Alterations in lipid metabolism and inflammatory processes are well established as potential risk factors in the development and progression of cardiovascular disease.1 However, with complications ranging from valve dysfunction to arrhythmia to myocardial infarction and stroke, the underlying mechanisms may be as varied as cardiovascular disease itself. On the other hand, the reoccurrence of common molecular and cellular pathways identified in the collective body of cardiovascular research could suggest shared initiators or mechanistic nodes between seemingly divergent processes, including lipid metabolism and inflammation. One area where this may hold true is cardiovascular calcification, in which dysregulated mineral metabolism in cardiovascular tissues leads to increased morbidity and mortality.
Calcification of soft tissues results from the deposition of calcium, largely in the form of hydroxyapatite in the vascular wall and/or valve leaflets. Previously thought to be a passive degenerative process, cardiovascular calcification has become increasingly apparent to be an active process initiated by many triggers. Recent studies have demonstrated variation in the gene LPA, which determines the plasma concentration of lipoprotein(a) (Lp(a); pronounced “L P little a”) to be associated with calcific aortic valve disease (CAVD).2,3 Lp(a) consists of a LDL-like particle in which apolipoprotein(a) is covalently bound to apolipoprotein B. Additionally, Lp(a) is a genetic risk factor for atherosclerotic events.4 As in atherosclerosis, calcifications in CAVD localize to areas with lipoprotein accumulation and inflammatory cell infiltration, suggesting a shared disease process.5 However, some noticeable differences do exist, including increased mechanical stresses and calcification involved valve obstruction in CAVD as opposed to microcalcifications leading atherosclerosis plaque rupture.6
In the current issue of Circulation, Bouchareb and Mahmut et al.7 propose a highly plausible mechanistic pathway through which Lp(a) and valve interstitial cell (VICs)-derived autotaxin (ATX) may induce valve calcification by regulating inflammation induced bone morphogenetic protein (BMP). This study connects lipid metabolism to inflammation and valve calcification, and in doing so identifies a pathway that may help lead to the development of CAVD therapeutics, an area with high unmet clinical need. Mathieu and colleagues had recently reported8 that lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme that utilizes oxidized phospholipids carried by Lp(a) to generate lysophosphatidylcholine (LPC), is both highly expressed in CAVD and to plays a role in the mineralization of VICs. The CAVD functional role of ATX, a key enzyme involved in the conversion of LPC to the signaling phospholipid, lysophosphatidic acid9 (LPA) has yet to be reported. ATX is a member of the ectophosphodiesterase/nucleotide phosphohydrolase (ENPP) family. It is notable that to varying extents ENPPs can hydrolyze ATP to generate pyrophosphate,10, 11 a known inhibitor of the bone and vascular smooth muscle calcification. However, in vitro analysis of ENPP substrate hydrolysis suggest that ATX is a poor nucleotide pyrophosphatase/phosphodiesterase, and unique among the ENPPs in acting as a phospholipase.11 As such, ATX phospholipase activity converting LPC to LPA, particularly in the context of elevated Lp(a), may play a greater role in CAVD development.
LPA is a potent extracellular signaling molecule with a diverse array of physiologic and pathologic actions including: induction of the mitogenic RAS-extracellular signal-regulated kinase pathway, the phosphoinositide 3-kinase (PI3K)-AKT cell survival pathway, Rho- and RAC-mediated cytoskeletal remodeling and cell migration, cell proliferation, vascular and neural development, phospholipase C activation leading to calcium mobilization, fibrosis, lymphocyte homing, and cytokine production.12 ATX acts locally, and signals through LPA generation and six LPA guanine-nucleotide-binding protein-coupled receptors (LPAR1-6), located on the surface of a wide variety of cells. Lp(a) can bind to a number of receptors including LDLR, LRPs, VLDLR, and SR-BI, although the extent to which it acts as a ligand can vary widely.13 Given the role of these and other cell surface receptors in cellular metabolism and trafficking, examination of the involvement of intracellular sorting processes including those affecting membrane composition and events such as endocytosis and exocytosis, may provide novel CAVD insight and should be examined in future studies.
In the current study by Bouchareb and Mahmut et al.,7 increased LPA, ATX lysophospholipase activity and protein abundance were found in mineralized human aortic valves. Increased ATX levels in valves were associated with oxidized lipids, increased remodeling score, and measurements of inflammation. As pointed out by the authors, one limitation of this study was that ATX was examined in human aortic valves with advanced end-stage pathology. As such a causative role of Lp(a) and VIC-derived ATX in the valve calcification process could not be clearly assigned from the human tissue studies alone. The authors hypothesized that LPC may induce valve calcification through NF-κB activation leading to IL-6 production and BMP2 signaling in VICs. To test whether NF-κB activation, IL-6, and BMP2 were involved in LPC mediated mineralization, Bouchareb and Mahmut et al.7 treated VICs with LPC, mineralizing medium (a combination of inorganic phosphate, insulin, and ascorbic acid), and inhibitors of BMP, NF-κB, LPARs, or silencing RNAs directed against ATX or IL-6. Disrupting any of these components strongly reduced/inhibited LPC enhanced mineralization. The human tissue and cell culture data were partially confirmed in this study with the use of an animal model of CAVD, LDLR−/−/ApoB100/100/IGFII transgenic mice, in which ATX was found to be increased in the aortic leaflets. Additionally, lysophosphatidic acid-treated mice showed a 1.7-fold increase in aortic valve leaflet calcification, along with an increase in BMP2. These results indicate that VICs and Lp(a)-derived ATX and oxidized lipids lead to increased LPA that acts to induce inflammation via LPARs, NF-κB activation, and ultimately results in IL-6 regulation of BMP mediated valve calcification.
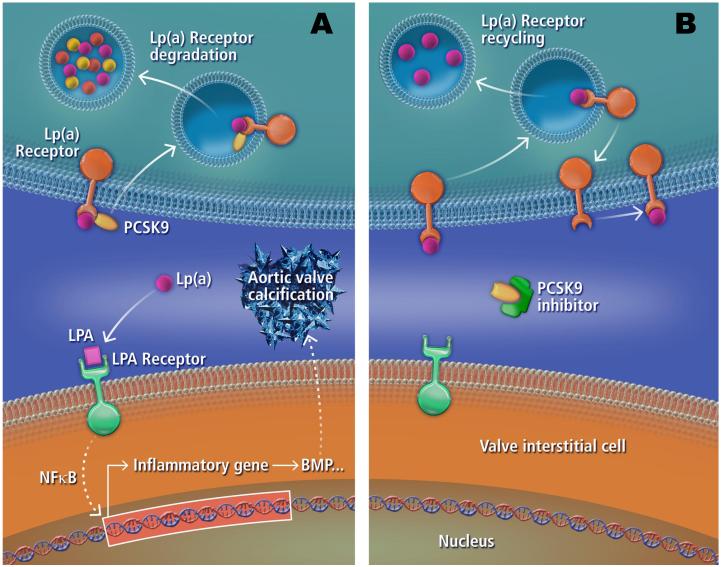
Approximately 50,000 valve replacements are performed annually in the United States for patients with severe aortic stenosis.5 Aside from valve replacement, there are currently no treatments that prevent or slow the progression of valve disease, which is responsible for more than 22,000 deaths each year in the United States.14 Bouchareb and Mahmut et al.7 correctly concluded that inhibition of ATX or blocking LPARs as potential novel CAVD therapies warrant future investigation. Some caution should be taken in this approach, as bone defects have been reported in LPA receptor modified mice.15 Similarly, assessment of targeting Lp(a) itself also seems warranted, although whether lowering Lp(a) levels can reduce the rate of incidence or progression of aortic valve disease remains to be determined. However, given that a common variant in Lp(a) was reported to increase the risk of developing aortic stenosis by more than 50%,2 Lp(a) targeting therapies should be explored further in a CAVD context. Of potential interest in this area is the recent development of PCSK9 based therapeutics, which significantly reduce major adverse cardiovascular events (death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, and unstable angina requiring hospitalization),16 in addition to reducing Lp(a) levels.17 Further analysis of whether reduction in Lp(a) levels via PCSK9 inhibition plays a role in reduced cardiovascular events;, particularly in relation to CAVD, may prove to be of importance (Figure 1). Outside of PCSK9 inhibition, niacin and the cholesteryl ester transfer protein inhibitor, anacetrapib, have been shown to reduce Lp(a) levels.18 Although whether these or similar compounds would act therapeutically in CAVD, or more specifically a CAVD at-risk subpopulation with elevated Lp(a), is unknown. HMG-CoA reductase inhibitors (statins), are known to act on both lipid metabolism and inflammation, but have largely shown a lack of therapeutic benefit in CAVD.19 However, it is worth pointing out that HMG-CoA reductase inhibitors have been reported to lower LDL cholesterol without reducing Lp(a),20 and while there are some conflicting reports showing both elevated and decreased Lp(a), several statin studies show no major changes. This result may not be too unexpected given that Lp(a) is reportedly a relatively poor ligand for LDLR,13 a receptor that serves as one of the major means of action on lipid metabolism following statin administration.
Figure 1.
Potential role of Lp(a) and PCSK9 in CAVD. Lp(a) may get taken up and metabolized by cells via Lp(a) receptors (receptors for which Lp(a) is a ligand). However, in the presence of PCSK9 some of these receptors may be internalized and degraded instead of recycled back to the cell surface (A). As such more Lp(a) derived oxidized lipids may be converted to LPA and be taken up by VICs via LPA receptors. This may lead to the production of inflammation related cytokines (e.g., IL6) through NF-κB nuclear localization that in turn increase BMP and/or other calcification inducing processes leading to valve calcification. PCSK9 inhibitors may act to block PCSK9 interaction with Lp(a) receptors, resulting in Lp(a) receptors being recycled back to the cell surface where they can take up more Lp(a) (B). Dashed arrows indicate multiple steps in the pathway.
In summary, the work of Bouchareb and Mahmut et al.7 builds on previous studies to identify a mechanistic pathway through which Lp(a) and ATX may be driving aortic valve calcification, and in doing such presents an important area of research worthy of additional investigation.
Acknowledgments
Funding Sources: Dr. Aikawa is supported by grants from the National Institutes of Health (R01HL114805; R01HL109506).
Footnotes
Disclosures: None.
References
- 1.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 2.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kalsch H, MuhleisenTW, Nothen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS. CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Schreiner PJ, Morrisett JD, Sharrett AR, Patsch W, Tyroler HA, Wu K, Heiss G. Lipoprotein[a] as a risk factor for preclinical atherosclerosis. Arter Throm Vas Bio. 1993;13:826–833. doi: 10.1161/01.atv.13.6.826. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease. Pathogesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 6.Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: microcalcification and atherosclerotic plaque vulnerability. Curr Opin Lipidol. 2014;25:327–332. doi: 10.1097/MOL.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lepine JL, Marie-Helene Laflamme, Hadji R, Couture C, Trahan S, Page S, Bosse Y, Pibarot P, Scipione CA, Romagnuolo R, Koschinsky ML, Arsenault BJ, Marette A, Mathieu P. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:XX–XXX. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 8.Mahnut A, Boulanger MC, El Husseini D, Fournier D, Bouchareb R, Despres JP, Pibarot P, Bosse Y, Mathieu P. Elevated expression of lipoprotein-assocaited phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol. 2014;63:460–469. doi: 10.1016/j.jacc.2013.05.105. [DOI] [PubMed] [Google Scholar]
- 9.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Indentification of human plasma lysophospholipase D, a lysophosphatidic acid producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 10.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NNP2) involves a single catalytic site. FEBS Lett. 2003;538:60–64. doi: 10.1016/s0014-5793(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 12.Moolenaar WH, Perrakis A. Insights into autotaxin: how to produce and present a lipid mediator. Nat Rev Mol Cell Biol. 2011;12:674–679. doi: 10.1038/nrm3188. [DOI] [PubMed] [Google Scholar]
- 13.Yang XP, Amar MJ, Vaisman B, Bocharov AV, Vishnyakova TG, Freeman LA, Kurlander RJ, Patterson AP, Becker LC, Remaley AT. Scavenger receptor-BI is a receptor for lipoprotein(a) J Lipid Res. 2013;54:2450–2457. doi: 10.1194/jlr.M038877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salles JP, Laurencin-Dalicieux S, Conte-Auriol F, Briand-Mesange F, Gennero I. Bone defects in LPA receptor genetically modified mice. Biochim Biophys Acta. 2013;1831:93–98. doi: 10.1016/j.bbalip.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. ODYSSEY LONG TERM Investigators. Efficacy and safety of Alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 17.Desai NR, Kohli P, Giugliano RP, O’Donoghue ML, Somaratne R, Zhou J, Hoffman EB, Huang F, Rogers WJ, Wasserman SM, Scott R, Sabatine MS. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128:962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 18.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth L, Ridker PM, Mora S. Lipoprotein(a) concentrations, Rosuvastatin therapy, and residual vascular risk. Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostner GM, Gavish D, Leopold B, Bolzano K, Weintraub MS, Breslow JL. HMG-CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 1989;80:1313–1319. doi: 10.1161/01.cir.80.5.1313. [DOI] [PubMed] [Google Scholar]