within FibGen was not released until substantial FibGen degradation occurred, suggesting that CHS within FibGen can be used to provide an additional reservoir for drug delivery in order to create a drug delivery strategy that provides early release of the infliximab directly mixed in FibGen and later release of infliximab (or another drug) loaded within the CHS. The application of drug-loaded FibGen shows promise for direct injection into painful IVDs or as an augmentation to microdiscectomy procedures. This and prior studies [32–34] demonstrated that FibGen offers promise to seal the AF, partially restore IVD height and biomechanical properties, and also to provide controlled and sustained release of infliximab or other drugs in order to reduce the levels of pro-inflammatory cytokines. Functionalized FibGen shows potential to address chronic inflammatory conditions and biomechanical dysfunction in the AF and motivates future organ culture and animal models to validate these findings in vivo, and eventual clinical trials to assess the value of this repair strategy for treating IVD herniation, degeneration, and back pain..
Acknowledgments
This work was funded by grants from the NIAMS/NIH (R01 AR057397), AO Foundation (Annulus Fibrosus Rupture Program), and from the Icahn School of Medicine at Mount Sinai (4D Technology Development Program).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Morakot Likhitpanichkul, Email: morakot.likhitpanichkul@mssm.edu.
Yesul Kim, Email: yesulkim89@gmail.com.
Olivia M Torre, Email: olivia.torre@icahn.mssm.edu.
Eugene See, Email: see.eugene81@gmail.com.
Zepur Kazezian, Email: zepur.kazezian@gmail.com.
Abhay Pandit, Email: abhay.pandit@nuigalway.ie.
Andrew C Hecht, Email: andrew.hecht@mssm.edu.
James C Iatridis, Email: james.iatridis@mssm.edu.
References
- 1.Guterl CC, See EY, Blanquer SB, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? The spine journal : official journal of the North American Spine Society. 2013;13(3):243–62. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature reviews Rheumatology. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 5.Kanna RM, Shetty AP, Rajasekaran S. Patterns of lumbar disc degeneration are different in degenerative disc disease and disc prolapse magnetic resonance imaging analysis of 224 patients. The spine journal : official journal of the North American Spine Society. 2014;14(2):300–7. doi: 10.1016/j.spinee.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 6.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178–81. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 7.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15(8):762–7. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Stefanakis M, Al-Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine. 2012;37(22):1883–91. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 9.Stefanakis M, Luo J, Pollintine P, Dolan P, Adams MA. ISSLS Prize winner: Mechanical influences in progressive intervertebral disc degeneration. Spine. 2014;39(17):1365–72. doi: 10.1097/BRS.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine. 2008;33(25):2789–800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder JE, Dettori JR, Brodt ED, Kaplan L. Disc degeneration after disc herniation: are we accelerating the process? Evidence-based spine-care journal. 2012;3(4):33–40. doi: 10.1055/s-0032-1328141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000;25(17):2165–70. doi: 10.1097/00007632-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. Eur Cell Mater. 2012;23:103–19. doi: 10.22203/ecm.v023a08. discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 14.Michalek AJ, Iatridis JC. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. The spine journal : official journal of the North American Spine Society. 2012;12(5):425–32. doi: 10.1016/j.spinee.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilke HJ, Ressel L, Heuer F, Graf N, Rath S. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine. 2013;38(10):E587–93. doi: 10.1097/BRS.0b013e31828ca4bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotz JC, Haughton V, Boden SD, et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology. 2012;264(1):6–19. doi: 10.1148/radiol.12110339. [DOI] [PubMed] [Google Scholar]
- 17.Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, Wuertz K. Age-related changes in human cervical, thoracal and lumbar intervertebral disc exhibit a strong intra-individual correlation. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2012;21(Suppl 6):S810–8. doi: 10.1007/s00586-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis research & therapy. 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis research & therapy. 2008;10(4):R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. The Journal of pathology. 2002;197(3):286–92. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 21.Peng B, Hao J, Hou S, et al. Possible Pathogenesis of Painful Intervertebral Disc Degeneration. Spine. 2006;31(5):560–6. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder M, Viezens L, Schaefer C, et al. Chemokine profile of disc degeneration with acute or chronic pain. Journal of neurosurgery Spine. 2013;18(5):496–503. doi: 10.3171/2013.1.SPINE12483. [DOI] [PubMed] [Google Scholar]
- 23.Korhonen T, Karpinnen J, Paimela L, et al. The Treatment of Disc Herniation-Induced Sciatica With Infliximab. Spine. 2005;30(24):2724–8. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen T, Karpinnen J, Paimela L, et al. The Treatment of Disc Herniation-Induced Sciatica With Infliximab. Spine. 2006;31(24):2759–66. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 25.Ohtori S, Miyagi M, Eguchi Y, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine. 2012;37(6):439–44. doi: 10.1097/BRS.0b013e318238af83. [DOI] [PubMed] [Google Scholar]
- 26.Genevay S, Finckh A, Zufferey P, Viatte S, Balague F, Gabay C. Adalimumab in acute sciatica reduces the long-term need for surgery: a 3-year follow-up of a randomised double-blind placebo-controlled trial. Annals of the rheumatic diseases. 2012;71(4):560–2. doi: 10.1136/annrheumdis-2011-200373. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SP, White RL, Kurihara C, et al. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Annals of internal medicine. 2012;156(8):551–9. doi: 10.7326/0003-4819-156-8-201204170-00397. [DOI] [PubMed] [Google Scholar]
- 28.Nakamae T, Ochi M, Olmarker K. Pharmacological inhibition of tumor necrosis factor may reduce pain behavior changes induced by experimental disc puncture in the rat: an experimental study in rats. Spine. 2011;36(4):E232–6. doi: 10.1097/BRS.0b013e3181d8bef3. [DOI] [PubMed] [Google Scholar]
- 29.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(Suppl 4):480–91. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorth DJ, Mauck RL, Chiaro JA, et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1beta-mediated degradation of nucleus pulposus in vitro. Arthritis research & therapy. 2012;14(4):R179. doi: 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Likhitpanichkul M, Gruen JA, Walter BA, Hecht AC, Iatridis JC. Synergistic pro-inflammatory effects of TNF-α and high strain on human annulus fibrosus cells is effectively inhibited by infliximab. Transactions of the Orthopaedic Research Society. 2014 Poster # 0745. [Google Scholar]
- 32.Guterl CC, Torre OM, Purmessur D, et al. Characterization of Mechanics and Cytocompatibility of Fibrin-Genipin Annulus Fibrosus Sealant with the Addition of Cell Adhesion Molecules. Tissue engineering Part A. 2014 doi: 10.1089/ten.tea.2012.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schek RM, Michalek AJ, Iatridis JC. Genipin-Crosslinked Hydrogel As A Potential Adhesive To Augment Intervertebral Disc Annulus Repair. Eur Cell Mater. 2011;21:373–83. doi: 10.22203/ecm.v021a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Likhitpanichkul M, Dreischarf M, Illien-Junger S, et al. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur Cell Mater. 2014;28:25–37. doi: 10.22203/ecm.v028a03. discussion-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browne S, Fontana G, Rodriguez BJ, Pandit A. A protective extracellular matrix-based gene delivery reservoir fabricated by electrostatic charge manipulation. Molecular pharmaceutics. 2012;9(11):3099–106. doi: 10.1021/mp300231d. [DOI] [PubMed] [Google Scholar]
- 36.Helary C, Browne S, Mathew A, Wang W, Pandit A. Transfection of macrophages by collagen hollow spheres loaded with polyplexes: a step towards modulating inflammation. Acta biomaterialia. 2012;8(12):4208–14. doi: 10.1016/j.actbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Ternant D, Mulleman D, Degenne D, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Therapeutic drug monitoring. 2006;28(2):169–74. doi: 10.1097/01.ftd.0000189901.08684.4b. [DOI] [PubMed] [Google Scholar]
- 38.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34(21):2338–45. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 39.Huang YC, Urban JP, Luk KD. Intervertebral disc regeneration: do nutrients lead the way? Nature reviews Rheumatology. 2014;10(9):561–6. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]