Abstract
Background
Malnutrition is common in patients with active tuberculosis (TB), yet little information is available on serial dietary intake or body composition in TB disease.
Objective
To evaluate macronutrient intake and body composition in individuals with newly diagnosed with TB over time.
Design
Adults with active pulmonary TB (n=191; 23 with multidrug resistant TB (MDR-TB) and 36 culture-negative household contacts (controls) enrolled in a clinical trial of high-dose cholecalciferol (vitamin D3) were studied. Macronutrient intake was determined at baseline, 8 and 16 weeks. Serial body composition was assessed by body mass index (BMI; kg/m2) and bioelectrical impedance analysis (BIA) to estimate fat mass and fat-free mass. Descriptive statistics, repeated measures ANOVA for changes over time and linear regression were used.
Results
At baseline, mean daily energy, protein, fat and carbohydrate (CHO) intakes were significantly higher, and body weight, BMI, fat-free mass and fat mass were significantly lower, between TB subjects and controls. These remained significant after adjusting for age, gender, employment status and smoking. In all TB subjects, baseline mean daily intakes of energy, fat and protein were adequate when compared to the US Dietary Reference Intakes and protein significantly increased over time (p<0.0001). Body weight, BMI, and fat and fat-free mass increased over time. MDR-TB patients exhibited lower body weight and fat-free mass over time, despite similar daily intake of kcal, protein, and fat.
Conclusions
Macronutrient intake was higher in TB patients than controls, but TB-induced wasting was evident. As macronutrient intake of TB subjects increased over time, there was a parallel increase in BMI, while body composition proportions were maintained. However, individuals with MDR-TB demonstrated concomitantly decreased body weight and fat-free mass over time versus drug-sensitive TB patients, despite increased macronutrient intake. Thus, MDR-TB appears to blunt anabolism to macronutrient intake, likely reflecting the catabolic effects of TB.
Introduction
Tuberculosis (TB) has long been associated with a decrease in body mass. The relationship between malnutrition and infection, especially in TB, is well established.(1-5) Evidence suggests that malnutrition, particularly low body mass index (BMI), can lead to secondary immune dysfunction that increases the host’s susceptibility to infections.(1, 3, 5, 6) Body weight changes during treatment have been explored as a simple biomarker for both disease severity and treatment outcomes.(4, 7) Further, it has been shown that TB patients with low BMI (<18.5 kg/m2) are at increased risk for TB-related mortality and treatment failure.(4, 7, 8) Despite the abundance of cross-sectional data on the relationship between drug-sensitive TB and body composition, there are few serial studies of body composition in TB(9, 10) or that combine dietary intake and body composition during anti-TB drug treatment. Also, the relationship between nutrient intake and body composition in individuals with multidrug resistant TB (MDR-TB) compared to those with drug-sensitive TB has not been reported.
Macronutrients, especially protein and energy intake, are critical factors involved in susceptibility to infection, but remain poorly studied in TB, a leading cause of infectious disease-related mortality worldwide.(1) Determination of accurate data regarding habitual dietary and nutrient intake has not been well characterized in patients with TB. The majority of studies in TB have measured body weight and BMI as a marker of nutrition status. While some studies suggest that nutritional supplementation may improve treatment outcomes in patients with active TB,(11, 12) a recent Cochrane review concluded there was insufficient evidence for specific nutritional supplementation recommendations in patients with active TB.(13) Given the limited and inconclusive data, we hypothesized that: 1) macronutrient intake would increase over time during anti-tuberculosis treatment in adults with TB and that this would be associated with concomitantly increased body weight and fat-free mass; and 2) high-dose vitamin D3 (Vit D) would result in increased fat-free mass versus placebo-treated subjects. Our objectives were thus to determine changes in macronutrient intake and body composition in adults with newly diagnosed TB, without or with MDR-TB, in the context of a randomized controlled clinical trial (RCT) of high-dose Vit D.
Subjects and Methods
Study Subjects
Subjects were derived from a double blind, randomized, controlled intent-to-treat trial (RCT) of high-dose vitamin D3 treatment of patients with active pulmonary TB disease (clinicaltrials.gov identifier NCT00918086). They were consecutively recruited between July 2009 and April 2012 from the Georgia National Center for Tuberculosis and Lung Diseases (NCTBLD) and an affiliated outpatient TB clinic in Tbilisi, Georgia.
Inclusion criteria were: 1) age ≥ 18 years; 2) newly diagnosed TB as determined by a positive AFB sputum smear and later confirmed by positive culture; 3) patient received ≤ 7 days of treatment with anti-TB drug therapy prior to entry; 4) subject has signed the informed consent. Exclusion criteria were: 1) patient had previous diagnosis of TB, known extra-pulmonary or MDR-TB at entry, or expected requirement for surgical resection of TB pulmonary granuloma; 2) patient is currently pregnant or lactating or has a history of hypercalcemia, nephrolithiasis, hyperparathyroidism, sarcoidosis, organ transplant, hepatic cirrhosis, seizures, or cancer in the past 5 years; 3) patient has a serum creatinine concentration >250 mmol/L or requires renal replacement therapy; 4); patient required corticosteroid use in the past 30 days; 5) current use of cytotoxic or immunosuppressive drugs; and 7) current incarceration.
Acid-fast bacilli (AFB) sputum smear-positive subjects (n=784) were assessed for eligibility. Of these, 345 did not meet inclusion/exclusion criteria and 240 additional subjects declined to participate (Supplemental Figure 1). A total of 100 subjects were randomized to receive Vit D and 99 were randomized to receive placebo for a total of 16 weeks, during which the study measurements were performed. After randomization, 192 subjects (97 in the Vit D group and 95 in the placebo group) were determined to have sputum cultures positive for Mycobacterium tuberculosis (Mtb), confirming pulmonary TB, and were thus eligible for this analysis. In one subject randomized to placebo, study endpoints were not determined; thus, a total of 97 subjects randomized to Vit D and 94 assigned to placebo were eligible at baseline. A similar number of subjects in each study group were lost to follow-up (18 in the Vit D group and 15 in the placebo group; respectively) (Supplemental Figure 1).
TB subjects receiving Vit D were given 50,000 IU of oral vitamin D3 weekly for 8 consecutive weeks, followed by 50,000 IU of vitamin D3 every two weeks for 8 consecutive weeks, for a total dose of 1.4 million IU vitamin D3 during the 16-week period of study. The TB control group received an identical placebo capsule at the same time points as the Vit D group.
When a newly diagnosed TB patient presented to the clinical physician co-investigators, after informed consent, the subject’s baseline data was obtained. We concomitantly attempted to enroll household contacts if these individuals accompanied the TB patient during the baseline visit day; thus, the 36 asymptomatic contacts enrolled were a convenience sample reference study group. These individuals were proven to be AFB sputum smear and culture negative and only baseline measurements were performed on these individuals.
Sputum Culture and Drug Susceptibility Testing
Two sputum specimens were obtained from all potential study subjects to confirm TB diagnosis. Direct sputum smears were examined by light microscopy. All sputum samples were sent to the NCTBLD National Reference Laboratory (NRL) for culture, using standard methodologies.(14) Drug susceptibility testing (DST) for first-line anti-TB drugs (isoniazid, rifampicin, ethambutol) and second-line drugs was done using absolute concentration method on solid media, as previously described.(15)
Nutritional and Body Composition Assessment
Dietary intakes were assessed for all study participants in the clinical trial at baseline, week 8 and week 16 of the clinical trial. A validated food/nutrient intake instrument which captures composition of specific foods and meal patterns common in Georgian culture was developed specifically for this RCT.(16) Trained study coordinators conducted one-on-one interviews using appropriate food model to determine all food intake of subjects in the three days prior to the specified study visits. After interviews were completed, data was entered into a web-based case report form (CRF) and sent to the US-based registered dietitian for review. Quantitative food intake data were subsequently analyzed using the Nutrition Data System for Research (NDSR) nutrient intake software (University of Minnesota, Minneapolis, MN). Final calculations were completed using NDSR version 2011. Mean daily intake of energy, total protein, fat and carbohydrate (CHO) were determined. The Georgia-specific nutrient tool over-reported energy, fat, carbohydrate and protein by 5.6%, 2.4%, 7.7% and 6.4% respectively compared to 24-hr recall.(16)
The body mass index [BMI; body weight (kg)/height (m2)] was calculated in all subjects using data obtained from a calibrated research stadiometer and digital body weight scale system (Tanita Inc; Arlington Heights, Illinois, USA) at baseline and on weeks 4, 8, 12, and 16. Subjects were clothed but without shoes. Time of day was not recorded, but most visits occurred in the morning. Bioelectrical impedance analysis (Bioelectrical Impedance Analyzer, Model Quantam X: RJL Systems, Clinton Township, MI, USA) (BIA) was used as outlined by the manufacturer to determine percent body fat mass and percent body fat-free mass at baseline and on weeks 4, 8, 12, and 16. In the pre-hoc study design, total body fat mass and fat-free mass were the primary BIA-derived body composition data. These were calculated using the total body weight of the subject at the respective time points. The BIA analyzer provided visual data on resistance, reactance, phase angle (PA), extracellular water, and intracellular water; bioelectrical impedance vectors (BIVA) data were not provided by the analyzer. The BIA data were manually entered into a computer spread sheet by investigators in Tbilisi and subsequently into a computer software algorithm program (provided by RJL Systems). The resultant derived total body fat mass and fat-free mass data were then manually uploaded in real-time into the web-based CRF. An electrical failure after study completion resulted in loss of the original source data (BIA-derived resistance, reactance, PA, extracellular water, and intracellular water) on the study computer. All measurements (food intake record and body composition) were done in an identical fashion by trained co-investigators blinded to the randomization; Food intake data was analyzed identically using NDSR in a blinded fashion by a registered dietitian investigator.
Ethics Statement
This study was approved by the Institutional Review Board of Emory University (Atlanta, GA, USA) and the Georgian NCTBLD Ethics Committee (Tbilisi, Georgia). All subjects provided written informed consent for participation in the study.
Statistical Analysis
For the parent RCT, a sample size of 110 participants per treatment arm provided 90% power to detect a difference of 16% in the participants with culture conversion at 8 weeks (two-sided z test with pooled variance; significance level = 0.05). This power calculation assumed that 75% of participants that received anti-TB drug + placebo were culture negative 8 weeks after entry and 90% of participants in the anti-TB drug + Vit D group.
Subjects that exhibited a markedly elevated baseline mean daily caloric intake (defined prehoc as mean daily caloric intake of > 6000 kcal/day) were excluded from analysis by the Tukey outlier method. Descriptive statistics (Student’s t-tests for continuous variables and chi-square tests and Fisher’s exact tests for categorical variables) were used where appropriate. PROC GLM was used to build models to determine differences in macronutrient and body composition variables between TB subjects and household contacts. Two models were developed; model I adjusted for age and gender, while model II adjusted for age, gender, employment status and smoking. We also tested for interactions with gender. Repeated measures analysis of variance was used to evaluate time and treatment group effects within the body composition and macronutrient variables and the Tukey method was used to correct for multiple comparisons. TB subjects were dichotomized by both sex and drug susceptibility to assess changes between those with and without MDR-TB. Time to appropriate treatment in MDR-TB subjects was defined as correct treatment within 56 days from the study baseline visit. Linear regression models using PROC MIXED were used to estimate the relationship between the outcome (time or drug susceptibility) and body composition and nutrient variables, and we assumed missing data was at random. The CONSORT diagram shows dropouts, but numbers vary in reported data because of missing data during a particular visit (i.e. some subjects, due to time-constraints, etc., did not have all endpoints determined at all serial visits); thus the number of subjects over time for specific endpoints does conform exactly to the CONSORT diagram. Also, serial data in the household contact (HC) reference group were not performed; thus, not reported. Outcome and covariate data on participants lost to follow-up were analyzed and were similar to participants that completed follow-up. All statistical analyses were done using SAS software, Version 9.3. (Cary, NC, USA) and a P-value of <0.05 was determined significant.
Results
Comparison of TB Subjects with Household Contacts
The demographic characteristics of 191 subjects with newly diagnosed pulmonary TB and 36 household contacts are shown in Table 1. A total of 4 subjects (≈ 2%) were co-infected with human immunodeficiency virus, consistent with previous low rates within the Georgian TB patient population.(15) The TB subjects’ mean age was 36 years, 64% were male, 47% were currently unemployed and the majority had an individual annual income less than 3,000 USD. Seventy-one percent of TB subjects were current tobacco smokers. The household contact cohort was older than the TB subjects (by 5 years on average), smoked less, and had modestly higher employment status but similar individual annual income (Table 1).
Table 1.
Subject Demographics
| Characteristic | TB Subjects (n=191) |
Household Contacts (n=36) |
P-value |
|---|---|---|---|
| Age [mean(SD)] | 36 (12) | 41 (14) | <0.01 |
| Male Sex | 123 (64%) | 12 (33%) | <0.01 |
| Ethnicity | |||
| Georgian | 176 (92%) | 33 (92%) | 0.37 |
| Other | 16 (8%) | 3 (8%) | |
| Education | |||
| Secondary or less | 83 (43%) | 11 (30%) | 0.13 |
| College | 28 (15%) | 10 (28%) | |
| University | 81 (42%) | 15 (42%) | |
| Employment Status |
|||
| Employed | 78 (41%) | 17 (47%) | <0.01 |
| Unemployed | 93 (47%) | 11 (31%) | |
| Other | 21 (12%) | 8 (22%) | |
| Annual Income | |||
| <1000 lari (~600USD) |
55 (28%) | 9 (25%) | 0.97 |
| 1000-5000 lari | 92 (48%) | 18 (50%) | |
| 5001-10,000 lari | 40 (21%) | 8 (22%) | |
| >10,000 lari | 5 (3%) | 1 (3%) | |
| Marital Status | |||
| Single | 83 (43%) | 4 (11%) | <0.01 |
| Married | 90 (47%) | 31 (86%) | |
| Divorced/Widow | 19 (10%) | 1 (3%) | |
| Current Smoker | 137 (71%) | 14 (39%) | <0.01 |
Unpaired Students t-test and chi-square tests and Fisher’s exact tests for categorical variables were used for analysis.
Subjects with TB reported consuming significantly greater amounts of total energy, protein, fat and CHO than the household contact reference group (Table 2). The number of study days when subjects reported consuming > 6000 kcal was 8 at baseline, 14 at week 8, and 8 at week 16. This was reflected in the overall significantly higher macronutrient intake of the TB disease cohort relative to the household contact group (Table 2). Total energy intake (kcal/day) was 17.6% higher in the TB subjects than the control group, while protein, total fat and CHO intake were 28.5%, 31.6%, and 32.1% higher than household contacts, respectively. These differences remained significant (p<0.05) even after adjusting for age, sex, employment status, and smoking for kcal, CHO, protein and fat. The increased caloric intake may be due to routine instructions given by TB physicians at the time of diagnosis to increase caloric intake as a treatment strategy.
Table 2.
Dietary Macronutrient Intake and Body Composition: Comparisons Between Subjects with TB and Household Contacts
| Characteristic | TB Subjects (n=191) |
Household Contacts (n=36) |
P- value |
Model I Adjusted P-value |
Model II Adjusted P-value |
|---|---|---|---|---|---|
|
Total Energy (kcal/day) (SD)[%BMR] |
3396 (1300) [236%] |
3001 (1040) [189%] |
<0.01 | <0.01 | <0.01 |
|
Protein (g/day) (SD)[%kcal] |
99 (42) [12%] |
88 (32) [12%] |
<0.01 | <0.01 | <0.01 |
|
Total Fat (g/day) (SD)[%kcal] |
134 (68) [36%] |
114 (44) [34%] |
<0.01 | <0.01 | 0.04 |
|
Carbohydrate (g/day) (SD)[%kcal] |
462 (174) [54%] |
392 (131) [52%] |
<0.01 | <0.01 | 0.01 |
| Body Composition | |||||
|
Body Weight (kg) |
62.4 (12.2) | 75.2 (17.5) | <0.01 | <0.01 | <0.01 |
| BMI (kg/m2) | 20.9 (3.6) | 26.7 (6.0) | <0.01 | <0.01 | <0.01 |
|
Fat-Free Mass (kg / % of total kg body weight) |
48.8 (10.2) / 79 (8) |
49.5 (12.5) / 68 (9) |
0.73 / <0.01 |
<0.01/ <0.01 |
0.02/ <0.01 |
|
Fat Mass (kg / % of total kg body weight) |
13.6 (8.6) / 21 (8) |
25.6 (11.1) / 32 (9) |
<0.01 /<0.01 |
<0.01/ <0.01 |
<0.01/ <0.01 |
Unpaired student’s t-tests were used for analysis. PROC GLM was used to build models to determine differences in macronutrient and body composition variables between TB subjects and household contacts. Model I adjusted for age and sex, while model II adjusted for age, sex, employment status and smoking. We also tested for interactions with sex.
Despite higher macronutrient dietary intake, subjects with TB demonstrated significantly lower body weight (18.6% lower), BMI (24.4% lower) and particularly fat mass (61.2% lower in grams; 41.5% as percentage of total mass); fat-free mass (1.4% higher in grams; 14.9% as a percentage of total body mass) was similar between the groups (Table 2). These differences remained significant even after adjusting for differences in age, sex, smoking and employment status.
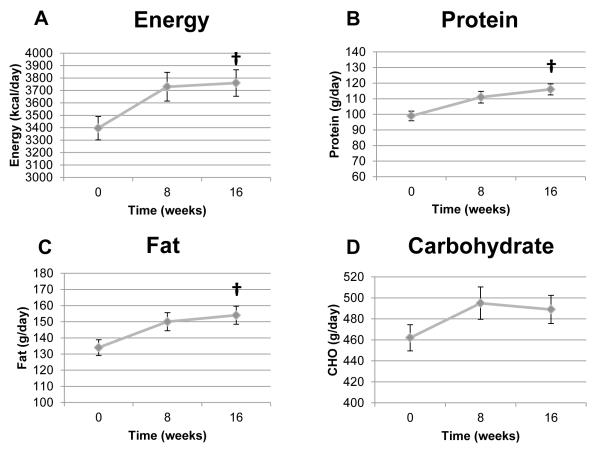
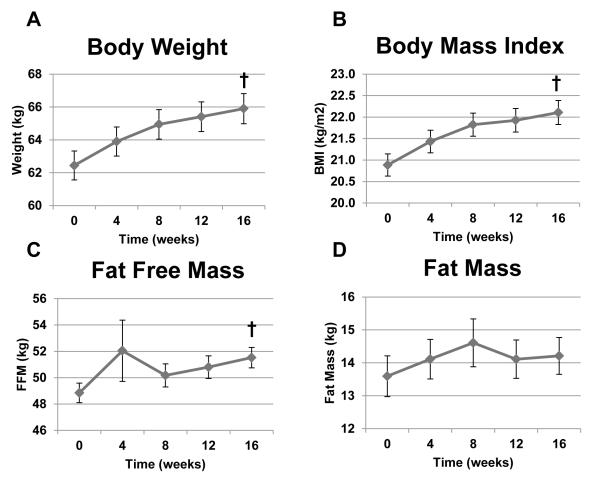
There were no differences in either macronutrient intake or the body composition indexes among TB patients who received high-dose vitamin D3 compared to those receiving placebo (Supplemental Table S1). Therefore, all TB subject data were combined for further analysis of subjects with TB. Mean daily intake of macronutrients at baseline, week 8 and week 16 is shown in Figure 1. Energy intake was 3396 kcal/day at baseline to 3724 kcal/day at week 8 (p=0.38) and then plateaued. Reported fat intake rose quantitatively from baseline to weeks 8 and 16, but these differences were not statistically significant. However, reported dietary protein intake significantly increased over the course of the 16-week study (p=0.030). CHO intake remained stable (Figure 1). Figure 2 shows serial body composition indexes in TB disease subjects. Body weight, BMI, and kilogram of fat-free mass significantly increased over time (all p<0.0001), while kilogram of fat mass remained constant (p=0.67).
Figure 1.
Mean daily reported energy, protein, fat and carbohydrate intake in TB patients over time. Panel A: Energy (kcal/day; p=0.0062). Panel B: Total protein (g/day; p<0.0001). Panel C: total fat (g/day; p=0.0043). Panel D: Total carbohydrate (g/day; p=0.1142). One-factor mixed-model repeated-measures analysis of variance was used for statistical analysis. Sample sizes were 191, 160, and 146 for time points 0, 8, and 16 weeks, respectively. Data as mean ± SEM. † Significant effect of time (p=0.030). CHO=carbohydrate.
Figure 2.
Body composition indexes in TB patients over time. Panel A: Body weight (kg, p<0.0001). Panel B: BMI (kg/m2, p<0.0001). Panel C: Fat-free mass (kg, p<0.0001). Panel D: Fat mass (kg, p=0.68). One-factor mixed-model repeated-measures analysis of variance was used for statistical analysis. Sample sizes were 191, 170, 165, 145, and 162 for time points 0, 4, 8, 12, and 16 weeks, respectively. Data as mean ± SEM. † Significant effect of time. BMI=body mass index; kg=kilograms.
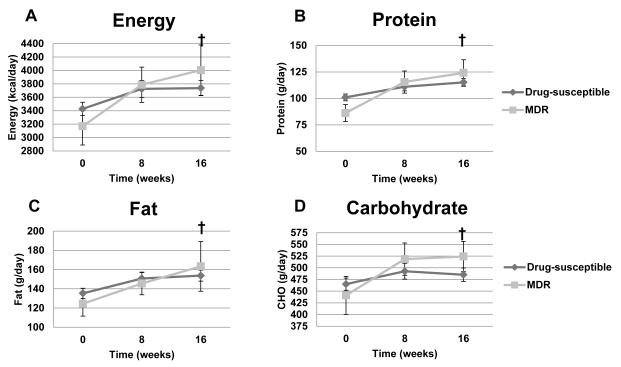
Mean daily dietary protein intake was significantly greater over time (p=0.0042) in both TB subject groups, but there were no significant differences between the drug-sensitive TB and the MDR-TB cohorts (Figure 3). Mean daily intake of energy, CHO and fat were each similar over time between drug-sensitive TB and MDR-TB subjects. When the MDR-TB group was dichotomized by median time to initiation of second-line anti-TB drug treatment regimen (<56 days n=11; >56 days n=12), there were no differences between groups at any time point.
Figure 3.
Mean daily energy, protein, fat and carbohydrate intake in TB patients as a function of drug susceptibility over time. Panel A: Energy (kcal/day, p=0.0077 effect of time; p=0.93 effect of drug susceptibility; p=0.43 for interaction). Panel B: Total protein (g/day, p=0.0003 effect of time; p=0.96 effect of drug susceptibility; p=0.16 for interaction). Panel C: total fat (g/day, p=0.0373 effect of time; p=0.87 effect of drug susceptibility; p=0.77 for interaction). Panel D: Total carbohydrate (g/day, p=0.0327 effect of time; p=0.67 effect of drug susceptibility; p=0.33 for interaction). Two-factor mixed-model repeated-measures analysis of variance was used for statistical analysis. Sample sizes in the drug-susceptible group were 168, 143, and 132 for time points 0, 8, and 16 weeks, respectively; sample sizes for the MDR-TB group were 23, 17, and 14 for time points 0, 8, and 16 weeks, respectively. † Significant effect of time. MDR-TB= Multi-drug resistant-TB.
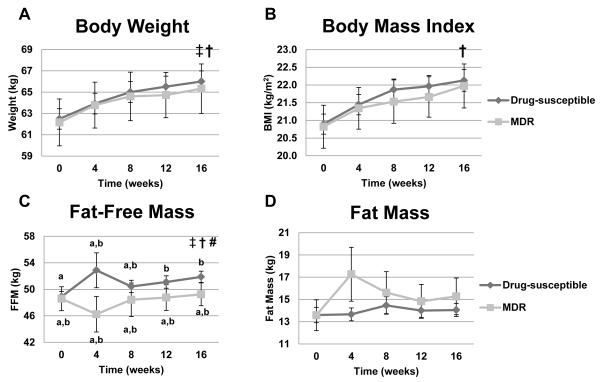
Body weight, BMI and fat-free mass significantly increased over time in TB disease subjects (Figure 4). However, this anabolic effect, presumably due to effective anti-TB drug therapy and TB clearance in the drug-sensitive TB cohort, was blunted in MDR-TB compared to drug-sensitive TB subjects. In the MDR-TB cohort, both changes in body weight and fat-free mass (a surrogate marker for lean body mass) were similar at baseline as the drug-sensitive cohort, but then essentially did not change over time, in marked contrast to the anti-TB drug sensitive subjects (Figure 4). Fat mass was not significantly altered over time or as a function of drug susceptibility. The fat-free mass data also showed a significant interaction effect of MDR-TB status and time (p = 0.044). In the drug-sensitive TB subjects, there was a significant difference from baseline (increased fat-free mass) at weeks 12 and 16 (p=0.0009 and p<0.0001, respectively) and between week 8 and 16 (p=0.044). In contrast, fat-free mass did not change over time in the MDR-TB patients. In addition, fat-free mass was significantly lower in the MDR-TB cohort than the drug-susceptible cohort at both week 4 and week 16 (p=0.046) (Figure 4).
Figure 4.
Body composition indexes in TB patients as a function of drug susceptibility over time. Panel A: Body weight (kg, p<0.0001 effect of time, p= 0.048 effect of drug susceptibility; p=0.91 for interaction). Panel B: BMI (kg/m2, p<0.0001 effect of time; p= 0.10 effect of drug susceptibility; p=0.76 for interaction). Panel C: Fat-free mass (kg, p =0.0502 effect of time, p =0.0070 effect of drug susceptibility and p= 0.0249 for interaction). Given the interaction effect, pairwise comparisons were made over time between the two TB groups at each time point for fat-free mass. Values for fat-free mass at individual time points within and between the two drug-susceptibility groups that do not share the same letters are significantly different. Panel D: Fat mass (kg, p =0.17 effect of time, p =0.69 effect of drug susceptibility and p= 0.68 for interaction). Two-factor mixed-model repeated-measures analysis of variance was used for statistical analysis. Sample sizes for drug susceptible group were 168, 153, 147, 131, and 145 for time points 0, 4, 8, 12, and 16 weeks, respectively; sample sizes for the MDR-TB group were 23, 17, 18, 14, and 17 for time points 0, 4, 8, 12, and 16 weeks, respectively. † Significant effect of time, ‡ Significant effect of drug-susceptibility,# significant interaction between time and drug-susceptibility.
Discussion
Body weight and BMI have been widely studied within the scope of TB.(4, 8-11, 18-20) However, our study adds to limited data on serial dietary intake and body composition changes during pulmonary TB.(9, 10) Our baseline body composition data show that despite the increased caloric and macronutrient intake, adults with recently diagnosed TB disease have significantly lower body weight and BMI, and markedly lower body fat and fat-free mass compared to asymptomatic adults from the same household. This suggests the possibility that enhanced lipolysis and skeletal muscle wasting occurs early in the disease course.
In the full cohort, body weight, BMI and fat-free mass increased over time with largely unchanged fat mass (from a low baseline percentage of body fat). This occurred in association with a significant increase in dietary protein and more modestly increased in energy and fat over time. Improved body weight with TB treatment is well known to occur.(9, 10, 18-20) All TB patients in Georgia are encouraged to increase their overall food intake as part of standard clinical care, but no specific nutrient supplements are provided. This, coupled with potentially improved appetite after initiation of anti-TB drugs likely resulted in the improved body mass over time. Whether fat-free mass/lean body mass or fat mass can be increased further in this population with supplementation with either certain foods or defined nutrient products would be of interest.
In contrast to our overall data, Schwenk et al studied serial body composition changes in 30 adults with TB in London (21 men, 9 women) at baseline (within 3 days of anti-TB drug initiation) and after 1 and 6 months of treatment.(9) Body composition, determined by duel-energy X-ray absorptiometry (DEXA) and deuterium bromide dilution to derive a four-compartment model, overall showed that the 10% gain in body weight at 6 months was largely due to a 44% gain in total body fat mass, while total body protein did not change significantly over time.(9) Sanchez et al used BIA to assess body composition in 24 adults with TB in Los Angeles, CA at baseline and weeks 4, 8, 16 and 24 after initiation of anti-TB drugs.(10) They found a 5.5% increase from baseline in body weight by week 4 and 15.6% by week 24; repletion of body weight was primarily as fat (increased by ≈ 2 and 5 kg by weeks 4 and 24, respectively), but fat-free mass increased by approximately 1 kg (2.0%) by week 4 and by ≈ 3 kg (6.6% by week 16); PA was shown to improve over time.(10) Neither of these latter studies provided concomitant dietary intake data or information on MDR-TB status. Our overall data on body weight and BMI changes are consistent with the data of Schwenk et al and Sanchez et al, while our BIA-derived fat-free mass is consistent with Sanchez et al.(9, 10)
Fat mass did not significantly increase over time in our TB disease subjects, and the reason for this is unclear at this time. A limitation of our data is that BIA-derived fat-free mass is not a direct measure of skeletal muscle mass and fat mass is better determined by DEXA and air-displacement plethysmography.(9) Fat-free mass is largely composed of lean tissue and primarily skeletal muscle, and may have been maintained in these individuals by the concomitant increase in energy and dietary protein sources. Studies in TB patients that measure lean body mass and other body composition compartments directly (e.g. DEXA coupled with deuterium bromide tests as reported in the TB cohort by Schwenk et al),(9), or ideally combined with air-displacement plethysmography to determine a four-compartment model, would be of interest. Additionally, serial studies of systemic inflammation,(10) skeletal muscle function,(21) metabolomic profiles(22) and protein and fat kinetic studies using stable isotopes(23) in TB would help to better define body composition changes, functional consequences and macronutrient metabolism in TB disease. Georgia has been designated by the WHO as a high-burden country for MDRTB.(15, 24, 25) To our knowledge, no previous study has evaluated dietary intake or body composition in patients with MDR-TB over time and in comparison to drug-sensitive subjects. One prior study showed that weight gain was associated with an improved clinical better response to MDR-TB drug treatment, which is consistent with our data in drug-sensitive subjects.(7) Despite a trend toward increased mean energy intake, individuals with MDR-TB gained significantly less body weight and fat-free mass. These data suggest that MDR-TB patients were more catabolic/less anabolic than the drug-sensitive TB patients, likely due to ineffective anti-TB drug treatment until second-line drugs were initiated approximately 8 weeks after diagnosis. Data on markers of inflammation, energy expenditure and metabolism would be of interest in this regard comparing MDR-TB with drug-sensitive subjects. Larger studies comparing these two TB disease cohorts over a longer period of time are also needed to confirm our data.
Our study has several limitations. Our cohort contained a small number of patients with MDR-TB, but this rate was consistent with the burden of MDR-TB in Georgia (11-12). Our cohort had a low rate of HIV (12-13) and we did not assess long-term relapse or cure rates as a function of our study endpoints. The nature of our food intake questionnaire did not allow us to control for weekend versus weekday differences in food intake. However, these TB patients are instructed to eat as much as possible and are generally not working during active disease, which diminishes differences between weekend days and weekdays. Only 24% of our sample was underweight (BMI < 18.5 kg/m2), which is a lower percentage of malnutrition than in TB cohorts from other lower-middle income countries.(19, 20, 26) Thus, our results cannot be generalized to TB populations with higher rates of malnutrition and food insecurity. There was a 14% loss to follow-up in our clinical trial; however, this is similar to other studies conducted in TB (9-10). In addition, the sample of 36 household contacts is a small convenience sample, representing those available contacts that consented to be studied when a TB subject entered the study. Although our dietary intake data was obtained from a validated tool,(16) self-reported intake is inherently prone to bias. In addition, the lack of data on PA and the specific body water compartments is a limitation of this report.
In conclusion, patients with recently diagnosed pulmonary TB in Georgia consume greater amounts of energy, protein, CHO and fat than asymptomatic controls without evidence of TB disease. However, TB patients exhibited lower body weight and BMI, which appeared to be largely explained by loss of body fat. Serial data showed a modest increase in macronutrient intake in TB patients which, in association with anti-TB drug therapy, is linked with significantly increased body weight, BMI and fat-free mass and maintenance of body fat and body composition proportions in the overall cohort. However, our data show, for the first time, that this anabolic response is blunted in individuals with MDR-TB, despite presumably adequate macronutrient intake per kilogram body weight. This strongly suggests that anabolism is less efficient in MDRTB, likely due to ongoing catabolic responses associated with poorly controlled TB. Additional studies on serial body composition and concomitant nutrient intake studies to guide nutritional intervention strategies in TB disease in general and also in MDR-TB are needed.(5, 13) Such studies will be of particular interest in centers where early diagnosis and thus earlier second-line drug intervention in MDR-TB is now possible.(27)
Supplementary Material
Supplemental Figure 1. CONSORT diagram of the progress through the phases (enrollment, intervention allocation, follow-up and data analysis) of the double-blind, randomized controlled trial comparing standard antimicrobial TB treatment plus high-dose vitamin D3 or standard antimicrobial TB treatment plus placebo.
Acknowledgments
The authors acknowledge the staff of the Georgian National Center for Tuberculosis and Lung Disease for their care of the study subjects. We also appreciate the efforts of Jessica A. Alvarez, Ph.D., R.D. and Jennifer L. Jones, Ph.D., R.D. for their helpful comments on the manuscript drafts. Sources of Support: This work was supported in part by National Institutes of Health grants UL1 TR000454 (Atlanta Clinical and Translational Science Institute), K24 DK096574 (TRZ), D43 TW007124 (HMB, RRK, TRZ), K23 AR054334 (VT), K23 AI103044 (RRK), R01 ES016731 (DPJ) and the Emory University Global Health Institute (TRZ, VT, HMB, UR, RRK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- (AFB)
Acid-fast bacilli
- (BIA)
Bioelectrical impedance analysis
- (BMI)
Body mass index
- (CHO)
Carbohydrate
- (CRF)
Case report form
- (IU)
International Units
- (kcal)
Kilocalories
- (Mtb)
Mycobacterium tuberculosis
- (MDR-TB)
Multidrug resistant tuberculosis
- (NCTBLD)
National Center for Tuberculosis and Lung Disease
- (NS)
Not significant
- (NRL)
National Reference Laboratory
- (RCT)
Randomized controlled trial
- (TB)
Tuberculosis
- (Vit D)
Vitamin D3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonnroth K, Williams BG, Cegielski JP, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–55. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 3.Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34(2):153–7. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 4.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Tuberculosis Trials Consortium. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174:344–8. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 5.Mupere E, Parraga IM, Tisch DJ, Mayanja HK, Whalen CC. Low nutrient intake among adult women and patients with severe tuberculosis disease in Uganda: a cross-sectional study. BMC Public Health. 2012;12:1050. doi: 10.1186/1471-2458-12-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Guideline: Nutritional Care and Support for Patients with Tuberculosis. World Health Organization; Geneva: 2013. [PubMed] [Google Scholar]
- 7.Gler MT, Guilatco R, Caoili JC, Ershova J, Cegielski JP, Johnson JL. Weight gain and response to treatment for multidrug-resistant tuberculosis. Am J Trop Med Hyg. 2013;89(5):943–9. doi: 10.4269/ajtmh.13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PloS one. 2013;8(10):e77979. doi: 10.1371/journal.pone.0077979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwenk A, Hodgson L, Wright A, Ward LC, Rayner CF, Grubnic S, et al. Nutrient partitioning during treatment of tuberculosis: gain in body fat mass but not in protein mass. Am J Clin Nutr. 2004;79(6):1006–12. doi: 10.1093/ajcn/79.6.1006. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez A, Azen C, Jones B, Louie S, Sattler F. Relationship of acute phase reactants and fat accumulation during treatment for tuberculosis. Tuberc Res Treat. 2011 doi: 10.1155/2011/346295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV-tuberculosis coinfection receiving directly observed short-course chemotherapy for tuberculosis. Trop Med Int Health. 2011;16(6):699–706. doi: 10.1111/j.1365-3156.2011.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paton NI, Chua Y, Earnest A, Chee CBE. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. Am J Clin Nutr. 2004;80(2):460–5. doi: 10.1093/ajcn/80.2.460. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. The Cochrane Library. 2011 Nov;9(11) doi: 10.1002/14651858.CD006086.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomtadze N, Aspindzelashvili R, Janjgava M, Mirtskhulava V, Wright A, Blumberg HM, et al. Prevalence and risk factors for multidrug-resistant tuberculosis in the Republic of Georgia: a population-based study. Int J Tuberc Lung Dis. 2009;13(1):68–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Frediani JK, Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, et al. A culture-specific nutrient intake assessment instrument in patients with pulmonary tuberculosis. Clin Nutr. 2013;32(6):1023–8. doi: 10.1016/j.clnu.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benova L, Fielding K, Greig J, Nyang’wa B, Casas EC, Silveria de Fonseca M, et al. Association of BMI category change with TB treatment mortality in HIV-positive smear-negative and extrapulmonary TB patients in Myanmar and Zimbabwe. PloS one. 2012;7(4):e35948. doi: 10.1371/journal.pone.0035948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Weight, body composition and handgrip strength among pulmonary tuberculosis patients: a matched cross-sectional study in Mwanza, Tanzania. Trans R Soc Trop Med Hyg. 2011;105:140–7. doi: 10.1016/j.trstmh.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Paton NI, Ng Y. Body composition studies in patients with wasting associated with tuberculosis. Nutrition. 2006;22:245–51. doi: 10.1016/j.nut.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30(2):135–42. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. 2013;32(2):309–13. doi: 10.1016/j.clnu.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization [cited 2012 May 3];WHO country profile 2012. Available from: http://www.who.int/countries/geo/en/
- 25.Mdivani N, Zangaladze E, Volkova N, Jibuti T, Shubladze N, Kutateladze T, et al. High Prevalence of Multidrug-Resistant Tuberculosis in Georgia. Int J Infect Dis. 2008;12(6):635–44. doi: 10.1016/j.ijid.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villamor E, Saathoff E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socioeconomic status, and severity of tuberculosis. Eur J Clin Nutr. 2006;60(2):163–71. doi: 10.1038/sj.ejcn.1602281. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization [cited 2012 May 3];WHO tuberculosis country profiles 2012. Available from: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=GE&outtype=pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CONSORT diagram of the progress through the phases (enrollment, intervention allocation, follow-up and data analysis) of the double-blind, randomized controlled trial comparing standard antimicrobial TB treatment plus high-dose vitamin D3 or standard antimicrobial TB treatment plus placebo.